Abstract
Objectives
The aim of the study was to quantify and compare craniofacial asymmetry in subjects with and without symptoms of temporomandibular joint disorders (TMDs).
Materials and Methods
A total of 126 adult subjects were categorized into two groups (63 with a TMDs and 63 without a TMDs), based on detection of symptoms using the Temporomandibular Joint Disorder-Diagnostic Index (TMD-DI) questionnaire. Posteroanterior cephalograms of each subject were traced manually and 17 linear and angular measurements were analyzed. Craniofacial asymmetry was quantified by calculating the asymmetry index (AI) of bilateral parameters for both groups.
Results
Intra- and intergroup comparisons were analyzed using independent t-test and Mann–Whitney U test, respectively, with a P<0.05 considered statistically significant. An AI for each linear and angular bilateral parameter was calculated; higher asymmetry was found in TMD-positive patients compared with TMD-negative patients. An intergroup comparison of AIs found highly significant differences for the parameters of antegonial notch to horizontal plane distance, jugular point to horizontal plane distance, antegonial notch to menton distance, antegonial notch to vertical plane distance, condylion to vertical plane distance, and angle formed by vertical plane, O point and antegonial notch. Significant deviation of the menton distance from the facial midline was also evident.
Conclusion
Greater facial asymmetry was seen in the TMD-positive group compared with the TMD-negative group. The mandibular region was characterized by asymmetries of greater magnitude compared with the maxilla. Patients with facial asymmetry often require management of temporomandibular joint (TMJ) pathology to achieve a stable, functional, and esthetic result. Ignoring the TMJ during treatment or failing to provide proper management of the TMJ and performing only orthognathic surgery may result in worsening of TMJ-associated symptoms (jaw dysfunction and pain) and re-occurrence of asymmetry and malocclusion. Assessments of facial asymmetry should take into account TMJ disorders to improve diagnostic accuracy and treatment outcomes.
Mild asymmetry of the craniofacial region is a common finding in human populations1. Symmetrical facial appearance may appear as skeletal asymmetry on radiographic examination, suggesting that soft tissues minimize subjacent asymmetry. Facial asymmetry can manifest as a part of a number of craniofacial syndromes, or it can develop as a result of trauma, pathology, or abnormal growth2. Minute facial asymmetry is acceptable in individuals with a normal facial appearance, but when the degree of asymmetry is severe and noticeable, it can negatively affect facial and smile esthetics, with major psychological and functional implications for patients2. Asymmetries in human facial structures can also affect the skeleton, muscles, and associated facial tissues3. From a clinical viewpoint, it is therefore crucial to determine the degree of dental, skeletal, soft-tissue, and functional involvement of the craniofacial components in facial asymmetry4.
The origin of facial asymmetry may be congenital, developmental, or acquired through temporomandibular disorders (TMDs)5, which are a disparate group of pathologies affecting the temporomandibular joint (TMJ), the jaw muscles, or both. They are the most common orofacial pain-inducing conditions of nondental origin. TMDs usually include several signs and symptoms, such as pain of the TMJ or jaw muscles, pain on mandibular movement, joint sounds, and locking or luxation of joints, as well as restricted mandibular movement. Other clinical signs related to TMJ pathology include occlusal instability, vertical facial asymmetry, and deviation of the chin to the affected side6.
Quantifying asymmetry helps provide objective distinctions between minor and major asymmetries. For diagnostic purposes and to evaluate treatment results, quantification can signify the amount of asymmetry. Asymmetry can be measured through qualitative analysis and visualization followed by direct quantitative measurement of the face or indirectly by measuring photographs or radiographs2.
The most commonly available tool to diagnose facial asymmetry is posteroanterior cephalometry (PAC)5. A PAC radiograph helps evaluate facial asymmetry by providing a frontal profile of the facial skeleton, which improves a diagnostician’s ability to correlate the right and left halves simultaneously. Because the right and left structures are apart from each other and equidistant from the film and X-ray sources, this minimizes the effects of unequal enlargement due to divergent rays and reduces distortion7.
Few studies have evaluated the relationship between craniofacial asymmetry and TMDs based on an asymmetry index (AI) or PAC radiographs, and it is unclear whether there is any clinically significant association between craniofacial asymmetry and TMD. The purpose of this study was to quantify and compare craniofacial asymmetry using PAC in subjects with and without symptoms of TMD.
Ethical approval for this investigation was obtained from the Institutional Ethics Committee of the Institute of Dental Sciences, Bareilly (No. IEC/103/2021). The purpose of the study was explained to, and informed consent obtained from, all participants.
This study was conducted from January to December 2021 with the cooperation of 126 male and female adult subjects aged between 18 and 28 years who had reported to the Department of Orthodontics and Dentofacial Orthopedics at Institute of Dental Sciences for orthodontic treatment. Subjects with any gross deformity of the facial skeleton due to trauma or congenital anomalies were excluded from the study.
After providing informed consent, each subject was asked to complete a questionnaire based on the Temporomandibular Joint Disorder-Diagnostic Index (TMD-DI)8. Using the resulting TMD-DI scores, assessment of TMD symptoms were made and the subjects were divided into two groups:
• Group I: total TMD-DI score >3 (TMD-positive)
• Group II: total TMD-DI score ≤3 (TMD-negative)
All PAC radiographs were taken by a single operator using a PAC machine (Allengers Smart PAN 2K150330009-D9). Subjects were positioned in the cephalostat with the sagittal plane at a right angle to the path of the X-rays, the Frankfort horizontal plane parallel to the floor, and the midsagittal plane perpendicular to the floor. Subjects were asked to stand straight and remain motionless while occluding with maximum intercuspation and their lips relaxed. The position of the head was fixed by placing ear rods into the ears, and the subjects were positioned 5 feet (standard) from the radiation source.
All PACs were hand-traced on 0.5-µm-thick matte tracing sheets using a sharp 0.5-mm pencil on a view box with trans-illuminated light. The choice of landmarks was based on parameters described for Ricketts and Grummons9, Grummons and Kappeyne van de Coppello10, and Reyneke11 analyses. The cephalometric landmarks, reference planes, and the parameters used in this study are listed in Table 1 and depicted in Fig. 1-4. Three additional points were used to define the vertical and horizontal reference planes (VP and HP): the intersection of the internal sphenoid margin (ISM) bone and medial orbital margin; the O point (the middle of the line connecting the right and left ISM), and the Z point (the intersections of the outer sphenoid bone and the lateral orbital margin). Two reference planes were constructed to calculate the AI: the horizontal plane was constructed by connecting the right and left Z point and the vertical reference plane (the facial midline) was constructed by drawing a line through the O point perpendicular to the horizontal reference plane.
After the construction of reliable horizontal and vertical reference planes, cephalometric analysis of all linear parameters (ANS-VP, Me-VP, Co-Ag, Co-Me, Co-HP, Ag-HP, J-HP, Ag-Me, Ag-VP, Co-VP, J-VP, ZA-VP) and angular parameters (Co-Ag-Me, Co-Go-Me, VP-O-J, VP-O-Ag, Z-Ag-ZA) was undertaken. A total of 17 parameters were analyzed to evaluate craniofacial asymmetry, including 5 vertical, 5 horizontal, 5 angular, and 2 midline skeletal parameters.
To quantify the craniofacial asymmetry in both the groups, an AI for bilateral measurements was calculated using a formula suggested by Habets et al.10,13.
AI=(R−L)/(R+L)×100%,
where R is the value of right side and L is the value of left side.
An AI was used to quantify facial asymmetry as an AI is easily applied and effective for calculating the asymmetry between right and left sides compared with linear differences, and is not affected by positioning error, distortion, or magnification. The measurements were repeated 3 times at 2-week intervals, and the mean value of each measurement was used. The observer was blinded to each patient’s symptoms of TMD during the cephalometric measurements.
Descriptive statistics were calculated as the mean and standard deviation (SD) for each parameter. Chi-square test and paired t-test were used for gender and age comparisons between the two groups, respectively. Intra- and inter-observer reliability were analyzed using Pearson correlation coefficient analysis. Mann–Whitney U test were performed to compare mean asymmetric indices between the groups. An independent t-test was used to analyze midline deviation. The alpha error was set at 0.05 and a P-value less than 0.05 (P<0.05) was considered statistically significant for all analyses. All analyses were performed using IBM SPSS Statistics software (ver. 20; IBM).
Group I (TMD-positive) comprised 37 females (58.7%) and 26 males (41.3%), and group II (TMD-negative) comprised 38 females (60.3%) and 25 males (39.7%).(Table 2) The ages of members of group I and group II ranged from 18 to 28 years, with a mean age of 22.49±2.50 years in the TMD-positive group, and 23.05±2.04 years in the TMD-negative group.(Table 3) Both groups had similar gender distributions.
The mean and SD of the AI of all the parameters of both the TMD-positive and TMD-negative groups and their between-group comparisons are listed in Table 4 and depicted in Fig. 5. A considerable amount of facial asymmetry was evident in various parameters for both groups. The maximum amount of asymmetry in the TMD-positive group was seen in Co-HP (11.38±9.83) followed by Ag-VP (5.42±3.36), VP-O-Ag (4.97±3.61), and Ag-Me (4.02±2.67). The maximum amount of asymmetry in the TMD-negative group was seen in Co-HP (9.50±7.29) followed by Z-Ag-ZA (3.45±3.41), VP-O-Ag (3.34±2.56), and J-VP (3.25±2.72). A highly significant difference (P=0.000) was seen in the AI of the TMD-positive group compared with the TMD-negative group for the Ag-HP and Ag-VP parameters. Other parameters in which the differences were statistically significant (P<0.05) were J-HP, Ag-Me, Co-VP, and VP-Ag-VP. The other parameters showed non-significant differences between the two groups.
The mean and SD of the midline skeletal parameters (i.e., VP-Me and VP-ANS) in both the TMD-positive and TMD-negative groups are listed in Table 5. An independent t-test was used to determine the differences between both the groups. There was a statistically significant difference (P=0.001) in the menton deviation between both the groups, with a midline deviation of 2.09±1.77 mm in the TMD-positive group and 1.20±1.04 mm in the TMD-negative group.
The ANS deviation from the facial mid-line measurement (i.e., the vertical plane to the ANS) was non-significant between the groups (P=0.140), although the deviation was slightly greater in the TMD-positive group.
Asymmetry appears to be an intrinsic characteristic of the human face, and minor asymmetry is considered normal3. Camouflage orthodontic treatment can effectively correct mild to moderate facial asymmetry. For severe facial asymmetry, a combination of orthodontic treatment and orthognathic surgery may be required14. Evaluation of craniofacial asymmetry plays an important role in planning accurate treatment outcomes, particularly when orthognathic surgery for various skeletal malocclusion and TMJ pathology is involved. A subject’s quality of life can be adversely affected by functional or pathological problems related to TMJ and facial asymmetry15. Asymmetry can result from a disturbance in the remodeling process of TMJ due to mandibular displacement. Multiple recent studies have reported that the main causative factor for mandibulofacial asymmetry is TMD signs and symptoms15. The present study was conducted to quantify and compare craniofacial asymmetry in subjects with and without TMD symptoms using an AI.
In our study, the greatest asymmetry was found in Co-HP followed by Ag-VP, VP-O-Ag, Ag-Me, and J-VP in the TMD-positive group and in Co-HP followed by Z-Ag-ZA, VP-O-Ag, and J-VP in the TMD-negative group. The greater AI value for Co-HP may be due to the higher standard deviation recorded for the parameter being indicative of a larger variation in Co-HP measurement among the group. An intra-group comparison of all parameters revealed that, although asymmetry was present in all the parameters, greater asymmetry was seen in mandibular parameters compared with maxillary parameters. This can be attributed to the fact that facial asymmetries manifest more commonly in the mandible (and chin), which form the skeletal support for soft tissues of the lower face and have longer periods of growth. The secondary role of the maxilla in asymmetry can be attributed to the rigid attachment of the maxilla to the stable region of synchondroses at the cranial base and the minimal soft tissue support it provides7. The AI of each parameter in the TMD-negative group was less than AI of respective parameters in the TMD-positive group.
An intergroup comparison of the AI was drawn between the craniofacial asymmetry of the TMD-positive and TMD-negative groups for each of the bilateral linear and angular parameters. Intergroup comparisons revealed highly significant (P<0.05) AIs for the Ag-HP, Ag-Me, Ag-VP, and VP-O-Ag, Co-VP, and J-HP parameters, consistent with the findings of Almăşan et al.16, who also found similar parameters contributed to asymmetry.
The highly significant difference we found for the Ag parameters (Ag-HP, Ag-Me, Ag-VP, and VP-O-Ag) may be due to the variation in Ag landmarks, which can be the result of deep antegonial notching that is seen most often on the affected side in TMD cases17. Kambylafkas et al.18 reported that symptomatic adults with unilateral TMD have mandibular notching on the affected side. Singer et al.19 stated that the clinical presence of a deep mandibular notch is an indication of diminished mandibular growth potential. Some studies19-21 indicate that, when the growth of the mandibular condyle fails to contribute to the lowering of the mandible, the masseter and medial pterygoid, continued growth causes the bone in the region of the angle to grow downward, producing antegonial notching. In other words, resorption that normally occurs below the gonial angle does not occur. Instead, a relative tension is generated between the angle and the muscle sling in which it is suspended, such that bone deposition occurs in the area under the angle posterior to the notch20. Some studies16,17,21,22 have reported that, if the antegonial notches in any one mandible are of different sizes, the mandible will develop some degree of asymmetry. The highly significant difference (P=0.018) for Ag-Me could be due to a deviation of Me to the affected side in the TMD-positive group. Several studies3,5,7,16,23 have shown that unilateral TMJ internal derangement results in a deviation of the menton to the affected side.
The highly significant asymmetry in the condylar region (i.e., Co-VP) in our study may be due to lateral resorptive changes in the condyle that result in mesial movement of the condylion in TMD subjects. The condyle point was found to be more displaced in subjects with a TMD2,16,24. Previous studies23,25 reported increased resorption of the middle or lateral portion of the mandibular condyle in joints with TMDs. Our results agree with those reported by Almăşan et al.16, who also found significant differences in the Co-VP parameter (P=0.05), although Sunitha et al.25 found only non-significant differences (P=0.12). Other parameters related to the condylion were non-significant in our study. On comparing the AI of the angular parameter Co-Go-Me between the two groups, there was a non-significant difference (P=0.508), indicating minor transverse asymmetry in the gonial region. These results may be the effect of a possible readjustment pattern of the mandible, including a compensatory mechanism at the gonial angle caused by internal derangement of the TMJ26. An overall comparison of the parameters related to mandibles showed statistically highly significant differences (P<0.05) for the Ag-HP, Ag-Me, Ag-VP, Co-VP, and VP-O-Ag parameters, indicating more transverse asymmetry of the mandible and contributing more to facial asymmetry rather than vertical asymmetry. These differences may be due to the fact that the vertical asymmetry of the mandible is usually matched by a compensatory mechanism or whatever remodeling takes place in TMD26.
When we compared the AI for the parameters related to the maxilla between the TMD-positive and TMD-negative groups, the vertical distance of the J-HP was highly significant (P=0.012), suggesting vertical asymmetry in the maxilla. The other parameters related to the maxilla that determine the horizontal asymmetry (ZA-VP, J-VP, and VP-O-J) were non-significant in our study, although the AI was higher in the TMD-positive group than in the TMD-negative group. More vertical asymmetry of the maxilla was evident, contributing to facial asymmetry rather than transverse asymmetry. Significant vertical asymmetry of the maxilla may be due to growth-adaptive changes taking place at the maxilla, with the asymmetries in the mandible being the last to complete in maxilla27.
For the AI of postural symmetry, differences in Z-Ag-ZA were found to be highly non-significant (P=0.816), indicating no significant tilt or rotation of the head in positioning subjects occurred while exposing them to PAC. This suggests that the asymmetry revealed in our study is not influenced by tilt or rotation of head.
On comparing midline skeletal parameters (Me and ANS to VP) between TMD-positive and TMD-negative groups, Me deviations were found to be highly significant (P=0.001). Boel et al.28, Rajpara et al.3, and Trpkova et al.2, also reported finding significant differences in Me deviations while studying facial asymmetry with PAC. Other studies have recognized asymmetric menton deviations of 2-4 mm, and a considerable amount of asymmetry (2.09 mm) was found in our study. The ANS deviation from the facial mid-line (VP-ANS) measurement was non-significant (P=0.140), although the deviation was slightly greater in the TMD-positive group.
Understanding the etiology of facial asymmetry is critical for orthodontists and other dental professionals planning appropriate treatment and management plans, and ensuring long-term stability. In our study, a positive association was found between facial symmetry and TMDs, indicating that TMJ disorders are either common causative factors or the result of facial asymmetries, particularly those affecting the mandible. Mandibular asymmetries can involve the symphysis, mandibular body, condyle, and ramus, and these changes can involve the position, size or volume. It is therefore important to quantify and determine which structures are involved, and whether the maxilla, mandible and/or another craniofacial region can be used to make a correct diagnosis. TMJ assessments in facial asymmetry subjects are of clinical importance as they play essential roles in the diagnosis and treatment of the condition. Treatment modality for correction of facial asymmetry (either orthodontic or orthopedic correction with or without orthognathic surgery) should be based on the patient’s age and the severity of the condition. Correction of facial asymmetry is essential in growing subjects as it may worsen if left untreated and could influence the modeling of TMJ, primarily on the affected side. For a growing patient, an asymmetric orthopedic correction can be the ideal treatment modality. For adult patients with severe facial asymmetry and where the growth has ceased, orthognathic surgery may be the optimal treatment approach. Surgical management of TMJ pathology is often needed to achieve a stable, functional, and esthetic result in subjects with facial asymmetry. Asymmetry can be corrected only with orthognathic surgery without TMJ surgery if facial asymmetry is unchanged and stable with no associated TMJ pathology. However, progressive facial asymmetry that is associated with the pathology of TMJ is indicative of an active disease process and may require TMJ surgery in addition to orthognathic surgery to accomplish an acceptable and stable outcome. Ignoring the TMJ during treatment or failing to provide proper management of the TMJ and performing only orthognathic surgery in such cases may result in worse TMJ-associated symptoms (jaw dysfunction and pain) and re-occurrence of asymmetry and malocclusion. Orthodontic treatment alone and other non-surgical TMJ treatments (e.g., medication, splints, chiropractic treatment, and physical therapy) may help manage TMJ symptoms but will not stabilize or eliminate TMJ pathology, which is necessary to achieve optimal functional outcomes.
A limitation of this study is that the TMD-DI considers only subjective symptoms rather than objective signs, making it a relatively weak instrument for the detection of TMD. We could have used other objective indices, such as Fonseca’s anamnestic index, the Helkimo Index, or the research diagnostic criteria for TMDs. However, self-application of TMD-DI was relatively less time-consuming and was expected to provide results that would not be influenced by measurement errors or examiner bias. Another limitation of the study was the potential for cephalometric errors, given the fact that our study was two-dimensional, and manual methods of analysis were used to evaluate the asymmetry of three-dimensional (3D) structures. Future studies should be based on clinical examinations of TMJ and radiographic evaluation utilizing a database of 3D images through CBCT to corroborate these findings, improve our knowledge in this subject, and dynamically visualize and quantify the relationship between TMD and facial asymmetry.
The results of this study lead to the following conclusion:
1. Facial asymmetry is positively associated with TMD symptoms, suggesting that TMJ should be evaluated in all the facial asymmetry cases to determine holistic and a functionally stable treatment plans.
2. Craniocaudal asymmetry was increased as the mandibular region showed asymmetry of higher magnitude compared with the maxilla.
3. The mandibular region showed more transverse asymmetry compared with vertical asymmetry.
Notes
Authors’ Contributions
A.P. participated in data collection and wrote the manuscript. P.B. and S.S. participated in the study design, coordinated and helped to draft the manuscript. A.K.C., A.G, and R.B. helped to draft manuscript. All authors read and approved the final manuscript.
References
1. Ryu SH, Chang HH. 2005; A study on usefulness of the reference line in diagnosis of the facial asymmetry. J Korean Assoc Oral Maxillofac Surg. 31:266–73.
2. Trpkova B, Major P, Nebbe B, Prasad N. 2000; Craniofacial asymmetry and temporomandibular joint internal derangement in female adolescents: a posteroanterior cephalometric study. Angle Orthod. 70:81–8. https://doi.org/10.1043/0003-3219(2000)070%3C0081:caatji%3E2.0.co;2. DOI: 10.1043/0003-3219(2000)070<0081:CAATJI>2.0.CO;2. PMID: 10730679.

3. Rajpara Y, Shyagali TR, Trivedi K, Kambalyal P, Sha T, Jain V. 2014; Evaluation of facial asymmetry in esthetically pleasing faces. J Orthod Res. 2:79–84. https://doi.org/10.4103/2321-3825.131118. DOI: 10.4103/2321-3825.131118.

4. Joshi H, Mehta F, Kumar A, Laddha S, Gandhi V. 2017; An assessment of skeletal craniofacial asymmetry in Gujarati population: an autocad study. IP Indian J Orthod Dentofacial Res. 3:148–53.
5. Sofyanti E, Boel T, Soegiharto B, Auerkari EI. 2018; TMD symptoms and vertical mandibular symmetry in young adult orthodontic patients in North Sumatra, Indonesia: a cross-sectional study. F1000Res. 7:697. https://doi.org/10.12688/f1000research.14522.2. DOI: 10.12688/f1000research.14522.2. PMID: 29946446. PMCID: PMC5998004.

6. Thilander B, Rubio G, Pena L, de Mayorga C. 2002; Prevalence of temporomandibular dysfunction and its association with malocclusion in children and adolescents: an epidemiologic study related to specified stages of dental development. Angle Orthod. 72:146–54. https://doi.org/10.1043/0003-3219(2002)072%3C0146:potdai%3E2.0.co;2. DOI: 10.1043/0003-3219(2002)072<0146:POTDAI>2.0.CO;2. PMID: 11999938.

7. Srivastava D, Singh H, Mishra S, Sharma P, Kapoor P, Chandra L. 2018; Facial asymmetry revisited: part I- diagnosis and treatment planning. J Oral Biol Craniofac Res. 8:7–14. https://doi.org/10.1016/j.jobcr.2017.04.010. DOI: 10.1016/j.jobcr.2017.04.010. PMID: 29556456. PMCID: PMC5854562.

8. Himawan LS, Kusdhany L, Ismail I. 2006; Diagnostic index for temporomandibular disorders in Indonesia. Thai J Oral Maxillofac Surg. 20(2):104–10.
9. Ricketts RM, Grummons D. 2003; Frontal cephalometrics: practical applications, part 1. World J Orthod. 4:297–316.
10. Grummons DC. Kappeyne van de Coppello MA. 1987; A frontal asymmetry analysis. J Clin Orthod. 21:448–65. PMID: 3476493.
11. Reyneke JP. 2010. Essentials of orthognathic surgery. 2nd ed. Quintessence Publishing;Hanover Park (IL):
12. Habets LL, Bezuur JN, van Ooij CP, Hansson TL. 1987; The orthopantomogram, an aid in diagnosis of temporomandibular joint problems. I. The factor of vertical magnification. J Oral Rehabil. 14:475–80. https://doi.org/10.1111/j.1365-2842.1987.tb00742.x. DOI: 10.1111/j.1365-2842.1987.tb00742.x. PMID: 3478455.

13. Habets LL, Bezuur JN, Naeiji M, Hansson TL. 1988; The Orthopantomogram, an aid in diagnosis of temporomandibular joint problems. II. The vertical symmetry. J Oral Rehabil. 15:465–71. https://doi.org/10.1111/j.1365-2842.1988.tb00182.x. DOI: 10.1111/j.1365-2842.1988.tb00182.x. PMID: 3244055.

14. Tseng YC, Yang YH, Pan CY, Chou ST, Ou KC, Chang HP. 2014; Treatment of adult facial asymmetry with orthodontic therapy or orthognathic surgery: receiver operating characteristic analysis. J Dent Sci. 9:235–43. https://doi.org/10.1016/j.jds.2013.03.007. DOI: 10.1016/j.jds.2013.03.007.

15. D'Ippolito S, Ursini R, Giuliante L, Deli R. 2014; Correlations between mandibular asymmetries and temporomandibular disorders (TMD). Int Orthod. 12:222–38. https://doi.org/10.1016/j.ortho.2014.03.013. DOI: 10.1016/j.ortho.2014.03.013. PMID: 24820702.
16. Almăşan OC, Băciuţ M, Hedeşiu M, Bran S, Almăşan H, Băciuţ G. 2013; Posteroanterior cephalometric changes in subjects with temporomandibular joint disorders. Dentomaxillofac Radiol. 42:20120039. https://doi.org/10.1259/dmfr.20120039. DOI: 10.1259/dmfr.20120039. PMID: 23253565. PMCID: PMC3729188.

17. Saglam AA, Sanli G. 2004; Condylar asymmetry measurements in patients with temporomandibular disorders. J Contemp Dent Pract. 5:59–65. DOI: 10.5005/jcdp-5-3-59. PMID: 15318257.

18. Kambylafkas P, Kyrkanides S, Tallents RH. 2005; Mandibular asymmetry in adult patients with unilateral degenerative joint disease. Angle Orthod. 75:305–10. https://doi.org/10.1043/0003-3219(2005)75[305:maiapw]2.0.co;2. DOI: 10.1043/0003-3219(2005)75[305:MAIAPW]2.0.CO;2. PMID: 15898365.

19. Singer CP, Mamandras AH, Hunter WS. 1987; The depth of the mandibular antegonial notch as an indicator of mandibular growth potential. Am J Orthod Dentofacial Orthop. 91:117–24. https://doi.org/10.1016/0889-5406(87)90468-9. DOI: 10.1016/0889-5406(87)90468-9. PMID: 3468794.

20. Egermark I, Carlsson GE, Magnusson T. 2005; A prospective long-term study of signs and symptoms of temporomandibular disorders in patients who received orthodontic treatment in childhood. Angle Orthod. 75:645–50. https://doi.org/10.1043/0003-3219(2005)75[645:aplsos]2.0.co;2. DOI: 10.1016/s0084-3717(08)70510-5. PMID: 16097235.

21. Lee KM, Hwang HS, Cho JH. 2014; Comparison of transverse analysis between posteroanterior cephalogram and cone-beam computed tomography. Angle Orthod. 84:715–9. https://doi.org/10.2319/072613-555.1. DOI: 10.2319/072613-555.1. PMID: 24325622. PMCID: PMC8650435.

22. Tai B, Goonewardene MS, Murray K, Koong B, Islam SM. 2014; The reliability of using postero-anterior cephalometry and cone-beam CT to determine transverse dimensions in clinical practice. Aust Orthod J. 30:132–42. PMID: 25549515.
23. Vig PS, Hewitt AB. 1975; Asymmetry of the human facial skeleton. Angle Orthod. 45:125–9. https://doi.org/10.1043/0003-3219(1975)045%3C0125:aothfs%3E2.0.co;2. DOI: 10.1043/0003-3219(1975)045<0125:AOTHFS>2.0.CO;2. PMID: 1054939.

24. Nicot R, Chung K, Vieira AR, Raoul G, Ferri J, Sciote JJ. 2020; Condyle modeling stability, craniofacial asymmetry and ACTN3 genotypes: contribution to TMD prevalence in a cohort of dentofacial deformities. PLoS One. 15:e0236425. https://doi.org/10.1371/journal.pone.0236425. DOI: 10.1371/journal.pone.0236425. PMID: 32726330. PMCID: PMC7390436.

25. Sunitha S, Mahesh KP, Patil K. 2016; Posteroanterior cephalometric analysis of facial asymmetry in temporomandibular joint disorders. Int J Curr Res. 8:27651–5.
26. Legrell PE, Nyquist H, Isberg A. 2000; Validity of identification of gonion and antegonion in frontal cephalograms. Angle Orthod. 70:157–64. https://doi.org/10.1043/0003-3219(2000)070%3C0157:voioga%3E2.0.co;2. DOI: 10.1043/0003-3219(2000)070<0157:VOIOGA>2.0.CO;2. PMID: 10833005.

27. Vig KW, Fields HW. 2000; Facial growth and management of orthodontic problems. Pediatr Clin North Am. 47:1085–123. https://doi.org/10.1016/s0031-3955(05)70259-5. DOI: 10.1016/S0031-3955(05)70259-5. PMID: 11059351.

28. Boel T, Sofyanti E, Sufarnap E. 2017; Analyzing menton deviation in posteroanterior cephalogram in early detection of temporomandibular disorder. Int J Dent. 2017:5604068. https://doi.org/10.1155/2017/5604068. DOI: 10.1155/2017/5604068. PMID: 28845159. PMCID: PMC5563424.

Fig. 1
Reference planes. Refer to Table 1 for the definition of landmarks. (HP: horizontal reference plane, VP: vertical reference plane, O: O point)
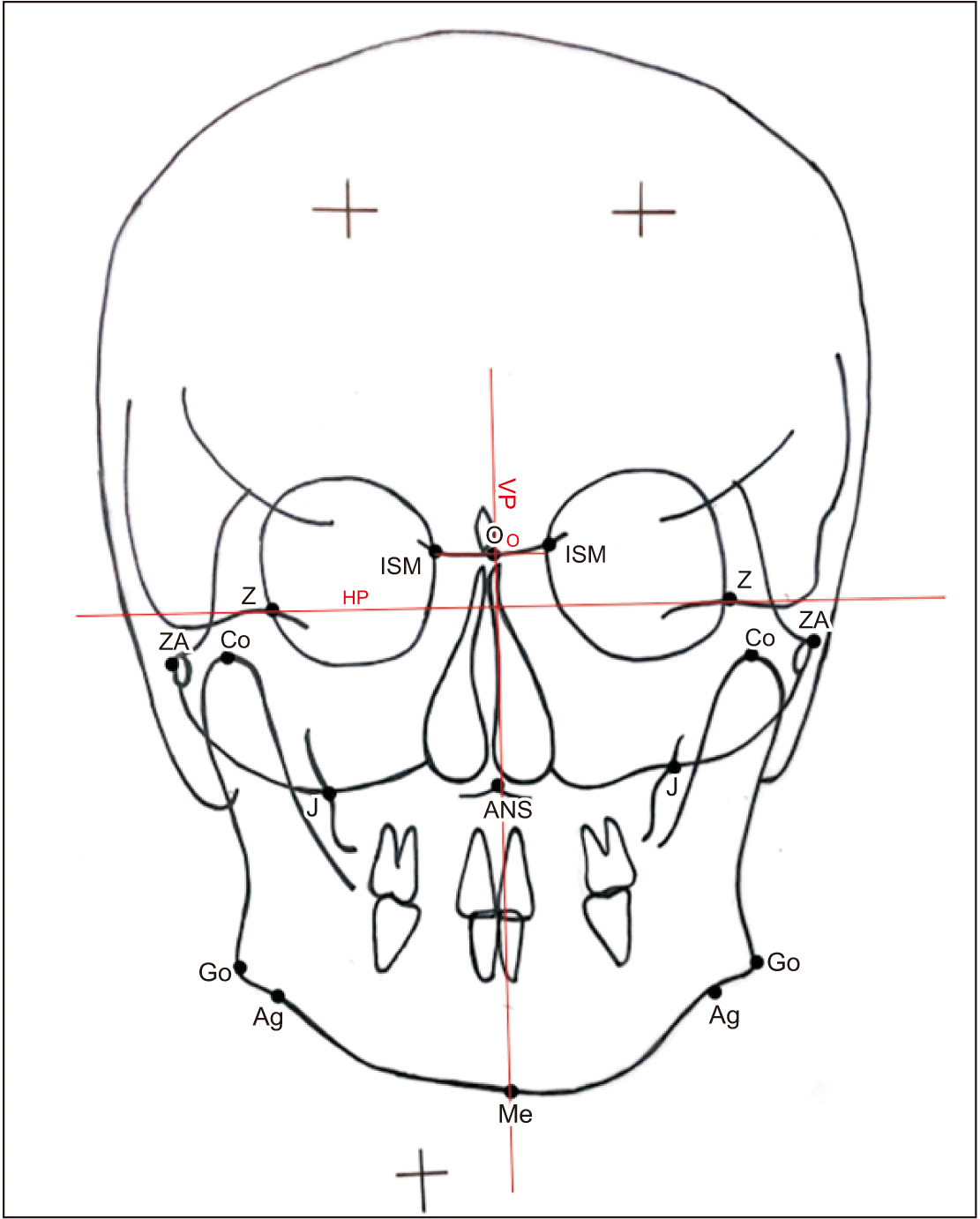
Fig. 2
Unilateral midline linear parameters. Refer to Table 1 for the definition of landmarks. (ANS-VP: anterior nasal spine to vertical plane, Me-VP: menton to vertical plane)
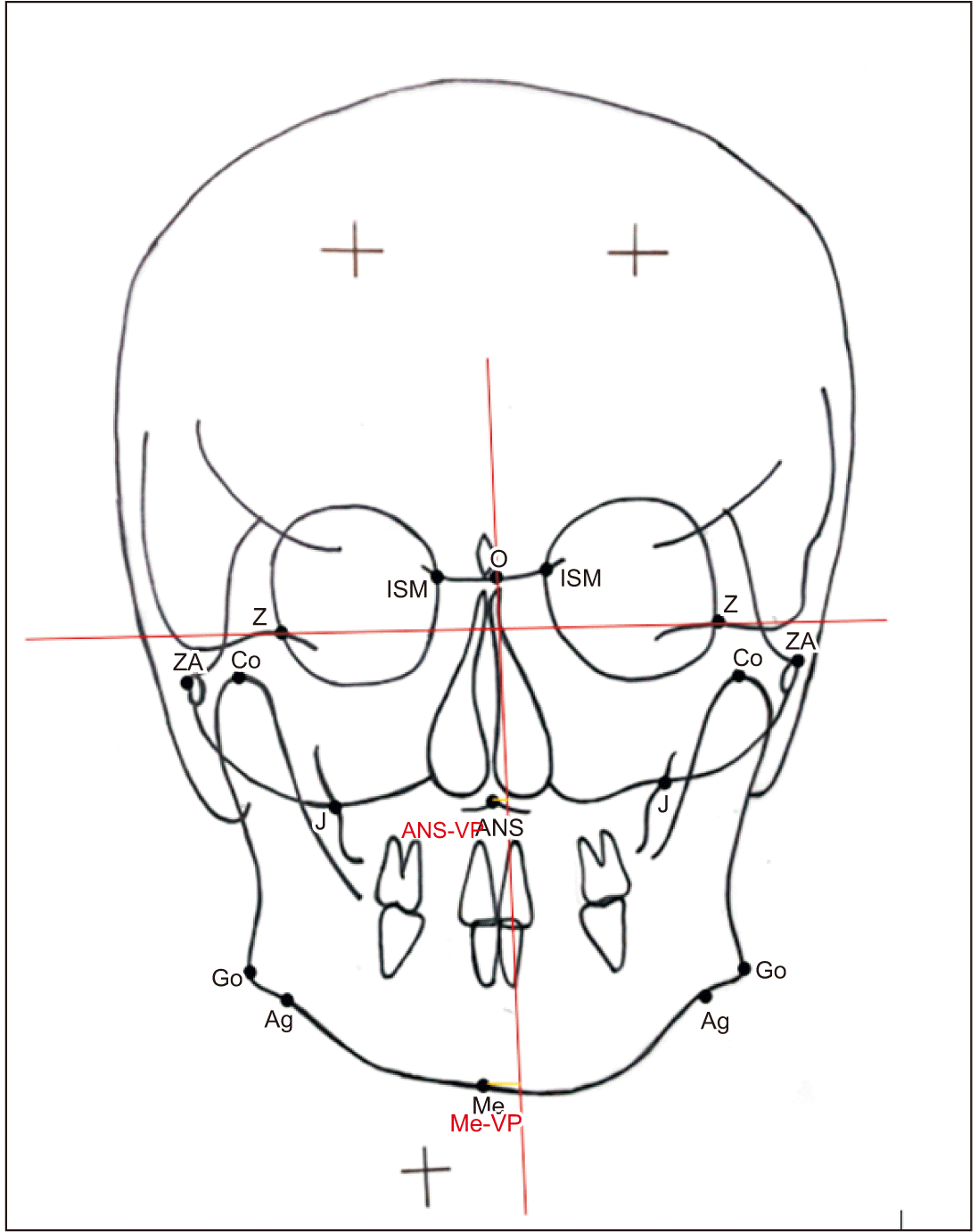
Fig. 3
Bilateral linear parameters. Refer to Table 1 for the definition of landmarks. (Co-VP: condylar superioris to vertical plane distance, ZA-VP: zygomatic arch to vertical plane distance, J-VP: jugulare to vertical plane distance, Ag-VP: antegonial notch to vertical plane distance, Ag-Me: antegonial notch to menton distance, ZA-VP’: zygomatic arch to vertical plane distance, Co-VP’: condylar superioris to vertical plane distance, J-VP’: jugulare to vertical plane distance, Ag-VP’: antegonial notch to vertical plane distance, Ag-Me’: antegonial notch to menton distance, Co-HP: condylion to horizontal plane distance, J-HP: jugulare to horizontal plane distance, Co-Ag: condylion to antegonial distance, Ag-HP: antegonial to horizontal plane distance, Co-Me: condylion to menton distance, Co-HP’: condylion to horizontal plane distance, J-HP’: jugulare to horizontal plane distance, Co-Ag’: condylion to antegonial distance, Ag-HP’: antegonial to horizontal plane distance, Co-Me’: condylion to menton distance)
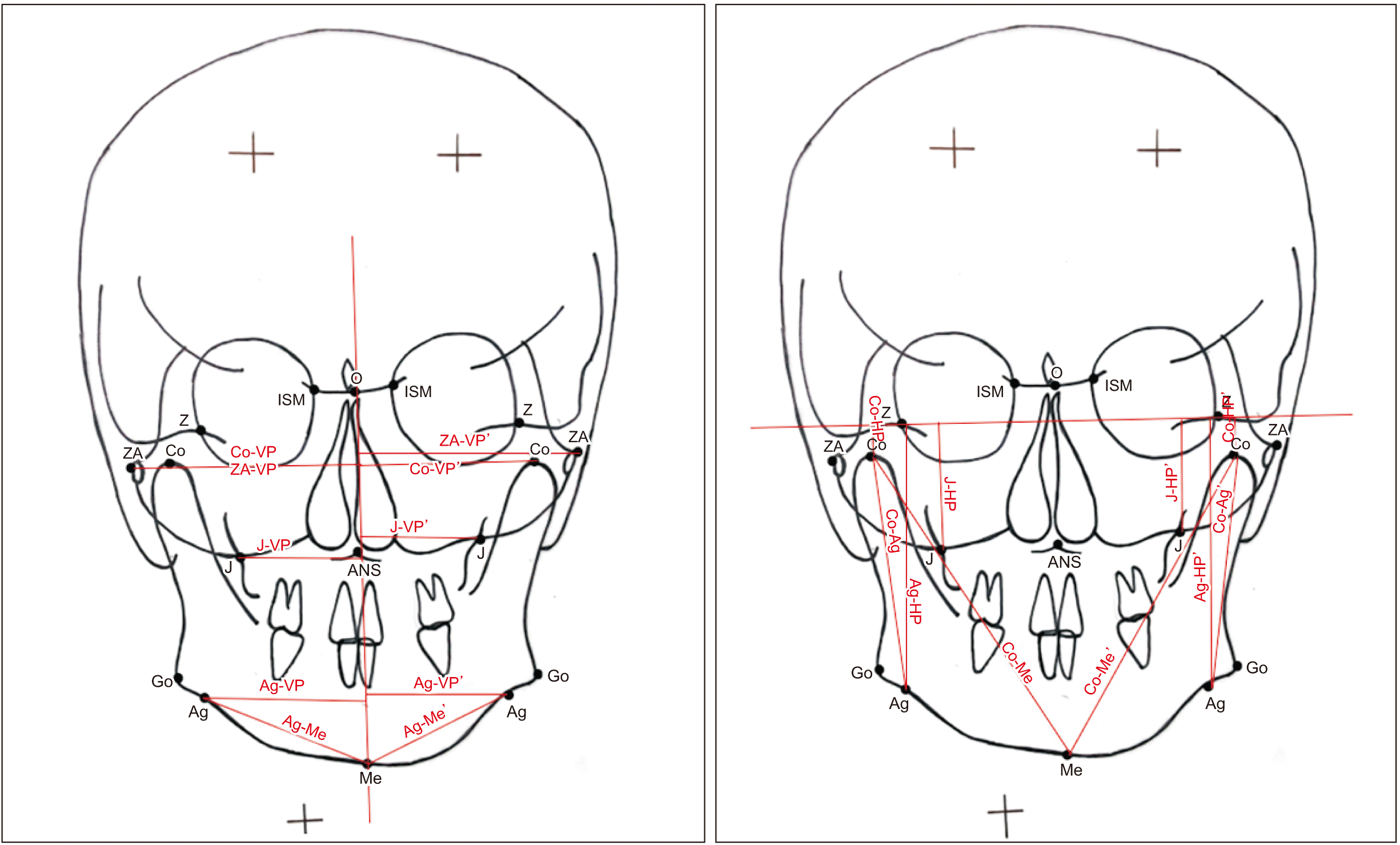
Fig. 4
Bilateral angular parameters. Refer to Table 1 for the definition of landmarks. (VP-O-J: angle between vertical plane and O-J line, Co-Ag-Me: angle between Co-Ag line and Ag-Me line, Z-Ag-ZA: angle between Z-Ag line and Ag-ZA line, VP-O-J’: angle between vertical plane and O-J line, Co-Ag-Me’: angle between Co-Ag line and Ag-Me line, Z-Ag-ZA’: angle between Z-Ag line and Ag-ZA line, VP-O-Ag: angle between vertical plane and O-Ag line, Co-Go-Me: angle between Co-Go line and Go-Me line, VP-O-Ag’: angle between vertical plane and O-Ag line, Co-Go-Me’: angle between Co-Go line and Go-Me line)
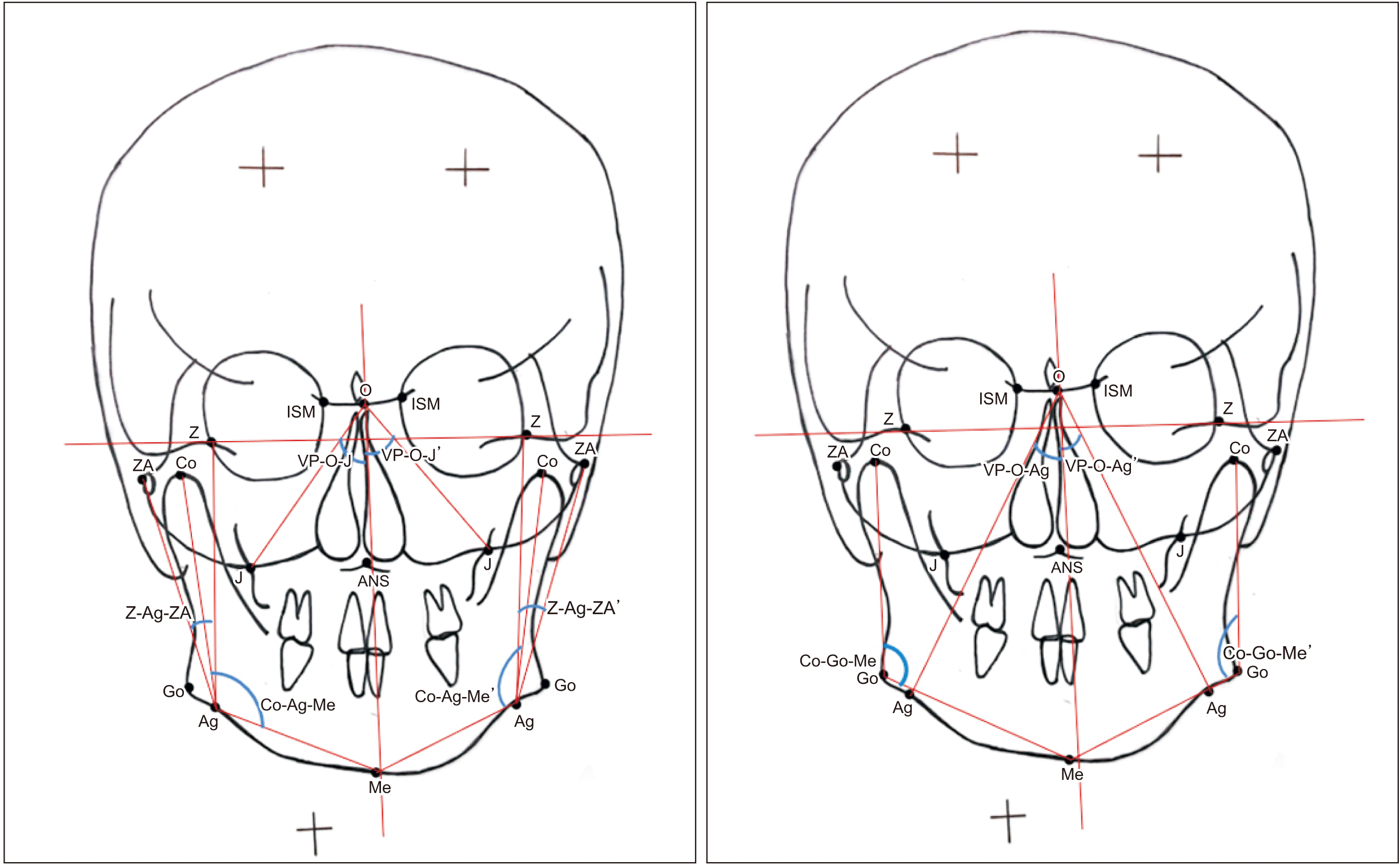
Table 1
Two-dimensional landmarks for asymmetry analysis
Table 2
Sex comparison of all subjects between TMD-positive and TMD-negative groups
| Variable |
TMD-positive (n=63) |
TMD-negative (n=63) |
Total | Value | df | P-value |
|---|---|---|---|---|---|---|
| Sex | ||||||
| Female | 37 (58.7) | 38 (60.3) | 75 | 0.033 | 1 | 0.856 |
| Male | 26 (41.3) | 25 (39.7) | 51 |
Table 3
Age comparison of all the subjects between TMD-positive and TMD-negative groups
| Variable | Particulars | Mean±SD | t | df | Mean difference | P-value |
|---|---|---|---|---|---|---|
| Age | TMD-positive | 22.49±2.50 | −1.365 | 124 | −0.556 | 0.175 |
| TMD-negative | 23.05±2.04 |
Table 4
Mean values of the asymmetry index of all linear and angular parameters for both TMD-positive and TMD-negative groups and their comparisons
| Parameter | Particulars | Mean±SD | Mann–Whitney U test | |
|---|---|---|---|---|
|
|
||||
| Statistic | P-value | |||
| Co-Ag | TMD-positive | 2.06±2.15 | 1,896.000 | 0.665 |
| TMD-negative | 1.59±1.10 | |||
| Co-Me | TMD-positive | 1.57±1.06 | 1,640.000 | 0.093 |
| TMD-negative | 1.39±1.79 | |||
| Co-HP | TMD-positive | 11.38±9.83 | 1,839.000 | 0.477 |
| TMD-negative | 9.50±7.29 | |||
| Ag-HP | TMD-positive | 1.56±1.39 | 1,164.000 | 0.000* |
| TMD-negative | 0.74±0.65 | |||
| J-HP | TMD-positive | 3.11±2.04 | 1,470.000 | 0.012* |
| TMD-negative | 2.20±1.85 | |||
| Ag-Me | TMD-positive | 4.02±2.67 | 1,498.500 | 0.018* |
| TMD-negative | 2.88±2.22 | |||
| Ag-VP | TMD-positive | 5.42±3.36 | 973.500 | 0.000* |
| TMD-negative | 2.59±2.18 | |||
| Co-VP | TMD-positive | 3.20±2.67 | 1,436.500 | 0.007* |
| TMD-negative | 2.01±1.40 | |||
| J-VP | TMD-positive | 3.39±2.74 | 1,977.000 | 0.971 |
| TMD-negative | 3.25±2.72 | |||
| ZA-VP | TMD-positive | 1.67±1.29 | 1,690.500 | 0.151 |
| TMD-negative | 1.43±1.52 | |||
| Co-Ag-Me | TMD-positive | 1.25±1.04 | 1,601.000 | 0.061 |
| TMD-negative | 0.91±0.80 | |||
| Co-Go-Me | TMD-positive | 1.29±1.30 | 1,849.000 | 0.508 |
| TMD-negative | 1.02±0.89 | |||
| VP-O-J | TMD-positive | 3.53±2.40 | 1,674.500 | 0.130 |
| TMD-negative | 3.07±2.87 | |||
| VP-O-Ag | TMD-positive | 4.97±3.61 | 1,442.000 | 0.008* |
| TMD-negative | 3.34±2.56 | |||
| Z-Ag-ZA | TMD-positive | 3.61±3.54 | 1,938.000 | 0.816 |
| TMD-negative | 3.45±3.41 | |||
Table 5
Mean values for unilateral midline skeletal parameters of TMD-positive and TMD-negative groups and their comparisons




 PDF
PDF Citation
Citation Print
Print



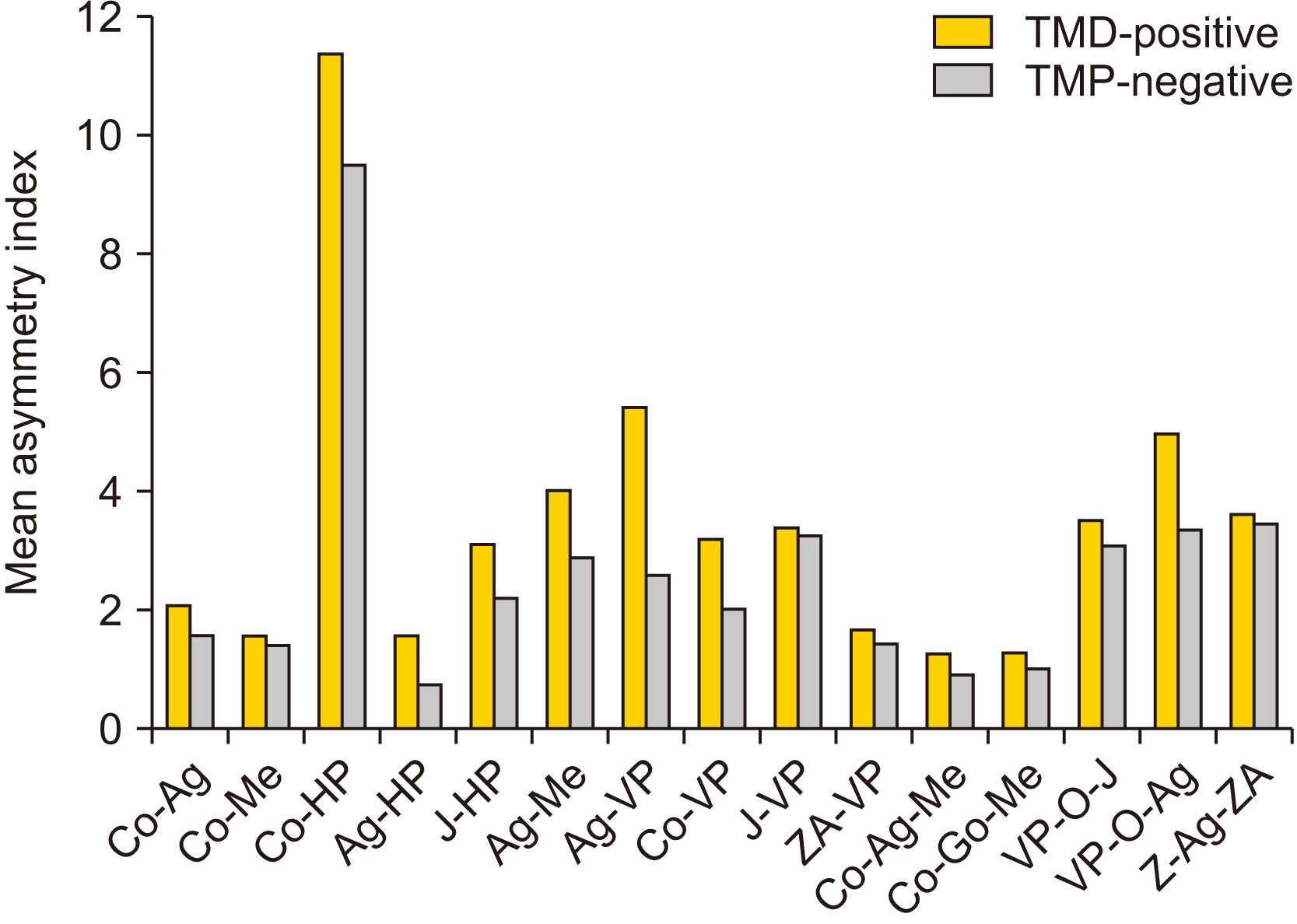
 XML Download
XML Download