Abstract
Schwannomas are benign tumors originating from myelinating cells constituting nerve sheaths but rarely contain cellular elements of the nerve. The authors encountered a 47-year-old female patient with a schwannoma on the anterior mandibular ramus arising from the buccal nerve, measuring 3 cm×4 cm. Surgical resection was performed with preservation of the buccal nerve via microsurgical dissection. After one month, the sensory function of the buccal nerve was recovered without complications.
Schwannoma is an encapsulated benign neurogenic tumor originating from the myelinating cells of the nervous system and composed entirely of neoplastic Schwann cells that rarely contain cellular elements, such as axons. Schwannoma is most often associated with the vestibular branch of cranial nerve VIII or the spinal cord and commonly occurs on the soft tissue of the head and neck, upper and lower extremities, and spine1. The Asia-Pacific population has a high incidence of schwannoma1.
The common clinical symptoms of mandibular schwannomas are swelling involving the body of the mandible, cortical thinning, and expansion. Schwannoma shows unspecific radiologic features as a well-defined monocular solid tumor with a thin sclerotic border. Schwannomas demonstrate typical magnetic resonance imaging (MRI) features of T1 iso-to-hypointensity, T2 hyperintensity, and postcontrast enhancement, including eccentric to the nerve fascicles and covering the peripheral nerve2. The final diagnosis is confirmed with histopathological or immunohistochemical evaluations. Surgical excision is the standard treatment of choice, and the prognosis is favorable because recurrence or malignant transformation is rare3.
Regarding the oral and maxillofacial region, the most frequent site of a schwannoma is the tongue, followed by the palate, buccal mucosa, gingiva, lips, and vestibule3,4. On the other hand, mandibular schwannomas are rare but can develop on the posterior along the mandibular branch of the trigeminal nerve5. Most have been reported to be the intra-osseous type, mostly related to the inferior alveolar neurovascular bundle, and occur 50% more frequently in females, at an average of 36.9 years (range, 8-77 years) of age6. The tumor can extend beyond the mandible in rare cases7. This paper reports a schwannoma originating from the buccal nerve, which was originally misdiagnosed as a salivary gland tumor on the mucosa around the anterior ramus on preoperative MRI.
This retrospective clinical study was approved by the Institutional Review Board of Gangnam Severance Hospital (IRB No. 3-2021-0298). The authors have read the Helsinki Declaration for research on humans and have followed the guidelines in this investigation. A 47-year-old female patient complained of swelling on the anterior ramus ascending branch. She had no history of trauma, bleeding, limitation of mouth opening, or pain. Although there appeared to be linea alba on the overlying mucosa, an intra-oral examination revealed firm and mobile swelling without pain or other symptoms. Radiography revealed expansion of the mandibular ramus and a well-defined heterogeneously enhancing mass, approximately 3 cm×4 cm in size, without aggressive destruction of the underlying bone.(Fig. 1. A) MRI showed a multifocal hypointense portion and a well-bounded lesion.(Fig. 1. B) Although no malignant appearance was detected on these images, the plan was to excise the tumor completely under general anesthesia because it had been misdiagnosed as a salivary gland tumor, and the patient refused incisional biopsy under local anesthesia due to fear. Laboratory investigations for routine preoperative screening did not yield anything significant.
Under general anesthesia, surgical excision was started by carefully dissecting the mass from the buccal mucosa on the anterior part of the mandibular ramus.(Fig. 2. A) The middle portion of the tumor was peripherally attached to the buccal nerve.(Fig. 2. B) A tumor fragment was sent for frozen biopsy to preserve the buccal nerve. Immunohistochemical staining confirmed a favorable schwannoma positive for the S-100 protein and vimentin. The tumor was excised via microsurgical dissection from the buccal nerve.(Fig. 2. C)
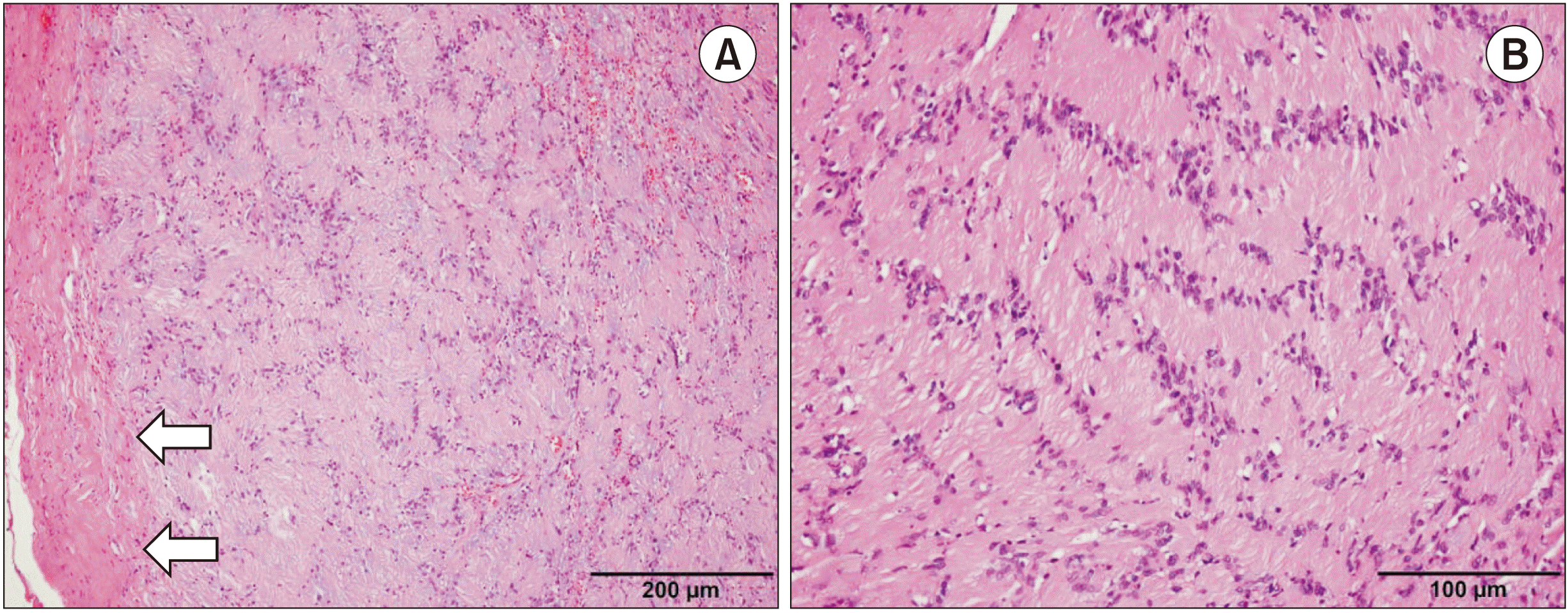
Pathological examination of the surgical specimen revealed a spindle cell proliferative lesion surrounded by a fibrous capsule and exhibiting a characteristic biphasic (Fig. 3. A) morphology. Most of the tumor was composed of compact spindle cells with indistinct cytoplasmic borders and a nuclear palisading pattern.(Fig. 3. B) A small portion of the tumor was composed of loosely arranged spindle cells in the myxoid stroma and irregularly spaced blood vessels (not shown). Neither nuclear atypia nor increased mitotic activity was observed. The final diagnosis of schwannoma was established from these histopathological findings and the S-100 positivity on immunohistochemistry.
Postoperatively, the patient complained of partial paresthesia of 70% and a limitation of mouth opening for approximately 30 mm, but these symptoms resolved after one month. The five-month follow-up examination showed no signs of recurrence or neuropathic symptoms.
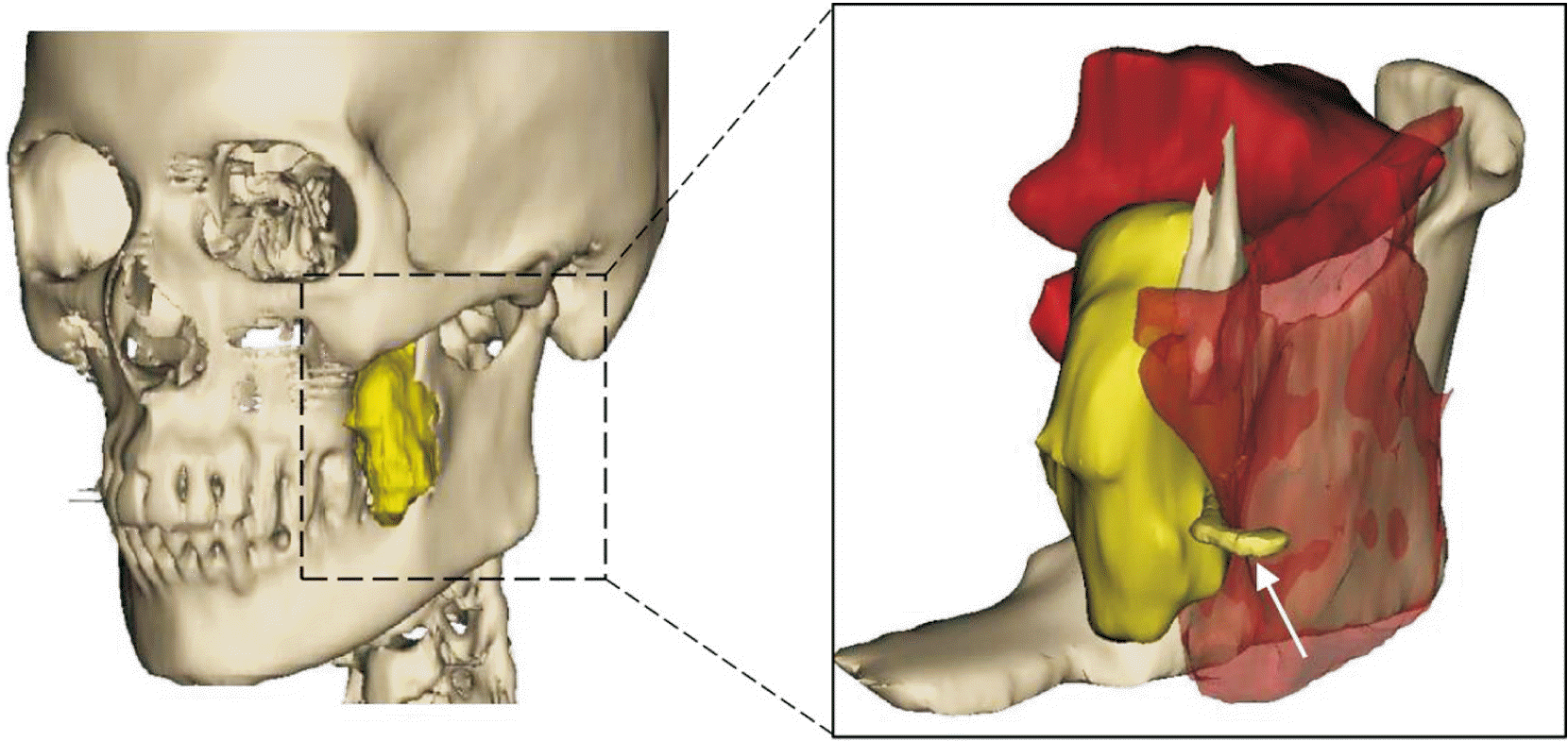
To the best of the authors knowledge, this is the first described oral schwannoma that originated from the buccal nerve. The authors could minimize the patient’s discomfort by preserving the nerve, which was not detected in the preoperative images. Hence, a three-dimensional diagram should be used when considering the nerve, as shown in Fig. 4.
Although the recurrence rate of schwannoma was reported to be 4%-6% after resection1, the rate was as high as 33.3% while preserving the facial nerve from jugular foramen schwannomas8. With regard to recurrence, the size of the schwannoma was suggested to be a critical risk factor, with risk increasing by 15.7% for every 1 cm increase in size (hazard ratio, 1.157; 95% confidential interval, 1.016-1.319)1. Therefore, long-term follow-up of patients with a large schwannoma will be needed to preserve the parental nerve.
In general, schwannomas are well-encapsulated and eccentric to the nerve fascicles, with few axons embedded in the tumor mass, and contain variable amounts of Antoni A and B areas. The Antoni A area is a compact cellular area characterized by well-aligned nuclei and interdigitating cellular processes. The intense laminin-positive staining in schwannomas is believed to represent the tight organization due to the adhesive properties of laminin, which also expressed the S-100 protein9. In contrast, the Antoni B area contains relatively few cells and loosely arranged cellular components. A schwannoma with a large portion of Antoni B tissues might be thin and wispy due to microcysts filled with basophilic mucin9.
The schwannoma of this patient was uniform with a solid parenchyma and was histologically revealed, during surgery by frozen section, as the favored type from the S-100 positive stain and dominant Antoni A areas. These features might allow a dissection from the buccal nerve. As this is the first report of schwannoma from the buccal nerve, the authors cannot suggest a long-term prognosis for recurrence. Change in malignant transformation of schwannomas is rare, and they rarely metastasize, though the lung is the most common site if metastasis occurs10. In conclusion, this case report showed that schwannoma can develop from the buccal nerve, and surgeons can minimize postoperative discomfort by preserving the nerve. In addition, long-term regular follow-up is needed.
Notes
Authors’ Contributions
J.K.K. conceived the idea and wrote the paper. J.K.H. and J.Y.K. were responsible for critical revision of the article. D.H. was responsible for the acquisition of the histologic data. J.K.K. and J.Y.K. were responsible for the laboratory or clinical/literature search, and analysis and interpretation of the data collected. All authors made substantial contributions to the discussion of content, reviewed and edited the manuscript before submission.
References
1. Fehlings MG, Nater A, Zamorano JJ, Tetreault LA, Varga PP, Gokaslan ZL, et al. 2016; Risk factors for recurrence of surgically treated conventional spinal schwannomas: analysis of 169 patients from a multicenter international database. Spine (Phila Pa 1976). 41:390–8. https://doi.org/10.1097/brs.0000000000001232. DOI: 10.1097/BRS.0000000000001232. PMID: 26555828. PMCID: PMC4769652.

2. Crist J, Hodge JR, Frick M, Leung FP, Hsu E, Gi MT, et al. 2017; Magnetic resonance imaging appearance of schwannomas from head to toe: a pictorial review. J Clin Imaging Sci. 7:38. https://doi.org/10.4103/jcis.jcis_40_17. DOI: 10.4103/jcis.JCIS_40_17. PMID: 29114437. PMCID: PMC5651654.

3. Kurup S, Thankappan K, Krishnan N, Nair PP. 2012; Intraoral schwannoma--a report of two cases. BMJ Case Rep. 2012:bcr1220115389. https://doi.org/10.1136/bcr.12.2011.5389. DOI: 10.1136/bcr.12.2011.5389. PMID: 22778466. PMCID: PMC3417034.

4. Shim SK, Myoung H. 2016; Neurilemmoma in the floor of the mouth: a case report. J Korean Assoc Oral Maxillofac Surg. 42:60–4. https://doi.org/10.5125/jkaoms.2016.42.1.60. DOI: 10.5125/jkaoms.2016.42.1.60. PMID: 26904498. PMCID: PMC4761576.

5. Yumuşakhuylu AC, Sari M, Topuz MF, Bağlam T, Binnetoğlu A. 2014; Trigeminal schwannoma extending into the parapharyngeal space. J Craniofac Surg. 25:e328–30. https://doi.org/10.1097/scs.0000000000000593. DOI: 10.1097/SCS.0000000000000593. PMID: 24978683.

6. Perkins D, Stiharu TI, Swift JQ, Dao TV, Mainville GN. 2018; Intraosseous schwannoma of the jaws: an updated review of the literature and report of 2 new cases affecting the mandible. J Oral Maxillofac Surg. 76:1226–47. DOI: 10.1016/j.joms.2017.12.017. PMID: 29360457.

7. Sun Z, Sun L, Li T, Ma X, Zhang Z. 2011; Intraosseous trigeminal schwannoma of mandible with intracranial extension. J Laryngol Otol. 125:418–22. https://doi.org/10.1017/s0022215110002707. DOI: 10.1017/S0022215110002707. PMID: 21269550.

8. Sanna M, Bacciu A, Falcioni M, Taibah A. 2006; Surgical management of jugular foramen schwannomas with hearing and facial nerve function preservation: a series of 23 cases and review of the literature. Laryngoscope. 116:2191–204. https://doi.org/10.1097/01.mlg.0000246193.84319.e5. DOI: 10.1097/01.mlg.0000246193.84319.e5. PMID: 17146395.

9. Wippold FJ 2nd, Lubner M, Perrin RJ, Lämmle M, Perry A. 2007; Neuropathology for the neuroradiologist: Antoni A and Antoni B tissue patterns. AJNR Am J Neuroradiol. 28:1633–8. https://doi.org/10.3174/ajnr.a0682. DOI: 10.3174/ajnr.A0682. PMID: 17893219. PMCID: PMC8134199.

10. Raper DMS, Sweiss F, Almira-Suarez MI, Helm G, Sheehan JP. 2013; Malignant peripheral nerve sheath tumor at the cerebellopontine angle treated with Gamma Knife radiosurgery: case report and review of the literature. J Radiosurg SBRT. 2:147–53. PMID: 29296354. PMCID: PMC5658887.
Fig. 1
Clinical images of schwannoma from the long buccal nerve. A. Swelling was noted on the anterior border of the ramus ascending branch. B. The long buccal nerve was detected from the middle portion of the tumor. C. The tumor was resected while preserving the long buccal nerve by dissecting it from the tumor.
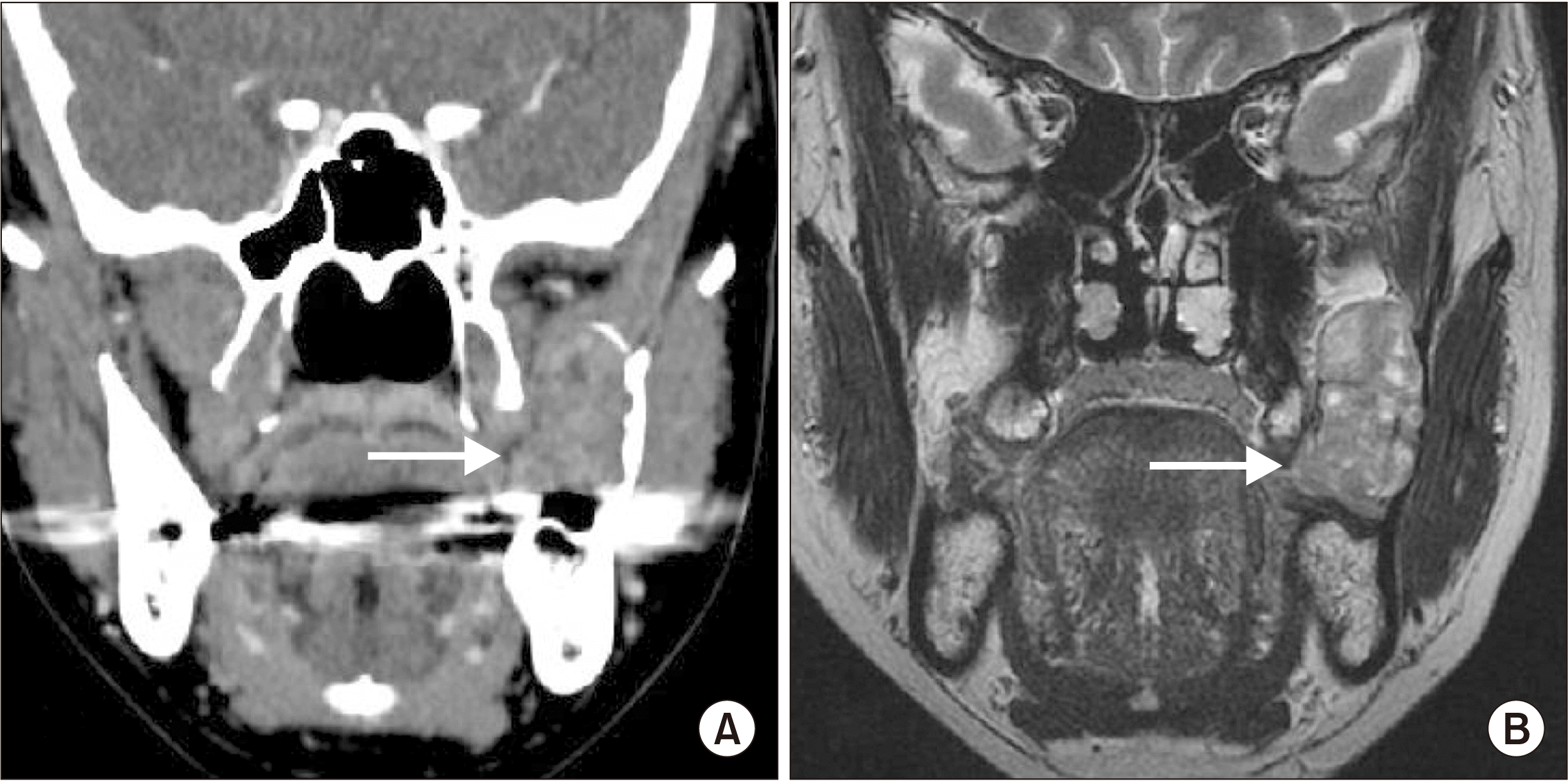
Fig. 2
Preoperative images. A. Computed tomography revealed a homogeneous mass, slightly less dense than the adjacent muscles, and expansion of the ramus with some irregular resorption borders (arrow). B. T2-weighted magnetic resonance imaging showed a multifocal hypointense portion, but the long buccal nerve was not visualized clearly (arrow).
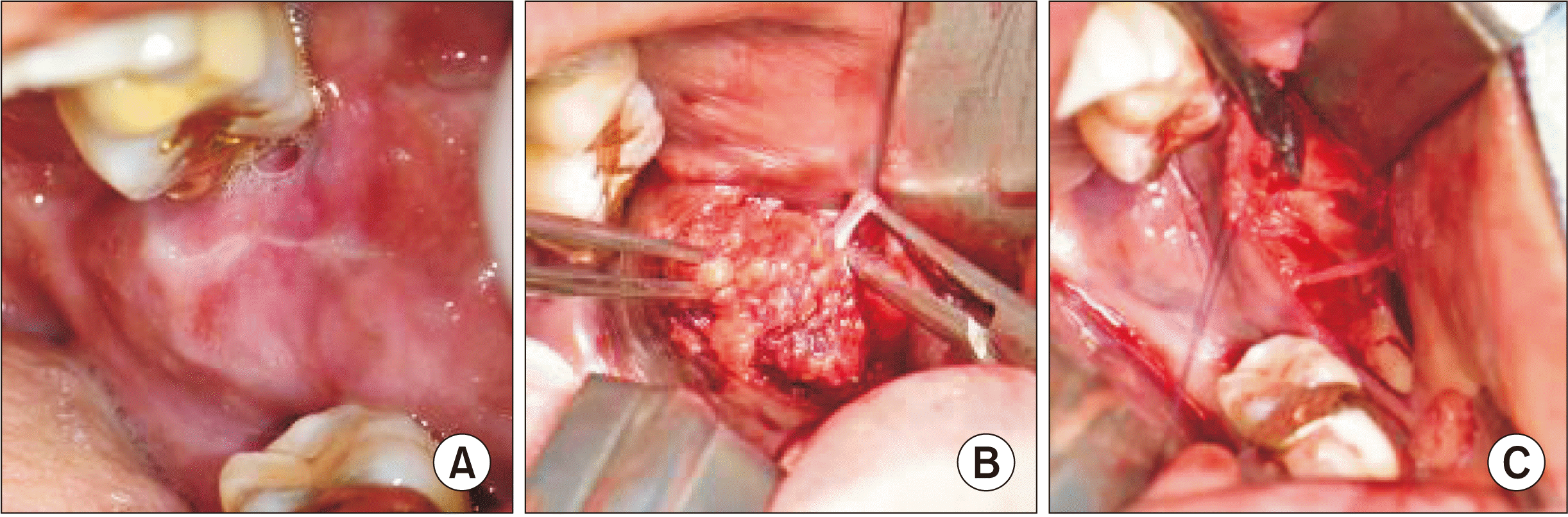
Fig. 3
Histopathology of the surgical specimen (H&E staining). A. The mass was composed of spindle cells with proliferated biphasic growth patterns and was surrounded by a well-formed fibrous capsule (arrows) (×100). Scale bar=200 µm. B. Compact cellular area, called an Antoni A area, showed characteristic nuclear palisading separated by fibrillary cell processes (×100). Scale bar=100 µm.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download