Abstract
Dental implants have been utilized for many years to treat individuals with missing teeth. To optimize the long-term success rate of such implants, new designs, surfaces, and materials have been analyzed. It is important for the clinician to have a background in the field of implant surface design, to be familiar with the strengths and limitations of the available options, and to be aware of the alterations in surface structure that may occur following installation. This article provides a detailed review of the structure and the surface characteristics of dental implants, the modifications of implant surface, as well as the methods of evaluating implant surface structure. Moreover, it provides information concerning the structural changes that may take place at the time of dental implant placement. It is important for clinicians to be aware of such changes to plan and execute implant procedures with the highest possible success and implant survival rates.
Go to : 
Dental implants are utilized to replace missing teeth in patients with partially or fully edentulous areas to improve function and appearance and enhance the patient’s quality of life1. Titanium is a silver-colored transition metal used for dental implants due to its physical and mechanical strength, corrosion resistance, chemical stability, and high biocompatibility. Titanium can become integrated with bone in a process known as “osseointegration,” which is necessary for long-term durability of dental implants2,3.
Studies have confirmed the high survival rates of implants over the last 50 years. The use of improved designs, surfaces, and materials has been a priority. Nevertheless, modern implants have increased roughness. This leads to having more prominent points on their surface, which are likely to break and detach from the implant body, during insertion into the bone.
Wennerberg et al.4 presented a method to quantitatively assess the outer surface of the fixture of dental implants. They examined roughness metrics before and after insertion for determining the degree of wear4. The shape of the implants and the heterogeneity of the bone tissue both have an impact on the shear forces created by friction of self-tapping implants on bone tissue to cause a dynamic shift of stresses along the implant fixture5.
There have been concerns about deterioration of dental implant material, allergic reactions, and chronic peri-implant inflammation, all of which can lead to implant failure6. Within the first 15 years after insertion, more than 11% of dental implants fail and must be removed7,8. Dental implants can fail for a variety of reasons, both biological and mechanical. These include peri-implantitis (inflammation around the implant that causes bone loss), degradation of structural materials and/or connections, flaws in the implant design, loss of patient bone density, surgical and/or prosthetic complications, and patient-specific conditions8,9.
Moreover, because placement precision is subjective, this operation requires a highly experienced practitioner. In difficult areas, such as regions of dense bone or zones of restricted vertical bone height, correct placement of the implant may not occur upon the first attempt, necessitating a screw-out and reinsertion.
This review provides a summary of dental implant structures and surface modifications, commercially available surface treatments, methods of evaluating dental implant surfaces, and possible changes in implant structure upon insertion.
Go to : 
Osseointegration, first described by professor Brånemark and colleagues, is direct contact (at the light microscope level) between living bone and the implant; “secondary implant stability” is another term for this biomechanical concept10.
Trauma to bone tissue occurs during the creation of an implant cavity and is followed by several stages of wound healing. Fibrin polymerization and formation of a blood clot are the initial results of the cellular and plasmatic hemostasis mechanisms. The blood clot acts as a scaffold for bone-forming cells, extracellular matrix (ECM) deposition, and neo-angiogenesis11,12.
Davies13 claimed that textured surfaces increase blood clot adherence and bone growth (contact osteogenesis) on the implant. This is most likely because a clot contracts away from a perfectly smooth machined surface, producing a micro-gap. When a micro-gap is present, new bone starts to form distant from the implant, as the osteogenic cells are unable to reach the implant surface (distant osteogenesis)13. Thus, osseointegration occurs more quickly on textured surfaces14,15. The quantity of new bone growth at the bone-to-implant interface largely determines a dental implant’s secondary stability16. By the end of the remodeling phase, bone has covered roughly 60%-70% of the implant surface15. The term “bone-to-implant contact” (BIC) represents this coverage and is frequently used in studies concerning osseointegration.
Conforming to the notion of mechano-transduction, bone remodeling occurs throughout one’s life12. The focus of research has been on creating novel topographies for implant surfaces that will improve osteoblastic migration, adhesion, proliferation, and differentiation.
Go to : 
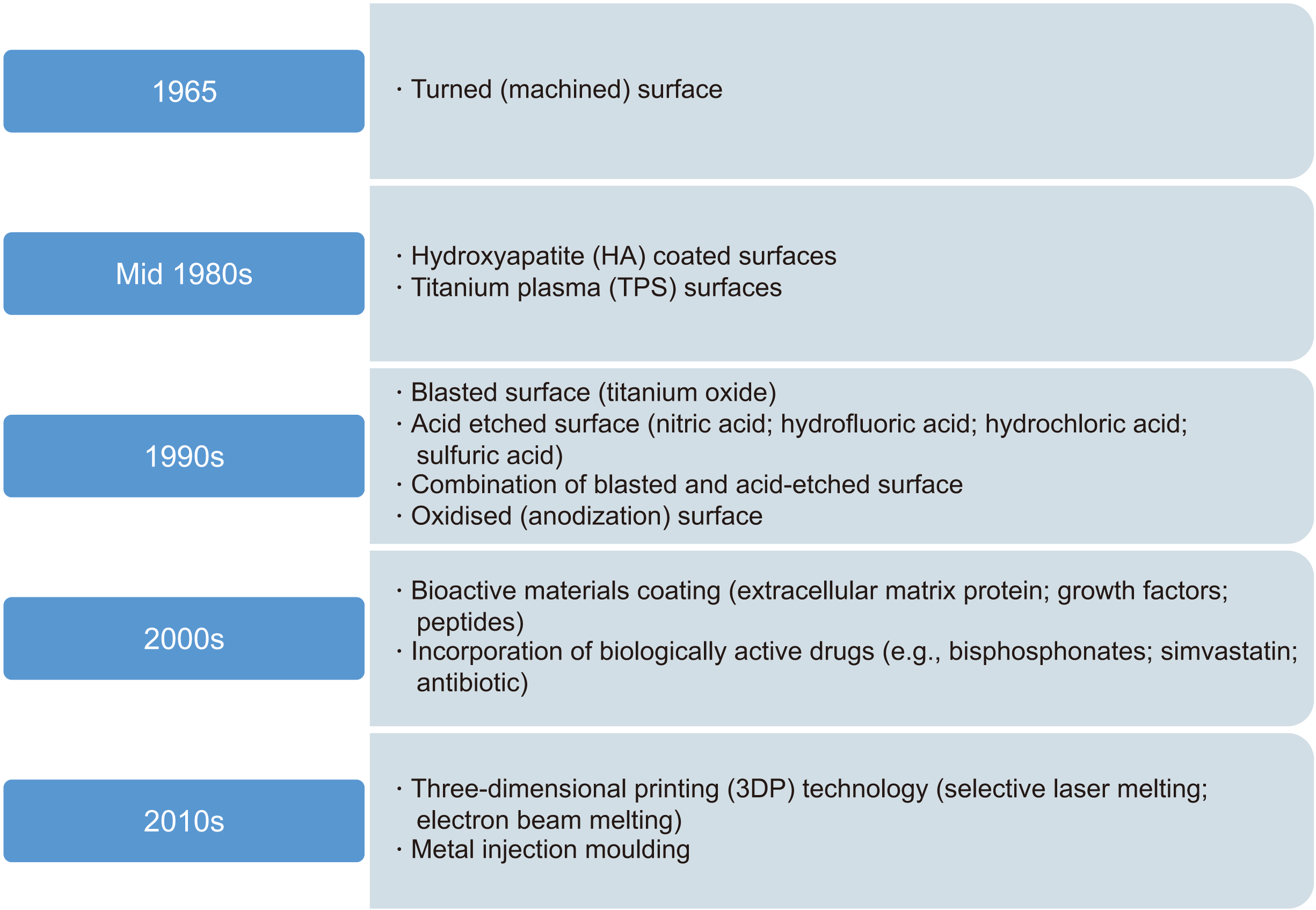
Recently, dental implants have been widely used for substitution of missing teeth. Titanium has proven to be a viable material for implant fabrication through a long history of usage17. For dental implants to successfully osseointegrate, primary implant stability is crucial and is affected by the implant surface. Therefore, several strategies for modifying implant surface structures and features have been implemented to improve primary implant stability and reduce bone healing time. The healing time has been shortened from 12-24 weeks to 6-8 weeks because of developments in implant surface technology18. Fig. 1 summarizes the evolution of dental implant surface modifications, which has been ongoing since 1965.
Beginning in the year 2000, dental implant research concerning the cellular interactions between implant surfaces and bone contact has been ongoing. Bioactive materials such as growth factors, peptides, and ECM protein, as well as biologically active drugs such as bisphosphonates, simvastatin, and antibiotics have been added to dental implants to enhance the interaction of the patient’s bone cells with the implant surfaces and to accelerate osseointegration.
Biomaterial research concerning dental implants has three therapeutic goals: (1) to increase stabilization of the implant by encouraging natural osseointegration, (2) to improve peri-implant soft tissue integration, and (3) to reduce peri-implantitis by inhibiting the adherence of bacteria to the surface of the implant. An important criterion is that the surface coating must not disintegrate during fixture insertion19.
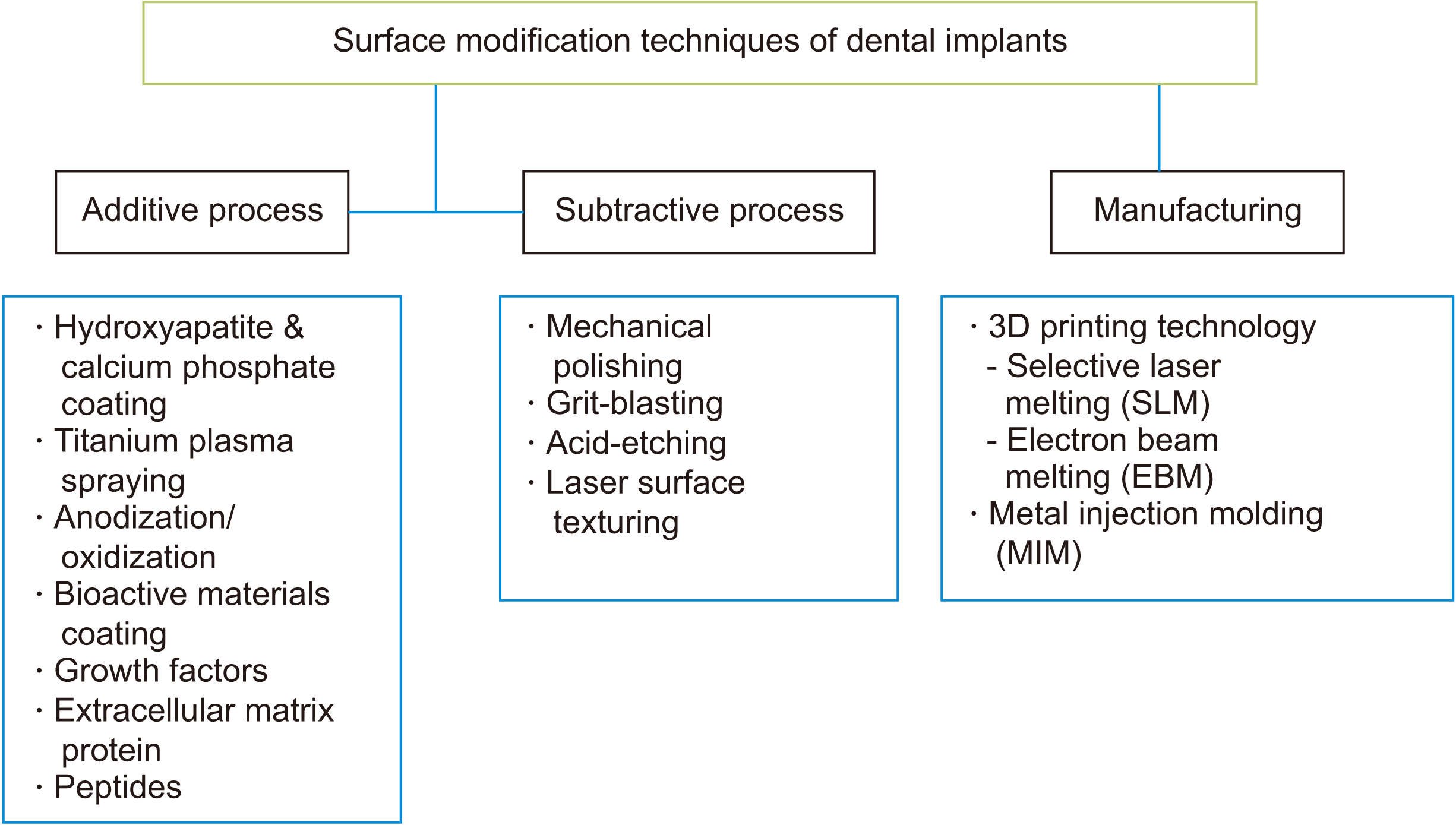
Another method is incorporating surface porosities via three-dimensional printing technology (such as selective laser melting, electron beam melting, or metal injection molding20). Porous implants have provided improved implant stability through osseointegration and osteo-conduction into the pores of the implants21. However, implant surface alterations using three-dimensional printing and metal injection molding are empirical and are largely at the laboratory stage, with only limited clinical results recorded.
As shown in Fig. 2, dental implant surface treatments can be classified as additive (e.g., hydroxyapatite-coated, titanium plasma sprayed) or subtractive (e.g., blasting, acid-etching, and oxidization)22. These procedures result in fixtures with varied surface roughness (Sa), as categorized in Table 1 and detailed in the following section. Surface modifications of dental implants can be classified into macro-, micro-, and nano-roughness23.
Dental implants with varying surface roughness (Sa)
Macro-roughness ranges from millimeters to microns. This scale is directly related to the shape of implants, including macro porous and threaded screws.
The micro-roughness range is from 1 to 100 microns. A micro-rough surface can be altered by processes such as machining, acid-etching, anodizing, sandblasting, and grit-blasting, as well as other coating treatments. A micro-rough surface affects the osseointegration process at the cellular level. A systematic review by Junker et al.11 noted that suitable surface structure at the micron level results in improved bone development and interlocking at the implant interface. Micro-topography is defined by pits, grooves, and protrusions, which affect biological processes at the bone-implant contact. Surface area may increase as a result of microtopography changes. BIC level has been found to be higher on micro-rough surfaces24.
(1) Sandblasted and acid-etched implants: a. Roxolid implants with SLA (sandblasted, large grit, acid-etched) surfaces (Straumann Holding AG) and b. Camlog Promote implants (Camlog), Dentium, and Osstem
The Straumann Holding AG surface mentioned above is created using grit sandblasting at 5 bar with 0.25-0.5 mm corundum particles to produce SLA macro-roughness25. The microtopographic surface structure is produced via high-temperature acid etching with HCl/H2SO4, which yields an active surface area with equal roughness and acceptable cell adhesion.
The Camlog, Dentium, and Osstem implants use a similar method. As mentioned above, they have surface roughness values in the micro-rough range, and the Sa value is 1.3 microns26.
(2) Grit-blasted, acid-etched, and neutralized implants: (e.g., implants using the FRIADENT plus surface (DENTSPLY Implants)
The FRIADENT plus surface is formed via large grit blasting (354-500 microns) in a temperature-controlled environment, followed by etching in hydrochloric, sulfuric, hydrofluoric, and oxalic acid and a special neutralizing technique. The micro-topography of the FRIADENT plus surface has a mean roughness of Ra=3.19 microns and spans numerous magnitude levels27. As a result of grit blasting, macro-roughness and amorphous micropores can be observed, with both 2- to 5-micron diameter micropores and a second layer of much smaller micropores.
The FRIADENT plus surface alters its wettability dynamically. When it comes into contact with ECM proteins, the originally hydrophobic surface transforms into a hydrophilic state with a 0° water contact angle28.
A current trend in dental implants is the use of nano-rough surfaces with ranges from 1-100 nanomicrons (0.001-0.1 microns). Such surfaces are modified using techniques such as discrete crystalline deposition (DCD), laser ablation, anodic oxidation, and titanium oxide blasted and acid-etched implants.
Implant roughness is believed to increase protein absorption and osteoblast adhesion, resulting in improved osseointegration29. This roughness value is expected to affect interactions between cells and implants at both the cellular and protein levels30.
Variations in both surface chemistry and surface roughness contribute to an increase in surface energy31. Consequently, changes in nano-topography have physical, chemical, and biological effects that may enhance osseointegration and result in improved osteogenic cell adhesion11,12. To improve outcomes in challenging clinical scenarios (such as cases involving immediate implantation after tooth extraction, early loading protocols, and patients who have compromised bone-healing or wound-healing abilities), additional advancements in dental implant surface design are needed32.
(1) DCD: 3iT3 dental implant (BIOMET 3i) and Osseotite surface (BIOMET 3i)
• Surface: A dual acid-etched titanium alloy implant modified using a nanoscale manufacturing strategy. This double acid-etched surface is coated with 20 to 100 nanometer-sized calcium phosphate (CaP) particles using the DCD solgel process. Compared to earlier CaP deposition methods, the CaP particles have better adhesion to the implant surface. With this method, the CaP particles cover nearly half the surface area. The Osseotite surface is more prone to bacterial adhesion than the NanoTite surface (BIOMET 3i)33,34.
(2) Laser ablation: Laser-Lok implant (BioHorizons) and PDL surface treatment (BiomateSwiss)
• Surface: With this implant type, the implant collar is fabricated with nanoscale roughness to improve how well the dental implant blends in with the nearby soft tissue. On the neck of the Laser-Lok implant, laser micromachining produces a pattern of micro- and nanoscale microchannels. It has been proposed that these microchannels act as a biologic seal by promoting connective tissue and bone adhesion and inhibiting epithelial downgrowth35.
A high energy density laser (up to 1,700°C) is used in the BiomateSwiss PDL laser surface treatment. This is a thermal processing method and is used to melt and evaporate a metal surface. This approach may generate unique three-dimensional pores with micro-nano and microchannel texture on the implant surface. The method is suited for adhesion and growth of osteocytes to improve the contact area of the bone and fixture, maximizing cell proliferation and osseointegration.
(3) Anodic oxidation: TiUnite (Nobel Biocare Holding AG)
• Surface: Anodic oxidation is a surface modification technique that electrochemically alters the TiO2 layer in standard titanium implants and increases its thickness range from 17-200 nanometers to 600-1,000 nanometers. A porous surface microstructure is created with pore sizes ranging from 1.3 to 2.0 mm2, a porosity of approximately 20%, and a small proportion of Sa=1 microns. Such an implant surface is referred to as “titanium porous oxide” (TPO) or “anodized titanium surface implants” (ASI)36. Anodic oxidation requires an electrical connection, with the implant acting as the anode. The nanoscale surface characteristics of the TPO implants, such as TiUnite, have been established. According to findings from cell research, anodic oxidation may provide a tight soft tissue seal by successfully transferring to the implant’s neck35. Titanium surfaces with nanostructures created via anodic oxidation promote human gingival fibroblast proliferation and adhesion, as well as ECM deposition35.
(4) Titanium oxide blasted and acid-etched implants: OsseoSpeed implants (DENTSPLY Implants) and TiOblast implants (DENTSPLY Implants)
• Surface: In 2004, the OsseoSpeed implant was made available for purchase by DENTSPLY Implants in Mannheim, Germany. This implant product is manufactured with consecutive subtractive processes, resulting in a unique surface roughness. Microscale surface roughness is produced by titanium oxide blasting. The nanostructure of the implant is subsequently sculpted using hydrofluoric acid etching. Surface fluoride accumulation is a pleiotropic side effect, encouraging early osseointegration in the host-implant contact area37. Cell experiments have shown that, in comparison to TiOblast implants, the OsseoSpeed surface improves mesenchymal stem cell osteogenesis and osteo-induction, as well as the branching cell shape of osteoblasts and the osteogenic gene expression profile (DENTSPLY Implants)36,38.
Go to : 
In addition to implant structure and surface roughness, implant surface wettability (or hydrophilicity) is an essential feature of osseointegration. The water contact angle, which varies from 0° with extremely hydrophilic surfaces to greater than 90° with hydrophobic surfaces, is an important variable. Protein structure and function are preserved by hydrophilic surfaces, whereas protein denaturation has been associated with conformational changes in hydrophobic implant textures. Protein adsorption is responsible for the cells’ capacity to bind more strongly to a hydrophilic implant surface than to a hydrophobic surface12. Implants with a dual surface and enhanced hydrophilicity were comparable to those with SLA surfaces in short-term osseointegration39.
Go to : 
Wennerberg et al.40 introduced quantitative assessment of the surface structure of dental implants in 1995. The current recommended instruments for measuring the surface structure can be divided into optical profiling instruments and scanning probe microscopes (SPM).
In general, optical profiling instruments are faster and have higher resolution than mechanical contact instruments. The two methods most suited for topographic characterization of oral implants are confocal laser scanning microscopy (CLSM) and a white light interferometer.
The authors of this review use CLSM with a special focus detecting system. With this system, the reflected light and the XYZ position of the helium-neon laser (He-Ne laser) are measured at the same time. The system adjusts the focus point by point regardless of previous measurements, reducing integration mistakes. The light entering the detector from out-of-focus details is reduced by two pinholes, resulting in fine vertical resolution. Furthermore, the CLSM approach is unlikely to overestimate surface roughness. The accuracy and reliability of this instrument have been investigated, and it was determined to be well-suited for topographic assessment of oral implants and other biomaterials41. The benefit of CLSM is that a large numerical aperture can be used, which is useful when measuring porous and/or inclined surfaces.
In a white light interferometer, a light beam is divided into two, one reflected from a reference plane and the other reflected from the surface of the sample to be measured. Surface irregularities induce phase changes in the reflected light; some waves will be augmented, while others will cancel each other out. The dark and light fringes are not straight and evenly spaced (as they are for optically flat surfaces) and the degree of fringe modulation is related to the surface height. Each point on the surface is measured independently, reducing integration errors. The main benefit of a white light interferometer is the potential to view and analyze implant structure features that are as tiny as a protein molecule. Such analysis allows investigation of the links between surface roughness and biological processes. Measurements can be performed in either air or liquid.
With an SPM, the contact between a sharp tip and the sample surface is measured. The tip is attached to a cantilever, the vertical movement of which during surface scanning is recorded. The most popular SPM techniques are scanning tunneling microscopy and atomic force microscopy (AFM), which are the best techniques for topographic analyses. AFM is the sole option for analysis of non-conductive surfaces and employs a very fine tip (radius 6 to 60 nano-microns) that is drawn across the surface at a consistent speed and pressure. There is also a tapping mode in which the tip oscillates above the surface, contacting it only at the bottom of its swing. A detection system monitors the position of the tip.
For many implant surfaces, the measurement area and the maximum measurement range in the vertical direction are insufficient for proper analysis. This implies that measurements are not always possible, or that they must be selective, limiting generalization of the measurement over the entire surface. Furthermore, because the threaded portion of dental implants cannot be examined non-destructively, such analysis is unsuitable, hindering evaluation of the most regularly used form of oral implants42. The SPM can be used in such situations to analyze structures as fine as a protein molecule based on the extremely high resolution of this technique. This method allows investigation of the links between surface roughness and biological processes. Measurements can be performed in either air or liquid27.
Using scanning electron microscopy (SEM) for structure evaluation provides the ability to investigate structures with a high aspect ratio, a wide depth of field, and high spatial resolution (down to the nanoscale range)43. SEM produces high-quality images and is better suited for morphologic rather than topographic analysis. SEM is primarily utilized as a comparison tool for topographic characterization, making it susceptible to subjective interpretations. A stereo pair of SEM images can be used to obtain height information, but this technique may reduce the high resolution that is otherwise the main advantage of this technology27.
Go to : 
The most important factor in cell response is surface roughness44. It is believed that the ideal surface for bone integration can only be achieved with a very accurate surface structure with an Ra value between 1 and 2 microns24. In addition, Shalabi et al.’s systematic evaluation45 discovered a beneficial effect of Ra/Sa from 0.5 to 8.5 microns on bone response. Although they were unable to provide a conclusive reason for this discrepancy, surface roughness measurements on oral implants are highly complicated. The numerous methods used in the available literature can lead to different outcomes45.
Go to : 
When evaluating an edentulous site for future implant placement, it is essential to look at the available bone volume overall. in addition, assessing the bone density or quality should be taken into consideration as well. The amount of accessible bone in an edentulous area affects treatment planning, implant design, surgical technique, healing time, and early progressive bone loading with prosthetic restorations. Researchers have been examining the classification of bone density and how it relates to dental implant therapy for the past three decades. In 1970, Linkow and Cherchève46 classified bone density into three groups: (1) Class I bone formation: Contains trabeculae with small, cancellated, evenly distributed spaces. (2) Class II bone formation: Reduced homogeneity in an osseous pattern, with noticeably larger cancellated spaces. (3) Class III bone formation: Considerable marrow-filled spaces between bone trabeculae, resulting in unsatisfactory implant stability (i.e., a loose-fitting implant).
Both Class I bone and Class II bone are suitable for implants, and Class I bone is considered ideal for implant restoration.
Four bone types have been presented, based on both radiographic inspection and the surgeon’s perception of resistance during implant preparation47. In contrast, Misch48 created four categories of bone density based on macroscopic cortical and trabecular bone characteristics. The Misch classification of bone density separates and categorizes four sections of the human jawbone.(Tables 2, 3) Furthermore, the density based on computed tomography scan is in Hounsfield units and can be used to quantify bone quality49,50. The bone type influences the design, drilling process, and insertion torque of dental implants.
Misch bone density classes, with associated bone quality description and density
The density and hardness of the bone, the size of the drill bits used, and the implant design all have an impact on the energy needed to install an implant. In dental implantology, this energy is called insertion torque and is measured in Newton centimeters (Ncm). The insertion torque needed to secure the implant into bone is based on the instrument tip and the friction created during insertion51.
The density and hardness of the bone, the size of the drill bit, and the implant design all have an impact on the insertion torque. Torque and bone density are related, with the D-1 bone type possessing the highest bone density. Use of drill bits with smaller dimensions and implants with tapered geometries can result in good stability and local compression52. Although a drill bit with a much smaller diameter than the implant may ensure initial primary stability, it can cause bone necrosis due to bone remodeling, which reduces primary stability53.
As they are placed into the bone, tapered implants cause higher compression than parallel implants. Tapered implants also require higher insertion torque than parallel implants due to lateral bone compression along the entire length of the implant during insertion.
In implant macro-designs with cutting edges, insertion torque is minimized54. The ideal insertion torque as well as its minimum and maximum limits have been the subject of numerous studies. To prevent over-compression or metallurgical issues, several implant manufacturers recommend the ideal insertion torque for quick loading and its upper limit of their implants.
Neugebauer et al.55 stated that insertion torques larger than 50 Ncm were excessive and should not be exceeded, and that 35 Ncm was ideal for initial loading. In addition, Duyck et al.56 hypothesized that insertion torques greater than 50 Ncm are more likely to result in peri-implant bone loss.
Go to : 
Implant installation causes stress on the bone and implant itself. According to the abrasion theory, abrasion occurs when two dissimilar surfaces collide with velocity and is affected by surface hardness, roughness, and velocity. Because bone has a lower hardness than titanium, it is more easily eroded, although titanium is not invulnerable at the micro level. Both compressive and tensile stress play a part in larger-scale thread abnormalities during the torquing process. Thread pitch and design (including form, width, depth, pitch, face, and helix angle) have an impact on insertion torque (angle and width). The amount of bone deformation caused by insertion torque is influenced by the amount of bone surrounding the implant and the degree of bone apposition. These factors will affect bone remodeling and the overall success of the implant57. For predictable results, the insertion torque should typically be greater than 30 Ncm to prevent implant micromovement and connective tissue formation56,58. When installing implants in dense bones, an extremely high insertion torque (above 50 Ncm) may occur, exerting compression stress on the neighboring bone and affecting osseointegration59. Additionally, some studies have found that shear force during implantation may change the characteristics of the surface of the implant60,61. Awareness of the biological response of soft tissue and bone (i.e., soft tissue adaptation and osseointegration) raised concerns about titanium wear during insertion. Comparison of roughness characteristics before and after insertion is a valid method for determining the level of wear62. A study with rabbits found after 12 weeks of healing a change in surface structure of unscrewed implant, demonstrating a reduced roughness after the removal of high-roughness implants4. In addition, studies on several types of implants have indicated loose titanium particles in the recipient bone. For instance, Meyer et al.63 found a detectable amount of titanium around titanium-plasma-sprayed surfaces but smaller amounts of residue adjacent to SLA, and smooth surfaces. In addition, Franchi et al.33 noted the presence of titanium particles around titanium plasma-sprayed implants 14 days after installation. Similar findings were seen in human studies, in which, six months postoperatively, titanium particles were noticed in the tissue covering implant fixtures64. These results indicate that a certain degree of implant wear occurs after placement in the oral cavity.
With regard to surface damage on dental implants after insertion into fresh cow rib bone, SEM images showed chipping of the porous structures along the surface, associated with fissures on the surface modification layer. In addition, delamination was observed when the exposed bulk of titanium was located along the sharp edges of the cutting threads. After implantation, the sharp peaks on the grit-blasted and acid-etched implants were diminished or removed, leaving smooth, flattened areas. Portions of the thick oxide coating were peeled from anodized implants, mostly in the apical region and on top of the threads, along with loose titanium particles60.
In another study, interferometer results revealed a decrease in the roughness of all dental implants61. A study from Salerno et al.65, on the other hand, reported no significant change in structure using AFM. In the authors’ opinion, AFM is ineffective for evaluating the structure of a dental implant because the probe cannot reach some areas around the thread, and AFM is more appropriate for analysis at the nano level, as it might not provide detail at the micro level, the functions of which are related to cell attachment and BIC65.
Another important by Franchi et al.33 noted that the threads of several implant fixtures were deformed in areas where the recipient bone showed fractures. This indicates that proper site preparation and avoidance of excessive insertion torque maintain both the bone and the implant since they hinder potential damage to the host bone (via fracture) and to the implant (via thread deformation).
Go to : 
A dental implant is a unique apparatus designed to replace missing teeth and to restore appearance and function to a level that is as close to normal as possible. It is important for the clinician to be familiar with the complex structure of dental implants. In addition, surgeons should be aware of the preferred drilling and insertion methods, as well as the possible changes in implant structure that may occur during the procedure. This knowledge will help to maximize the success and survival rates of dental implants.
Go to : 
Notes
Authors’ Contributions
P.A., B.M., and N.A. wrote the manuscript. P.N.N. and P.L. collected the information for Figures 1 and 2 and participated in writing the relevant paragraphs. T.K., N.W., and N.A. provided the idea of this article, supervised on the project, and critically revised the manuscript.
Go to : 
References
1. De Bruyn H, Christiaens V, Doornewaard R, Jacobsson M, Cosyn J, Jacquet W, et al. 2017; Implant surface roughness and patient factors on long-term peri-implant bone loss. Periodontol 2000. 73:218–27. https://doi.org/10.1111/prd.12177. DOI: 10.1111/prd.12177. PMID: 28000269.

2. Adell R, Lekholm U, Rockler B, Brånemark PI. 1981; A 15-year study of osseointegrated implants in the treatment of the edentulous jaw. Int J Oral Surg. 10:387–416. https://doi.org/10.1016/s0300-9785(81)80077-4. DOI: 10.1016/S0300-9785(81)80077-4. PMID: 6809663.

3. Berbel LO, Banczek EDP, Karoussis IK, Kotsakis GA, Costa I. 2019; Determinants of corrosion resistance of Ti-6Al-4V alloy dental implants in an in vitro model of peri-implant inflammation. PLoS One. 14:e0210530. https://doi.org/10.1371/journal.pone.0210530. DOI: 10.1371/journal.pone.0210530. PMID: 30703125. PMCID: PMC6354969.

4. Wennerberg A, Albrektsson T, Ulrich H, Krol JJ. 1992; An optical three-dimensional technique for topographical descriptions of surgical implants. J Biomed Eng. 14:412–8. https://doi.org/10.1016/0141-5425(92)90087-2. DOI: 10.1016/0141-5425(92)90087-2. PMID: 1405559.

5. Guan H, van Staden RC, Johnson NW, Loo YC. 2011; Dynamic modelling and simulation of dental implant insertion process-a finite element study. Finite Elem Anal Des. 47:886–97. https://doi.org/10.1016/j.finel.2011.03.005. DOI: 10.1016/j.finel.2011.03.005.

6. Albrektsson T, Brånemark PI, Hansson HA, Lindström J. 1981; Osseointegrated titanium implants. Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop Scand. 52:155–70. https://doi.org/10.3109/17453678108991776. DOI: 10.3109/17453678108991776. PMID: 7246093.

7. Albrektsson T, Buser D, Chen ST, Cochran D, DeBruyn H, Jemt T, et al. 2012; Statements from the Estepona consensus meeting on peri-implantitis, February 2-4, 2012. Clin Implant Dent Relat Res. 14:781–2. https://doi.org/10.1111/cid.12017. DOI: 10.1111/cid.12017. PMID: 23205721.

8. Adler L, Liedholm E, Silvegren M, Modin C, Buhlin K, Jansson L. 2016; Patient satisfaction 8-14 years after dental implant therapy - a questionnaire study. Acta Odontol Scand. 74:423–9. https://doi.org/10.1080/00016357.2016.1177661. DOI: 10.1080/00016357.2016.1177661. PMID: 27136739.

9. Testori T, Clauser C, Deflorian M, Capelli M, Zuffetti F, Fabbro MD. 2016; A retrospective analysis of the effectiveness of the longevity protocol for assessing the risk of implant failure. Clin Implant Dent Relat Res. 18:1113–8. https://doi.org/10.1111/cid.12428. DOI: 10.1111/cid.12428. PMID: 27271293.

10. Albrektsson T, Jacobsson M. 1987; Bone-metal interface in osseointegration. J Prosthet Dent. 57:597–607. https://doi.org/10.1016/0022-3913(87)90344-1. DOI: 10.1016/0022-3913(87)90344-1. PMID: 3298630.

11. Junker R, Dimakis A, Thoneick M, Jansen JA. 2009; Effects of implant surface coatings and composition on bone integration: a systematic review. Clin Oral Implants Res. 20 Suppl 4:185–206. https://doi.org/10.1111/j.1600-0501.2009.01777.x. DOI: 10.1111/j.1600-0501.2009.01777.x. PMID: 19663965.

12. Terheyden H, Lang NP, Bierbaum S, Stadlinger B. 2012; Osseointegration--communication of cells. Clin Oral Implants Res. 23:1127–35. https://doi.org/10.1111/j.1600-0501.2011.02327.x. DOI: 10.1111/j.1600-0501.2011.02327.x. PMID: 22092345.

13. Davies JE. 2003; Understanding peri-implant endosseous healing. J Dent Educ. 67:932–49. DOI: 10.1002/j.0022-0337.2003.67.8.tb03681.x. PMID: 12959168.

14. Glauser R, Schüpbach P, Gottlow J, Hämmerle CH. 2005; Periimplant soft tissue barrier at experimental one-piece mini-implants with different surface topography in humans: a light-microscopic overview and histometric analysis. Clin Implant Dent Relat Res. 7 Suppl 1:S44–51. https://doi.org/10.1111/j.1708-8208.2005.tb00074.x. DOI: 10.1111/j.1708-8208.2005.tb00074.x. PMID: 16137087.

15. Cochran D, Oates T, Morton D, Jones A, Buser D, Peters F. 2007; Clinical field trial examining an implant with a sand-blasted, acid-etched surface. J Periodontol. 78:974–82. https://doi.org/10.1902/jop.2007.060294. DOI: 10.1902/jop.2007.060294. PMID: 17539708.

16. Schwartz Z, Nasazky E, Boyan BD. 2005; Surface microtopography regulates osteointegration: the role of implant surface microtopography in osteointegration. Alpha Omegan. 98:9–19. PMID: 16122142.
17. Jorge JR, Barão VA, Delben JA, Faverani LP, Queiroz TP, Assunção WG. 2013; Titanium in dentistry: historical development, state of the art and future perspectives. J Indian Prosthodont Soc. 13:71–7. https://doi.org/10.1007/s13191-012-0190-1. DOI: 10.1007/s13191-012-0190-1. PMID: 24431713. PMCID: PMC3634937.

18. Peñarrocha-Oltra D, Peñarrocha-Diago M, Canullo L, Covani U, Peñarrocha M. 2014; Patient-reported outcomes of immediate versus conventional loading with fixed full-arch prostheses in the maxilla: a nonrandomized controlled prospective study. Int J Oral Maxillofac Implants. 29:690–8. https://doi.org/10.11607/jomi.3516. DOI: 10.11607/jomi.3516. PMID: 24818209.

19. Dharmayanti AWS, Dubey R, Dubey NK, Deng WP. Pal K, Banerjee I, Sarkar P, Kim D, Deng WP, Dubey NK, editors. 2020. Implant surface modification strategies through antibacterial and bioactive components. Biopolymer-based formulations: biomedical and food applications. Elsevier;p. 647–73. DOI: 10.1016/B978-0-12-816897-4.00026-6.

20. Bencharit S, Byrd WC, Hosseini B. 2015; Immediate placement of a porous-tantalum, trabecular metal-enhanced titanium dental implant with demineralized bone matrix into a socket with deficient buccal bone: a clinical report. J Prosthet Dent. 113:262–9. https://doi.org/10.1016/j.prosdent.2014.09.022. DOI: 10.1016/j.prosdent.2014.09.022. PMID: 25702965. PMCID: PMC4380789.

21. Andani MT, Shayesteh Moghaddam N, Haberland C, Dean D, Miller MJ, Elahinia M. 2014; Metals for bone implants. Part 1. Powder metallurgy and implant rendering. Acta Biomater. 10:4058–70. https://doi.org/10.1016/j.actbio.2014.06.025. DOI: 10.1016/j.actbio.2014.06.025. PMID: 24956564.

22. Albrektsson T, Wennerberg A. 2004; Oral implant surfaces: part 2--review focusing on clinical knowledge of different surfaces. Int J Prosthodont. 17:544–64. PMID: 15543911.
23. Albrektsson T, Wennerberg A. 2004; Oral implant surfaces: part 1--review focusing on topographic and chemical properties of different surfaces and in vivo responses to them. Int J Prosthodont. 17:536–43. PMID: 15543910.
24. Dohan Ehrenfest DM, Coelho PG, Kang BS, Sul YT, Albrektsson T. 2010; Classification of osseointegrated implant surfaces: materials, chemistry and topography. Trends Biotechnol. 28:198–206. https://doi.org/10.1016/j.tibtech.2009.12.003. DOI: 10.1016/j.tibtech.2009.12.003. PMID: 20116873.

25. Wennerberg A, Galli S, Albrektsson T. 2011; Current knowledge about the hydrophilic and nanostructured SLActive surface. Clin Cosmet Investig Dent. 3:59–67. https://doi.org/10.2147/cciden.s15949. DOI: 10.2147/CCIDEN.S15949. PMID: 23674916. PMCID: PMC3652359.

26. Dohan Ehrenfest DM, Vazquez L, Park YJ, Sammartino G, Bernard JP. 2011; Identification card and codification of the chemical and morphological characteristics of 14 dental implant surfaces. J Oral Implantol. 37:525–42. https://doi.org/10.1563/aaid-joi-d-11-00080. DOI: 10.1563/AAID-JOI-D-11-00080. PMID: 21728785.

27. Wennerberg A, Albrektsson T. 2000; Suggested guidelines for the topographic evaluation of implant surfaces. Int J Oral Maxillofac Implants. 15:331–44. PMID: 10874798.
28. Rupp F, Scheideler L, Rehbein D, Axmann D, Geis-Gerstorfer J. 2004; Roughness induced dynamic changes of wettability of acid etched titanium implant modifications. Biomaterials. 25:1429–38. https://doi.org/10.1016/j.biomaterials.2003.08.015. DOI: 10.1016/j.biomaterials.2003.08.015. PMID: 14643618.

29. Wennerberg A, Albrektsson T, Andersson B, Krol JJ. 1995; A histomorphometric and removal torque study of screw-shaped titanium implants with three different surface topographies. Clin Oral Implants Res. 6:24–30. https://doi.org/10.1034/j.1600-0501.1995.060103.x. DOI: 10.1034/j.1600-0501.1995.060103.x. PMID: 7669864.

30. Barfeie A, Wilson J, Rees J. 2015; Implant surface characteristics and their effect on osseointegration. Br Dent J. 218:E9. https://doi.org/10.1038/sj.bdj.2015.171. DOI: 10.1038/sj.bdj.2015.171. PMID: 25766196.

31. Coelho PG, Jimbo R, Tovar N, Bonfante EA. 2015; Osseointegration: hierarchical designing encompassing the macrometer, micrometer, and nanometer length scales. Dent Mater. 31:37–52. https://doi.org/10.1016/j.dental.2014.10.007. DOI: 10.1016/j.dental.2014.10.007. PMID: 25467952.

32. Kligman S, Ren Z, Chung CH, Perillo MA, Chang YC, Koo H, et al. 2021; The impact of dental implant surface modifications on osseointegration and biofilm formation. J Clin Med. 10:1641. https://doi.org/10.3390/jcm10081641. DOI: 10.3390/jcm10081641. PMID: 33921531. PMCID: PMC8070594.

33. Franchi M, Bacchelli B, Martini D, Pasquale VD, Orsini E, Ottani V, et al. 2004; Early detachment of titanium particles from various different surfaces of endosseous dental implants. Biomaterials. 25:2239–46. https://doi.org/10.1016/j.biomaterials.2003.09.017. DOI: 10.1016/j.biomaterials.2003.09.017. PMID: 14741589.

34. Arciola CR, Selan L, Battaglia R, Imbriani M, Rizzo S, et al. Rodriguez y Baena R. 2012; Evaluation of bacterial adhesion on machined titanium, Osseotite® and Nanotite® discs. Int J Artif Organs. 35:754–61. https://doi.org/10.5301/ijao.5000143. DOI: 10.5301/ijao.5000143. PMID: 23065893.

35. Nevins M, Kim DM, Jun SH, Guze K, Schupbach P, Nevins ML. 2010; Histologic evidence of a connective tissue attachment to laser microgrooved abutments: a canine study. Int J Periodontics Restorative Dent. 30:245–55. PMID: 20386781.
36. Zechner W, Tangl S, Fürst G, Tepper G, Thams U, Mailath G, et al. 2003; Osseous healing characteristics of three different implant types. Clin Oral Implants Res. 14:150–7. https://doi.org/10.1034/j.1600-0501.2003.140203.x. DOI: 10.1034/j.1600-0501.2003.140203.x. PMID: 12656873.

37. Smeets R, Stadlinger B, Schwarz F, Beck-Broichsitter B, Jung O, Precht C, et al. 2016; Impact of dental implant surface modifications on osseointegration. Biomed Res Int. 2016:6285620. https://doi.org/10.1155/2016/6285620. DOI: 10.1155/2016/6285620. PMID: 27478833. PMCID: PMC4958483.

38. Coelho PG, Granjeiro JM, Romanos GE, Suzuki M, Silva NR, Cardaropoli G, et al. 2009; Basic research methods and current trends of dental implant surfaces. J Biomed Mater Res B Appl Biomater. 88:579–96. https://doi.org/10.1002/jbm.b.31264. DOI: 10.1002/jbm.b.31264. PMID: 18973274.

39. Kim HG, Yun PY, Kim YK, Kim IH. 2021; Comparison of sandblasted and acid-etched surface implants and new hydrophilic surface implants in the posterior maxilla using a 3-month early-loading protocol: a randomized controlled trial. J Korean Assoc Oral Maxillofac Surg. 47:175–82. https://doi.org/10.5125/jkaoms.2021.47.3.175. DOI: 10.5125/jkaoms.2021.47.3.175. PMID: 34187957. PMCID: PMC8249194.

40. Wennerberg A, Albrektsson T, Andersson B. 1995; An animal study of c.p. titanium screws with different surface topographies. J Mater Sci Mater Med. 6:302–9. https://doi.org/10.1007/BF00120275. DOI: 10.1007/BF00120275.

41. Wennerberg A, Ohlsson R, Rosén BG, Andersson B. 1996; Characterizing three-dimensional topography of engineering and biomaterial surfaces by confocal laser scanning and stylus techniques. Med Eng Phys. 18:548–56. https://doi.org/10.1016/1350-4533(95)00005-4. DOI: 10.1016/1350-4533(95)00005-4. PMID: 8892239.

42. Baró AM, García N, Miranda R, Vázquez L, Aparicio C, Olivé J, et al. 1986; Characterization of surface roughness in titanium dental implants measured with scanning tunnelling microscopy at atmospheric pressure. Biomaterials. 7:463–6. https://doi.org/10.1016/0142-9612(86)90036-0. DOI: 10.1016/0142-9612(86)90036-0. PMID: 3790677.

43. Wieland M, Textor M, Spencer ND, Brunette DM. 2001; Wavelength-dependent roughness: a quantitative approach to characterizing the topography of rough titanium surfaces. Int J Oral Maxillofac Implants. 16:163–81. PMID: 11324205.
44. Gittens RA, McLachlan T, Olivares-Navarrete R, Cai Y, Berner S, Tannenbaum R, et al. 2011; The effects of combined micron-/submicron-scale surface roughness and nanoscale features on cell proliferation and differentiation. Biomaterials. 32:3395–403. https://doi.org/10.1016/j.biomaterials.2011.01.029. DOI: 10.1016/j.biomaterials.2011.01.029. PMID: 21310480. PMCID: PMC3350795.

45. Shalabi MM, Gortemaker A, Van't Hof MA, Jansen JA, Creugers NH. 2006; Implant surface roughness and bone healing: a systematic review. J Dent Res. 85:496–500. https://doi.org/10.1177/154405910608500603. DOI: 10.1177/154405910608500603. PMID: 16723643.

46. Linkow L, Cherchève R. 1970. Theories and techniques of oral implantology. Mosby;St. Louis (MO): p. 1. DOI: 10.1177/154405910608500603.
47. Adell R. 1985; Tissue integrated prostheses in clinical dentistry. Int Dent J. 35:259–65. PMID: 3912327.
48. Misch CE. 1988; Bone character: second vital implant criterion. Dent Today. 7:39–40.
49. Choi YJ, Jun SH, Song YD, Chang MW, Kwon JJ. Subburaj K, editor. 2011. CT scanning and dental implant. CT scanning - techniques and applications. IntechOpen;DOI: 10.5772/19250.

50. Seriwatanachai D, Kiattavorncharoen S, Suriyan N, Boonsiriseth K, Wongsirichat N. 2015; Reference and techniques used in alveolar bone classification. J Interdiscipl Med Dent Sci. 3:172. DOI: 10.4172/2376-032X.1000172.

51. Turkyilmaz I, McGlumphy EA. 2008; Influence of bone density on implant stability parameters and implant success: a retrospective clinical study. BMC Oral Health. 8:32. https://doi.org/10.1186/1472-6831-8-32. DOI: 10.1186/1472-6831-8-32. PMID: 19025637. PMCID: PMC2614413.

52. Checa S, Prendergast PJ. 2010; Effect of cell seeding and mechanical loading on vascularization and tissue formation inside a scaffold: a mechano-biological model using a lattice approach to simulate cell activity. J Biomech. 43:961–8. https://doi.org/10.1016/j.jbiomech.2009.10.044. DOI: 10.1016/j.jbiomech.2009.10.044. PMID: 19954779.

53. Alam K, Qamar SZ, Iqbal M, Piya S, Al-Kindi M, Qureshi A, et al. 2023; Effect of drill quality on biological damage in bone drilling. Sci Rep. 13:6234. https://doi.org/10.1038/s41598-023-33381-y. DOI: 10.1038/s41598-023-33381-y. PMID: 37069203. PMCID: PMC10110507.

54. Freitas AC Jr, Bonfante EA, Giro G, Janal MN, Coelho PG. 2012; The effect of implant design on insertion torque and immediate micromotion. Clin Oral Implants Res. 23:113–8. https://doi.org/10.1111/j.1600-0501.2010.02142.x. DOI: 10.1111/j.1600-0501.2010.02142.x. PMID: 21426405.

55. Neugebauer J, Traini T, Thams U, Piattelli A, Zöller JE. 2006; Peri-implant bone organization under immediate loading state. Circularly polarized light analyses: a minipig study. J Periodontol. 77:152–60. https://doi.org/10.1902/jop.2006.040360. DOI: 10.1902/jop.2006.040360. PMID: 16460238.

56. Duyck J, Corpas L, Vermeiren S, Ogawa T, Quirynen M, Vandamme K, et al. 2010; Histological, histomorphometrical, and radiological evaluation of an experimental implant design with a high insertion torque. Clin Oral Implants Res. 21:877–84. https://doi.org/10.1111/j.1600-0501.2010.01895.x. DOI: 10.1111/j.1600-0501.2010.01895.x. PMID: 20528892.

57. Rosa MB, Albrektsson T, Francischone CE, Filho HO, Wennerberg A. 2013; Micrometric characterization of the implant surfaces from the five largest companies in Brazil, the second largest worldwide implant market. Int J Oral Maxillofac Implants. 28:358–65. https://doi.org/10.11607/jomi.2791. DOI: 10.11607/jomi.2791. PMID: 23527375.

58. Rodrigo D, Aracil L, Martin C, Sanz M. 2010; Diagnosis of implant stability and its impact on implant survival: a prospective case series study. Clin Oral Implants Res. 21:255–61. https://doi.org/10.1111/j.1600-0501.2009.01820.x. DOI: 10.1111/j.1600-0501.2009.01820.x. PMID: 19958375.

59. Tabassum A, Meijer GJ, Wolke JG, Jansen JA. 2010; Influence of surgical technique and surface roughness on the primary stability of an implant in artificial bone with different cortical thickness: a laboratory study. Clin Oral Implants Res. 21:213–20. https://doi.org/10.1111/j.1600-0501.2009.01823.x. DOI: 10.1111/j.1600-0501.2009.01823.x. PMID: 20070754.

60. Mints D, Elias C, Funkenbusch P, Meirelles L. 2014; Integrity of implant surface modifications after insertion. Int J Oral Maxillofac Implants. 29:97–104. https://doi.org/10.11607/jomi.3259. DOI: 10.11607/jomi.3259. PMID: 24451859.

61. Senna P, Antoninha Del Bel Cury A, Kates S, Meirelles L. 2015; Surface damage on dental implants with release of loose particles after insertion into bone. Clin Implant Dent Relat Res. 17:681–92. https://doi.org/10.1111/cid.12167. DOI: 10.1111/cid.12167. PMID: 24283455. PMCID: PMC4420732.

62. Robert-Perron E, Blais C, Thomas Y, Pelletier S, Dionne M. 2005; An integrated approach to the characterization of powder metallurgy components performance during green machining. Mater Sci Eng A. 402:325–34. https://doi.org/10.1016/j.msea.2005.05.019. DOI: 10.1016/j.msea.2005.05.019.

63. Meyer U, Bühner M, Büchter A, Kruse-Lösler B, Stamm T, Wiesmann HP. 2006; Fast element mapping of titanium wear around implants of different surface structures. Clin Oral Implants Res. 17:206–11. https://doi.org/10.1111/j.1600-0501.2005.01184.x. DOI: 10.1111/j.1600-0501.2005.01184.x. PMID: 16584417.

64. Flatebø RS, Johannessen AC, Grønningsaeter AG, Bøe OE, Gjerdet NR, Grung B, et al. 2006; Host response to titanium dental implant placement evaluated in a human oral model. J Periodontol. 77:1201–10. https://doi.org/10.1902/jop.2006.050406. DOI: 10.1902/jop.2006.050406. PMID: 16805683.

65. Salerno M, Itri A, Frezzato M, Rebaudi A. 2015; Surface microstructure of dental implants before and after insertion: an in vitro study by means of scanning probe microscopy. Implant Dent. 24:248–55. https://doi.org/10.1097/id.0000000000000244. DOI: 10.1097/ID.0000000000000244. PMID: 25853585.

Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download