Abstract
Objective
Systemic lupus erythematosus (SLE) is an autoimmune disease, characterized by the production of autoantibodies and high cholesterol levels. HMG-CoA (3-hydroxy-3-methylglutaryl-coenzyme A) reductase inhibitors have exhibited anti-inflammatory effects in several clinical trials. We conducted this study to evaluate the effect of rosuvastatin on inflammatory responses in lupus-prone mice.
Methods
MRL/lpr mice were intraperitoneally injected with rosuvastatin (10 mg/kg, n=4) or vehicle (2% dimethyl sulfoxide, n=4) five times a week from 13 to 17 weeks of age. The serum levels of low-density lipoprotein (LDL) cholesterol and autoantibodies were measured, as well as the urine levels of albumin. Renal tissues were stained for histopathological analysis. Concentrations of key inflammatory cytokines were measured in the serum, and messenger RNA (mRNA) levels in target organs (kidney, spleen, and lymph nodes) were evaluated.
Results
Rosuvastatin treatment significantly decreased serum LDL cholesterol concentration in MRL/lpr mice. However, the clinical manifestations and autoantibody titres did not improve with rosuvastatin treatment. In addition, serum inflammatory cytokines and proteinuria did not change. Histopathological analysis of the kidneys revealed no improvement. When assessing the expression of mRNA, treatment with rosuvastatin decreased tumor necrosis alpha and interleukin-17 concentration in spleen and kidney tissue and in the kidneys and lymph nodes of MRL/lpr mice, respectively.
Systemic lupus erythematosus (SLE) is an autoimmune disease in which dysregulation of the immune system causes inflammation and attacks the body’s organ systems, resulting in tissue damage [1]. SLE is caused by a failure in regulating the production of pathogenic autoantibodies and the formation of immune complexes [2]. Representative autoantibodies found in SLE are antinuclear antibody (ANA) and anti-double-stranded DNA (dsDNA) antibody [3]. Anti-dsDNA antibodies are a key SLE classification criterion [4].
It is known that about 50% of people with SLE will experience lupus nephritis (LN) [5]. Urinary albumin is generally known to serve as a marker of LN [6]. In SLE, a cytokine imbalance contributes to immune dysfunction, inflammation, and organ damage. Interferon (IFN) secretion is induced by immune complexes and induces up-regulation of several inflammatory proteins that account for IFN signaling. Thus, patients with SLE are known to exhibit elevated serum IFN-α levels [7]. In addition, the concentrations of T cell-derived cytokines, including interleukins (IL)-6 and IL-17, and inflammatory cytokines, including tumor necrosis factor-alpha (TNF-α), are known to be increased in the serum [8].
Cholesterol levels are often high in patients with SLE and cardiovascular problems or kidney involvement [9]. The 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase inhibitors (statins) are used as a form of dyslipidaemia treatment that acts as a rate-limiting step in cholesterol biosynthesis and reportedly provides benefits in primary and secondary cardiovascular disease prevention in large randomized clinical trials [10]. In addition, statin treatment reportedly reduces inflammation and pro-inflammatory cytokine levels [11]. Rosuvastatin is a HMG-CoA reductase inhibitor that reduces cholesterol levels [12]. Based on this background, rosuvastatin was expected to reduce the inflammatory response in lupus-prone mice, and the effect of rosuvastatin was analyzed using MRL/lpr mice, a representative model of SLE.
All animal procedures were reviewed and approved by the Animal Ethics Committee of Researchers’ institution (approval number: 2018-0029), and maintained under non-pathogenic conditions for mouse acclimatization according to the guidelines of the Animal Facility of Researchers’ institution. All female MRL/lpr (36~40 g) mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). The mice were acclimatized for one week after purchase, equally divided into groups according to body weight, and four mice were assigned to one of the two groups. From 13 to 17 weeks of age, five times a week for a total of four weeks, the control (vehicle, n=4) and experimental group (rosuvastatin-treated, n=4) were administered 2% dimethyl sulfoxide (DMSO) and 10 mg/kg rosuvastatin in 2% DMSO intraperitoneally, respectively. All mice were weighed once per week during the rosuvastatin treatment period. At the end of the experimental period, 17-week-old mice were anesthetized with isoflurane and blood and urine samples were collected immediately before sacrifice. In addition, the major SLE target organs (kidney, spleen, and lymph nodes) were collected immediately after sacrifice.
Concentrations of major SLE markers in mouse serum were analyzed using enzyme-linked immunosorbent assay (ELISA) using mouse ANA (MyBioSource, San Diego, CA, USA) and anti-dsDNA antibody (MyBioSource). Serum cytokine concentration was measured using an IL-6 mouse ELISA kit (Enzo Life Sciences, Farmingdale, NY, USA). Mouse urine samples collected to examine renal function were used to analyze urine albumin concentration using an albumin ELISA kit (Alpco Diagnostics, Salem, NH, USA). All ELISA experiments were performed according to the manufacturer’s instructions.
The low-density lipoprotein (LDL) cholesterol levels in serum were determined using a Cholesterol Assay Kit (AbCam, Cambridge, UK), according to standard protocols.
Immediately after sacrifice, unilateral kidneys isolated after washing with phosphate-buffered saline were fixed in 4% paraformaldehyde at 4°C overnight, embedded in paraffin, and 2-μm kidney sections were stained with haematoxylin and eosin (H&E) and periodic acid–Schiff (PAS) reagents according to standard protocols.
Messenger RNAs (mRNAs) collected from major SLE target organs, including the spleen, kidney, and lymph nodes, were extracted using the RNeasy Mini Kit (Qiagen, Valencia, CA, USA) according to the manufacturer's instructions. The mRNA isolated from each tissue was used to synthesize cDNA using the SuperScript III First-Strand Synthesis System (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's instructions. The synthesized cDNA was converted with a Rotor-Gene SYBR Green polymerase chain reaction (PCR) Kit (Qiagen) and amplified using a real-time PCR method. Real-time PCR was repeated 40 times to determine the mRNA expression of TNF-α, IFN-α, IFN-γ, IL-6, and IL-17 after heating at 95°C for 5 minutes, 60°C for 30 seconds, and 60°C for 1 minute. Quantified individual mRNA expression was normalized to that of 18S, and the control or rosuvastatin group mice were delineated as relative mRNA expression set at 1.0.
To confirm the significance of major markers of SLE and cytokine levels between the control and rosuvastatin groups, the levels were verified using the Mann–Whitney U test method for non-parametric data using PASW Statistics 18.0 (IBM Corp., Armonk, NY, USA) program. Statistical significance was set at less than p<0.05.
The therapeutic efficacy of rosuvastatin was evaluated in MRL/lpr mice, a well-known model of SLE characterized by high levels of circulating antibodies and inflammatory cytokines that cause autoimmune diseases similar to human SLE. As MRL/lpr mice spontaneously developed SLE over several months, we treated 13-week-old female MRL/lpr mice with rosuvastatin (10 mg/kg) by IP injection five times a week for four weeks. There were no significant differences in skin lesions and body weight after rosuvastatin treatment (data not shown). In MRL/lpr mice, rosuvastatin administration markedly reduced LDL cholesterol levels (Figure 1). However, there was no significant change in serum ANA and anti-dsDNA antibody levels, which are major indicators of lupus disease, and there was no difference in expression of the inflammatory cytokine IL-6.
To determine whether treatment with rosuvastatin in the MRL/lpr mouse model improved renal tissues, we performed histopathological analysis of renal tissues using H&E and PAS staining in each group of mice. Analysis of the glomerular size, surrounding inflammatory cells, mesangial cell population, and endothelial cell proliferation showed no change between the control and rosuvastatin groups (Figure 2). Additionally, there were no significant differences in the albumin concentration in mouse urine.
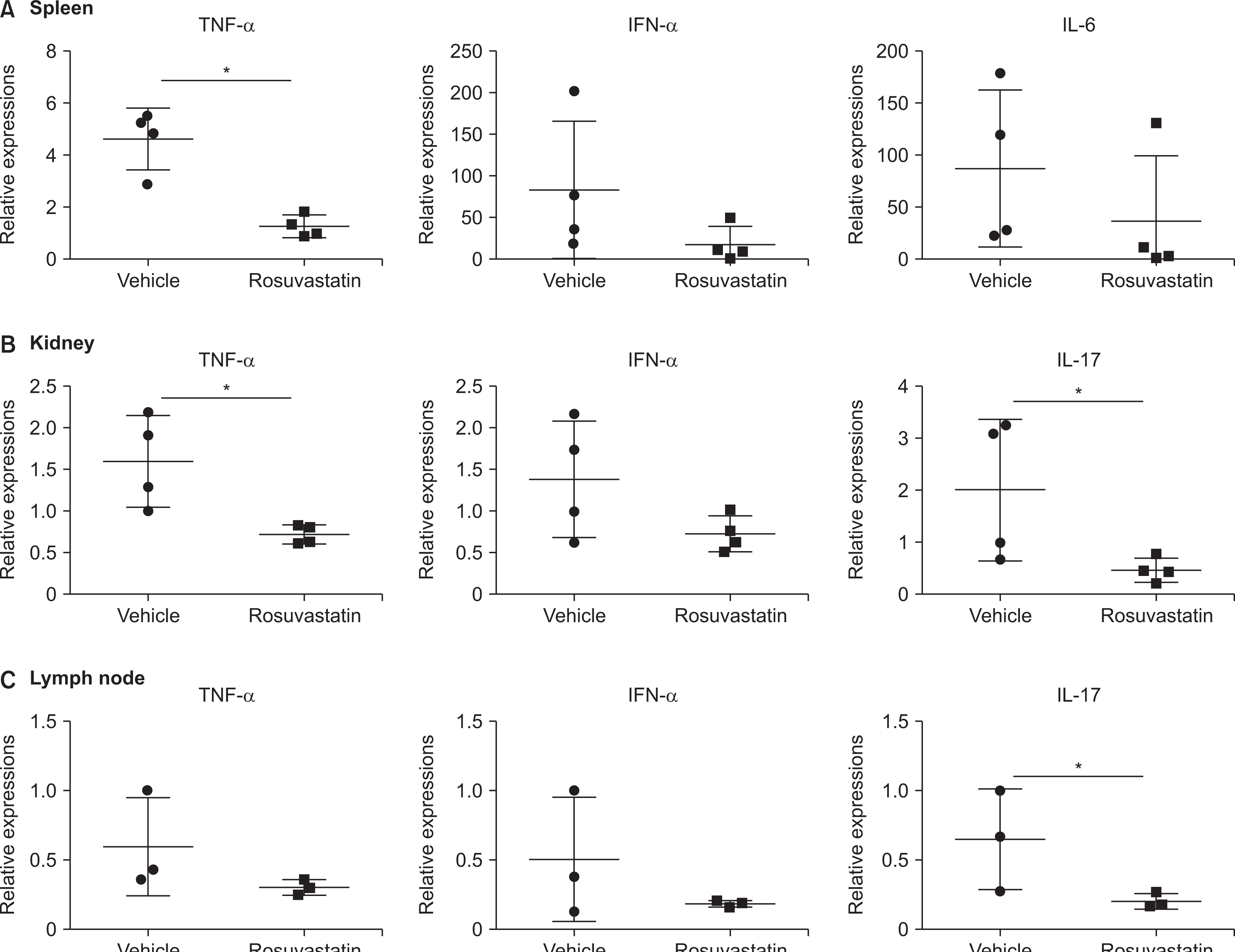
Next, we examined the mRNA levels of rosuvastatin-induced changes in inflammatory cytokines in the spleen, kidney, and lymph nodes, which are the major organs of MRL/lpr mice. The expression of TNF-α in the spleen and kidney tissues was significantly decreased in the rosuvastatin group (Figure 3). In addition, the expression of IL-17 in the kidneys and lymph nodes was significantly decreased in the rosuvastatin group.
Patients with SLE have high cholesterol levels and a higher prevalence of cardiovascular disease due to premature or accelerated atherosclerosis [13]. Statins, currently the most widely used treatment for dyslipidaemia, inhibit cholesterol synthesis in the liver as a HMG-CoA reductase inhibitors, thus, regulating cholesterol production [14]. Eight statins are currently in use: lovastatin, pravastatin, simvastatin, fluvastatin, atorvastatin, mevastatin, rosuvastatin, and pitavastatin [15]. Several studies have reported the use of statins in animal models of SLE. In New Zealand black×New Zealand white (NZB/W) F1 mice, treatment with atorvastatin at a concentration of 30 mg/kg/day decreased serum IgG and anti-dsDNA antibody levels, and reduced kidney damage [16]. Furthermore, simvastatin treatment in gld.ApoE–/– mice improved lymphadenopathy, renal disease, and pro-inflammatory cytokine levels [17].
Based on this background, the effect of rosuvastatin was investigated by predicting that it would reduce the inflammatory response in MRL/lpr mice, a representative SLE model. In the treated group, MRL/lpr mice were injected with rosuvastatin (10 mg/kg) for four weeks, starting at week 13. On week 17, the mice were sacrificed, and serum, urine, kidney, spleen, and lymph nodes were extracted. The results showed that rosuvastatin significantly reduced serum LDL cholesterol levels, but ELISA analysis revealed that ANA, serum dsDNA antibody, serum IL-6, and urine albumin levels were not significantly different in the treated group compared to the control group. In addition, the histological findings did not differ between the two groups. However, the mRNA expression of TNF-α was decreased in the spleen and kidney, and that of IL-17 was decreased in the kidney and lymph nodes of rosuvastatin-treated MRL/lpr mice.
This study has two main limitations: first, the concentration of rosuvastatin used in this study (10 mg/kg) may have been insufficient. Contrary to reports of the suppression of disease development when atorvastatin was administered at a dose of 30 mg/kg, another study reported no improvement in SLE in mice administered atorvastatin at 10 mg/kg for a longer period [18]. This finding suggests that alleviation of SLE by rosuvastatin may occur in a dose-dependent manner in the MRL/lpr model. Additionally, it is possible that rosuvastatin did not improve SLE because of the limited injection period used in this study. In future studies, the results may be improved by administering a period longer than four weeks in this study. Second, autoimmunity in MRL/lpr mice occurs due to defects in Fas-mediated apoptosis of T cells [19]. Statins are known to modulate cellular functions such as cell proliferation and apoptosis through the inhibition of the formation downstream of intermediates in cholesterol synthesis [20]. Therefore, it is assumed that the efficacy of the rosuvastatin could be neutralized when used on MRL/lpr models in which abnormal apoptosis occurs. Therefore, we need to validate rosuvastatin using various lupus-prone mice in future studies. In addition, this study has limited the power of the study to detect statistical significance due to its small sample size (n=4 per group), although it is a pilot study.
In MRL/lpr mice, IP injection of rosuvastatin for four weeks significantly reduced blood LDL cholesterol levels. In addition, as a result of analyzing the mRNA levels of major target tissues of SLE, rosuvastatin decreased TNF-α in the spleen and kidney tissues and decreased IL-17 in the kidney and lymph nodes. However, rosuvastatin did not significantly improve SLE symptoms or activity markers. Therefore, our data suggest that rosuvastatin is insufficient to ameliorate SLE and inflammatory cytokines in MRL/lpr mice.
Notes
FUNDING
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HR16C0001).
AUTHOR CONTRIBUTIONS
Conception and design of study: W.Y.B. and C.H.S. Acquisition of data: W.Y.B., S.M.L., and S.W.L. Analysis and/or interpretation of data: W.Y.B., S.M.L., and S.W.L. Drafting the manuscript: W.Y.B. and C.H.S. Revising the manuscript critically for important intellectual content: C.H.S. All authors read and approved the final manuscript.
REFERENCES
1. D'Cruz DP, Khamashta MA, Hughes GR. 2007; Systemic lupus erythematosus. Lancet. 369:587–96. DOI: 10.1016/S0140-6736(07)60279-7. PMID: 17307106.
2. Isenberg DA, Manson JJ, Ehrenstein MR, Rahman A. 2007; Fifty years of anti-ds DNA antibodies: are we approaching journey's end? Rheumatology (Oxford). 46:1052–6. DOI: 10.1093/rheumatology/kem112. PMID: 17500073.

3. Elkon K, Casali P. 2008; Nature and functions of autoantibodies. Nat Clin Pract Rheumatol. 4:491–8. DOI: 10.1038/ncprheum0895. PMID: 18756274. PMCID: PMC2703183.

4. Tan EM, Cohen AS, Fries JF, Masi AT, McShane DJ, Rothfield NF, et al. 1982; The 1982 revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 25:1271–7. DOI: 10.1002/art.1780251101. PMID: 7138600.

5. Almaani S, Meara A, Rovin BH. 2017; Update on lupus nephritis. Clin J Am Soc Nephrol. 12:825–35. DOI: 10.2215/CJN.05780616. PMID: 27821390. PMCID: PMC5477208.

6. Gasparin AA, Pamplona Bueno de Andrade N, Hax V, Tres GL, Veronese FV, Monticielo OA. 2019; Urinary biomarkers for lupus nephritis: the role of the vascular cell adhesion molecule-1. Lupus. 28:265–72. DOI: 10.1177/0961203319826695. PMID: 30712490.

7. Ytterberg SR, Schnitzer TJ. 1982; Serum interferon levels in patients with systemic lupus erythematosus. Arthritis Rheum. 25:401–6. DOI: 10.1002/art.1780250407. PMID: 6176248.

8. Ohl K, Tenbrock K. 2011; Inflammatory cytokines in systemic lupus erythematosus. J Biomed Biotechnol. 2011:432595. DOI: 10.1155/2011/432595. PMID: 22028588. PMCID: PMC3196871.

9. Sinicato NA, da Silva Cardoso PA, Appenzeller S. 2013; Risk factors in cardiovascular disease in systemic lupus erythematosus. Curr Cardiol Rev. 9:15–9. DOI: 10.2174/1573403X11309010003.

10. Schönbeck U, Libby P. 2004; Inflammation, immunity, and HMG-CoA reductase inhibitors: statins as antiinflammatory agents? Circulation. 109(21 Suppl 1):II18–26. DOI: 10.1161/01.CIR.0000129505.34151.23. PMID: 15173059.
11. Musial J, Undas A, Gajewski P, Jankowski M, Sydor W, Szczeklik A. 2001; Anti-inflammatory effects of simvastatin in subjects with hypercholesterolemia. Int J Cardiol. 77:247–53. DOI: 10.1016/S0167-5273(00)00439-3. PMID: 11182189.

12. White CM. 2002; A review of the pharmacologic and pharmacokinetic aspects of rosuvastatin. J Clin Pharmacol. 42:963–70. DOI: 10.1177/009127000204200902. PMID: 12211221.

13. Lu X, Wang Y, Zhang J, Pu D, Hu N, Luo J, et al. 2021; Patients with systemic lupus erythematosus face a high risk of cardiovascular disease: a systematic review and Meta-analysis. Int Immunopharmacol. 94:107466. DOI: 10.1016/j.intimp.2021.107466. PMID: 33636561.

14. Corsini A, Bellosta S, Baetta R, Fumagalli R, Paoletti R, Bernini F. 1999; New insights into the pharmacodynamic and pharmacokinetic properties of statins. Pharmacol Ther. 84:413–28. Erratum. DOI: 10.1016/S0163-7258(99)00045-5. PMID: 10665838.

15. de Pádua Borges R, Degobi NAH, Bertoluci MC. 2021; Choosing statins: a review to guide clinical practice. Arch Endocrinol Metab. 64:639–53. DOI: 10.20945/2359-3997000000306.
16. Lawman S, Mauri C, Jury EC, Cook HT, Ehrenstein MR. 2004; Atorvastatin inhibits autoreactive B cell activation and delays lupus development in New Zealand black/white F1 mice. J Immunol. 173:7641–6. DOI: 10.4049/jimmunol.173.12.7641. PMID: 15585892.

17. Aprahamian T, Bonegio R, Rizzo J, Perlman H, Lefer DJ, Rifkin IR, et al. 2006; Simvastatin treatment ameliorates autoimmune disease associated with accelerated atherosclerosis in a murine lupus model. J Immunol. 177:3028–34. DOI: 10.4049/jimmunol.177.5.3028. PMID: 16920939. PMCID: PMC2752011.

18. Graham KL, Lee LY, Higgins JP, Steinman L, Utz PJ, Ho PP. 2008; Failure of oral atorvastatin to modulate a murine model of systemic lupus erythematosus. Arthritis Rheum. 58:2098–104. DOI: 10.1002/art.23605. PMID: 18576356. PMCID: PMC4143381.

19. Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, et al. 1992; Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 176:1693–702. DOI: 10.1084/jem.176.6.1693. PMID: 1460426. PMCID: PMC2119469.

20. O'Grady S, Crown J, Duffy MJ. 2022; Statins inhibit proliferation and induce apoptosis in triple-negative breast cancer cells. Med Oncol. 39:142. DOI: 10.1007/s12032-022-01733-9. PMID: 35834073. PMCID: PMC9283343.
Fig. 1
No change in SLE major markers by rosuvastatin treatment. (A) dsDNA antibodies, (B) ANA, (C) IL-6, and (D) LDL-cholesterol in the serum of MRL/lpr mice treated with rosuvastatin were determined using ELISA. All experiments were performed in duplicate wells (n=3~4 mice/group). The Mann–Whitney U test was used to determine differences between groups. SLE: systemic lupus erythematosus, dsDNA: anti-double-stranded DNA, ANA: antinuclear antibodies, IL-6: interleukin-6, ELISA: enzyme-linked immunosorbent assay, LDL: low-density lipoprotein. *p<0.05.
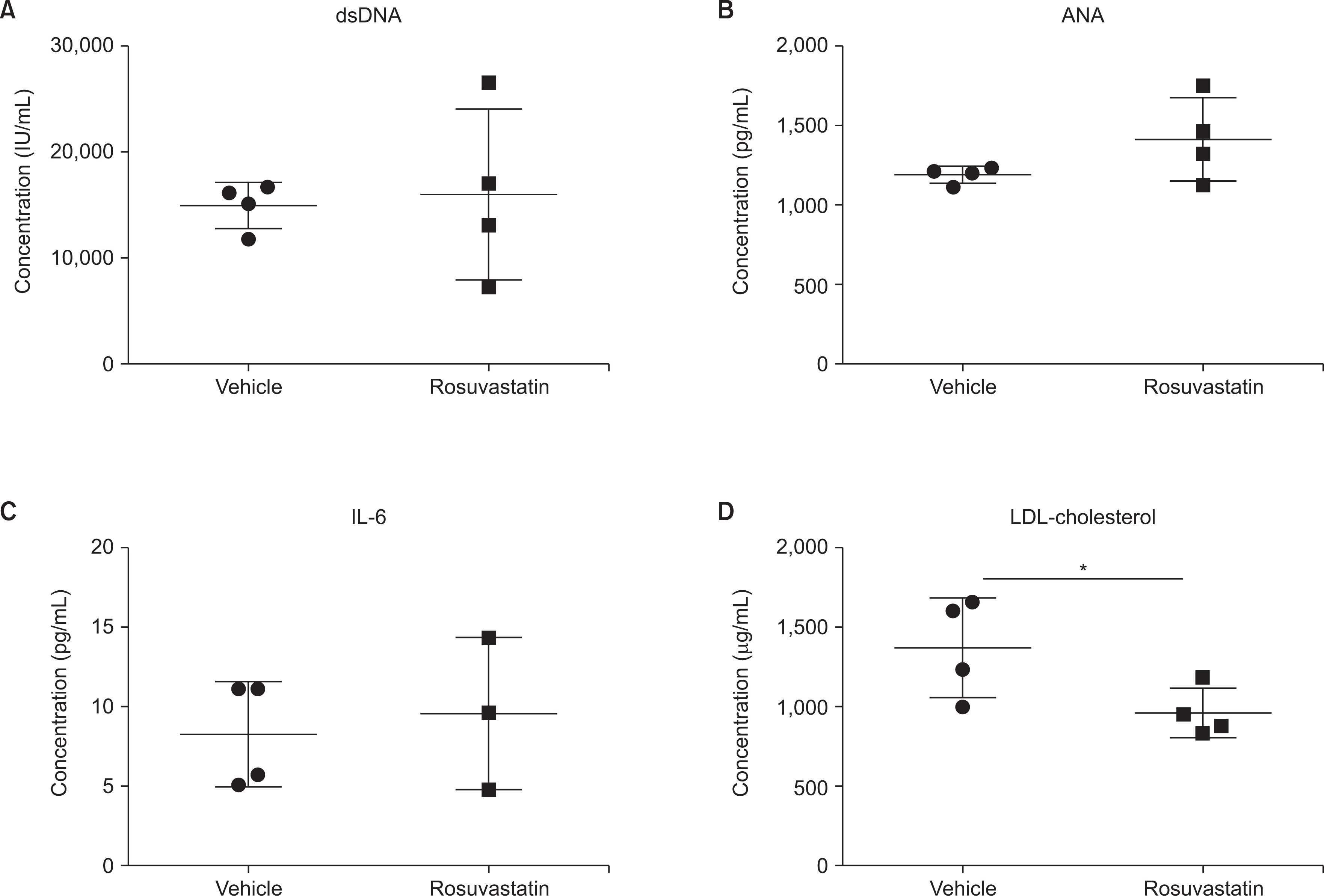
Fig. 2
Rosuvastatin did not improved LN in MRL/lpr mice. (A) Representative photographs of H&E staining (top panels) and PAS staining (bottom panels) of rosuvastatin-treated kidneys (×200). (B) Albumin content in the urine as determined using ELISA (n=4 micegroup). LN: lupus nephritis, H&E: hematoxylin and eosin, PAS: periodic acid–Schiff, ELISA: enzyme-linked immunosorbent assay.

Fig. 3
Reduction of several inflammatory cytokines in key target tissues during SLE in MRL/lpr mice. The level of pro-inflammatory cytokines in the spleen (A), kidney (B), and lymph node (C) tissues of MRL/lpr mice treated with rosuvastatin was verified at the messenger RNA level. The Mann–Whitney U test was used to determine differences between groups. TNF: tumor necrosis factor-alpha, IFN: interferon-alpha, IL-6: interleukin-6, SLE: systemic lupus erythematosus. *p<0.05.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download