Abstract
Objective
The objective of this study was to evaluate the safety and efficacy of light-emitting diode therapy (LEDT) in the management of pain and stiffness in patients with refractory hand tenosynovitis to non-steroidal anti-inflammatory drugs.
Methods
A total of 12 patients were enrolled in the study and received LEDT twice a week for four weeks. Sociodemographic, clinical, and laboratory data were collected, and the visual analog scale (VAS) pain and stiffness scores of each hand were assessed every two weeks. The thickness of the flexor tendon in the patients’ hand was evaluated using ultrasonography. To investigate the molecular effects of LEDT, we measured the expression levels of type III collagen in tendon cells, with and without LEDT treatment.
Results
After undergoing LEDT, participants showed clinically significant improvements in VAS pain scores at weeks 2, 4, and 8 compared to their baseline, and in VAS stiffness scores at weeks 4 and 8. According to the ultrasonography results, there was a decreasing tendency in tendon thickness for each finger in week 8 compared to the baseline, but the difference was not statistically significant. No adverse events were reported. Additionally, our results indicated a significant increase in type III collagen levels in the LEDT group compared to the control group (1.48±0.18 vs. 0.99±0.02, p=0.031), indicating a potential molecular mechanism for the observed clinical improvements.
Hand tenosynovitis is a common disorder that affects the flexor sheaths in the fingers. It is characterized by localized pain, dysfunction, inflammation, and degeneration of soft tissue due to repetitive overuse or injury [1,2]. A population-based study reported that upper extremities pain disorder is usual, with the lifetime prevalence of discomfort in the wrist or the hand being 9%~17% [3].
Currently, several therapeutic managements are practiced in clinical circumstances, but their effects are limited. Non-steroidal anti-inflammatory drugs (NSAIDs) are largely used in the treatment of hand tenosynovitis, but are not sufficiently effective [4]. A few studies explored the therapeutic effect of local corticosteroid injections, but they were found to have efficacy only in 57% of patients, and the relapse rate within one year after the injections was as high as 56% [5,6].
Recently, several studies have suggested visible light irradiation as an alternative to pharmacological treatments [7-10]. A literature review on tendon lesions in rats reported that light-emitting diodes therapy (LEDT) promoted the repair of an injured calcaneal tendons by significantly reducing inflammation [10]. Martins et al. [8] reported that LEDT played a role in increasing the activity of antioxidant enzymes and levels of interleukin (IL)-10. These results are promising, but they are limited to animal models and illustrate little about the impact of LEDT in humans.
To date, the scientific evidence supporting the therapeutic effect of LEDT remains insufficient. Despite its efficacy in treating pain and inflammation, little is known about the impact of LEDT in humans. Since no study has explored the efficacy of LEDT to those who are unresponsive to NSAIDs, we aimed to investigate the effectiveness, tolerability, and safeness of LEDT in hand tenosynovitis patients.
We evaluated patients who presented with hand tenosynovitis at the Division of Rheumatology, Department of Internal Medicine at Chonnam National University Hospital, Korea, from August 2019 to November 2019. All the participants complained of hand pain, and none of them exhibited any inflammatory autoimmune disease, such as rheumatoid arthritis.
The inclusion criteria were: (1) patient age of 30~65 years, (2) a confirmed diagnosis of hand tenosynovitis by a rheumatologist, (3) pain with a score of ≥4 on the visual analog scale (VAS), (4) a regimen of the prescribed maximal dose of NSAIDs for 2 weeks, and (5) a signed informed consent after a thorough explanation from the physicians. The exclusion criteria were: (1) rheumatologic disorders including rheumatoid arthritis, (2) previous treatment of steroids and/or disease-modifying anti-rheumatic drugs, (3) photosensitivity or the currently consumption of drugs that increase photosensitivity, (4) pregnant or currently lactating, and (5) failure to meet the inclusion criteria.
Finally, we included 12 participants in the study. All participants were provided with informed consent and the study got approval by the Institutional Review Board/Ethics Committee of Chonnam National University Hospital (CNUH-2019-251). This clinical trial for the medical device was approved by the Korean Ministry of Food and Drug Safety (2019-979).
This study was conducted over 8 weeks, comprising a 4-week LEDT course and a four-week follow-up course for each patient. To investigate the effectiveness of LEDT, we evaluated the VAS scores for pain and stiffness every 2 weeks. We assessed the VAS score for pain at the time of enrollment (pretreatment) as well as in week 2 (during treatment), week 4 (immediately after treatment), and week 8 (4 weeks after treatment). The rheumatologists among the present group of researchers evaluated the thickness of the flexor tendons in both hands using ultrasonography at the time of enrollment (pretreatment) and in week 8 (4 weeks posttreatment). For the entire duration of this trial, the use of any medication except for NSAIDs is strictly prohibited.
All the patients were planned to undergo two 20-minute LEDT sessions per week for 4 consecutive weeks. Conventional LED devices (BELLALUX, MD-031R) with wavelengths of 850 nm and 592 nm were used for therapy (Supplementary Figure 1).
The primary outcome to demonstrate the efficacy of LEDT was an alteration in the VAS pain score from the time of enrollment to treatment (week 4). The VAS pain scale ranges from 0 (thoroughly absence of pain) to 10 (worst intolerable pain). Similarly, the VAS stiffness scale ranges from 0 (thoroughly absence of stiffness) to 10 (worst intolerable stiffness).
The secondary efficacy outcome was tendon thickness. We examined the improvements in the indices from the time of enrollment to 4 weeks after treatment (week 8). Expert rheumatologists independently assessed the mean thickness of the first to fifth flexor tendons of all enrolled patients’ hands using ultrasonography. Tendon thickness was assessed using the longitudinal view with images taken around the A1 pulley. We then compared the baseline and posttreatment thickness of each tendon.
Safety outcomes were assessed by checking on the presence and frequency of adverse events during the study. We closely watched potential adverse events by asking participants after each time point and during the follow-up period and marking their responses on a checklist.
Tissue samples were obtained from patients who had undergone arthroscopic rotator cuff repair at our institute and had provided consent. The supraspinatus tendon tissue samples were washed with phosphonate-buffered saline and cut into small pieces. They were then treated with 3 mg/mL of collagenase (Sigma, St. Louis, MO, USA) in Dulbecco’s modified Eagle’s medium (DMEM) at 37°C for 16 hours. After enzymatic digestion, DMEM was added to suppress collagenase activity, and the samples were filtered through 70 μm-sized cell strainers. The filtered cell suspension was centrifuged at 1,500 rpm for 5 minutes, and the cell pellets were resuspended and cultured in low-glucose DMEM (DMEM low glucose; Gibco, Eggenstein, Germany) supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin at 37°C in a 5% CO2 atmosphere. The medium was changed every 3 days, and cells at passages 3 or 4 were used in the study. The tenocytes were seeded (5×104) in 96-well plates and incubated overnight. The medium was then replaced, and 10 μg/mL of lipopolysaccharide (LPS; Invitrogen, Waltham, MA, USA) was added to the cells, which were further incubated for 24 hours. Afterward, the cells were treated with 36 J/cm2 of LED with wavelengths of 850 nm and 592 nm for 20 minutes periods over 3 days.
After RNA extraction using TRIzol reagent (Invitrogen) following the manufacturer’s protocol, cDNA was synthesized from 1 μg of total RNA using the QuantiTect Reverse Transcription Kit (QIAGEN, Hilden, Germany). Gene expression levels were quantified by RT-qPCR using the SYBR Green PCR Master Mix Kit (QIAGEN). The PCR reaction conditions comprised an initial step of 15 minutes at 95°C, followed by 55 cycles of denaturation at 94°C for 15 seconds, annealing at 55°C for 30 seconds, and extension at 72°C for 30 seconds. The Thermal Cycler Dice Real Time System lll (Takara, Kusatsu, Japan) was used for RT-qPCR. The primer sequences used were as follows: type III collagen fwd:5’-GTTTTCAGTTTAGCTACGGC-3’, rev:5’-CTTCAGGGCCTTCTTTACAT-3’.
Statistical analyses were conducted using SPSS for Windows (ver. 20; IBM Corp., Armonk, NY, USA). To identify the efficacy of LEDT, paired t-tests were used to compare the means at the time of enrollment with those at diverse time points after the treatment. We used the Wilcoxon matched-pairs signed rank test to compare the RNA expression levels between the LPS only and LPS with LEDT groups and calculated the corresponding p-values. A p-value of <0.05 was considered statistically significant.
The baseline characteristics of the 12 patients with hand tenosynovitis enrolled in this study, are described in Table 1. The mean age of the enrolled patients was 57.08±5.81 years, and the mean disease duration was 4.83±9.53 months. The mean baseline VAS score was 7.00±1.21, and the mean VAS stiffness score was 7.58±0.90. All our patients underwent their assigned LEDT sessions over 4 consecutive weeks.
Table 2 shows the outcome measurements from the values at baseline to the values at specific time points. The VAS pain score significantly improved during week 2 of the treatment (mean change from baseline: –0.583; 95% confidence interval [CI]: –1.008 to –0.159; p=0.036) and immediately after treatment (week 4) (–1.333; 95% CI: –2.161 to –0.506; p=0.015) and was maintained at 4 weeks posttreatment (week 8) (–1.875; 95% CI: –2.733 to –1.017; p=0.003). The VAS stiffness score saw a significant decrease in weeks 4 (–1.083; 95% CI: –1.871 to –0.295; p=0.036) and 8 (–1.250; 95% CI: –2.154 to –0.346; p=0.033) as compared with the baseline. After LEDT, the VAS pain slope of all participants was significantly reduced at week 8 as compared with the baseline (p<0.05) (Figure 1A). The VAS stiffness slope significantly decreased from weeks 4 to 8 (p<0.05) (Figure 1B).
Following LEDT, there was a decreasing tendency in flexor tendon thickness for each finger in both hands during week 8 compared to the baseline. Nevertheless, when comparing the baseline and week 8 values, the change was not statistically significant (data not shown). Notably, there were no adverse events or dropouts in this study.
To investigate the molecular effects of LEDT, we measured the expression levels of type III collagen in tendon cells. Our results showed a significant increase in the levels of type III collagen in the LPS with LEDT group compared to the LPS-only group (mean±standard error of mean, 1.48±0.18 vs. 0.99±0.02, p=0.031, as shown in Figure 2).
We found a meaningful reduction in pain and stiffness in patients with hand tenosynovitis after LEDT. The treatment was well tolerated, and no adverse events were reported.
Our study demonstrated that hand pain and stiffness were significantly reduced in patients with hand tenosynovitis who were refractory to NSAIDs treatment after LEDT. Most previous trials of hand tenosynovitis have been based on pharmacological or invasive interventions. NSAIDs have been selected by physicians as the initial treatment, but their effects are limited, and adverse events and patients’ underlying conditions must be considered [4]. A recent systematic review found that only half of the patients had effective results after corticosteroid injections [5], and over half had recurrence within several months of the injections [6]. In addition, adverse events of glucocorticoid injections are uncommon, but contain permanent pigmentation change at the location of injection, fat necrosis, infection, and tendon rupture [11]. Considering steroid injection, it may have certain limitations that need to be taken into account. On the other hand, we performed LEDT as a nonpharmacological and noninvasive treatment in hand tenosynovitis patients. We evaluated patients at multiple time points to explore the efficacy of LEDT and found that it may have a crucial role in improvement of pain and stiffness in patients with hand tenosynovitis. Remarkably, we did not find any adverse events of LEDT. This study suggests that LEDT could be a useful and safe therapeutic modality in clinical practice.
Several studies have described the effect of LEDT on tenocytes in murine models [12,13]. In a progressive osteoarthritis rabbit model, low-level laser therapy slowed down the degradation of type II collagen and aggrecan [13]. In another animal study, LEDT induced type III collagen production, which is a vital step in tendon healing [12,14]. In the present study, we measured type III collagen RNA levels of tendon structural molecules and found that they increased after LEDT. This result agreed with those of previous research, which demonstrated that LEDT might enhance the healing process by increasing collagen molecules in damaged tendons. Taken together, our results indicate that LEDT shows an effect that promotes the formation of collagen fibers. Therefore, this outcome could be a pathophysiological basis for clinical improvement.
Despite these promising findings, this study has some limitations. First, the participants could recognize their therapies, and they can have great expectations for treatment advantages, such as a placebo effect, might have affected the assessments of this result because of the lack of patients blinding. Additionally, other factors such as education and warnings for avoidance of hand use could have influenced the outcome. Second, this study included a small number of patients who could not be representative of all hand tenosynovitis patients. To overcome these limitations, we undertook an independent investigation utilizing objective scientific measures, including tenocyte experiments.
In conclusion, although this study did not establish an optimal treatment regimen due to limitations in sample size and treatment variables such as number of sessions and treatment interval, the results suggest that LEDT may be a useful alternative treatment modality for refractory hand tenosynovitis. Further research with larger sample sizes and controlled treatment regimens may provide more definitive results.
Supplementary data can be found with this article online at https://doi.org/10.4078/jrd.2023.0004.
Notes
REFERENCES
1. Hubbard MJ, Hildebrand BA, Battafarano MM, Battafarano DF. 2018; Common soft tissue musculoskeletal pain disorders. Prim Care. 45:289–303. DOI: 10.1016/j.pop.2018.02.006. PMID: 29759125.

2. Jeanmonod R, Harberger S, Waseem M. 2022; In: StatPearls. Treasure Island. StatPearls Publishing.
3. Walker-Bone KE, Palmer KT, Reading I, Cooper C. 2003; Soft-tissue rheumatic disorders of the neck and upper limb: prevalence and risk factors. Semin Arthritis Rheum. 33:185–203. DOI: 10.1016/S0049-0172(03)00128-8. PMID: 14671728.

4. Giugale JM, Fowler JR. 2015; Trigger finger: adult and pediatric treatment strategies. Orthop Clin North Am. 46:561–9. DOI: 10.1016/j.ocl.2015.06.014. PMID: 26410644.
5. Fleisch SB, Spindler KP, Lee DH. 2007; Corticosteroid injections in the treatment of trigger finger: a level I and II systematic review. J Am Acad Orthop Surg. 15:166–71. DOI: 10.5435/00124635-200703000-00006. PMID: 17341673.

6. Rozental TD, Zurakowski D, Blazar PE. 2008; Trigger finger: prognostic indicators of recurrence following corticosteroid injection. J Bone Joint Surg Am. 90:1665–72. DOI: 10.2106/JBJS.G.00693. PMID: 18676896.

7. Lim W, Lee S, Kim I, Chung M, Kim M, Lim H, et al. 2007; The anti-inflammatory mechanism of 635 nm light-emitting-diode irradiation compared with existing COX inhibitors. Lasers Surg Med. 39:614–21. DOI: 10.1002/lsm.20533. PMID: 17868110.

8. Martins DF, Turnes BL, Cidral-Filho FJ, Bobinski F, Rosas RF, Danielski LG, et al. 2016; Light-emitting diode therapy reduces persistent inflammatory pain: role of interleukin 10 and antioxidant enzymes. Neuroscience. 324:485–95. DOI: 10.1016/j.neuroscience.2016.03.035. PMID: 27001179.

9. de Melo CA, Alves AN, Terena SM, Fernandes KP, Nunes FD, da Silva Dde F, et al. 2016; Light-emitting diode therapy increases collagen deposition during the repair process of skeletal muscle. Lasers Med Sci. 31:531–8. DOI: 10.1007/s10103-016-1888-9. PMID: 26873500.

10. Nascimento LDES, Nascimento KFES, Pessoa DR, Nicolau RA. 2019; Effects of therapy with light emitting diode (LED) in the calcaneal tendon lesions of rats: a literature review. ScientificWorldJournal. 2019:6043019. DOI: 10.1155/2019/6043019. PMID: 30853864. PMCID: PMC6377949.

11. Ryzewicz M, Wolf JM. 2006; Trigger digits: principles, management, and complications. J Hand Surg Am. 31:135–46. DOI: 10.1016/j.jhsa.2005.10.013. PMID: 16443118.

12. Chen MH, Huang YC, Sun JS, Chao YH, Chen MH. 2015; Second messengers mediating the proliferation and collagen synthesis of tenocytes induced by low-level laser irradiation. Lasers Med Sci. 30:263–72. DOI: 10.1007/s10103-014-1658-5. PMID: 25231827.

13. Wang P, Liu C, Yang X, Zhou Y, Wei X, Ji Q, et al. 2014; Effects of low-level laser therapy on joint pain, synovitis, anabolic, and catabolic factors in a progressive osteoarthritis rabbit model. Lasers Med Sci. 29:1875–85. DOI: 10.1007/s10103-014-1600-x. PMID: 24890034.

14. de Souza TO, Mesquita DA, Ferrari RA, Dos Santos Pinto D Jr, Correa L, Bussadori SK, et al. 2011; Phototherapy with low-level laser affects the remodeling of types I and III collagen in skeletal muscle repair. Lasers Med Sci. 26:803–14. DOI: 10.1007/s10103-011-0951-9. PMID: 21761120.

Fig. 1
(A) Slope of VAS pain scores by time in all patients (pretreatment, during treatment, 4 weeks posttreatment). The slope was significantly reduced at week 8 from the baseline. (B) Slope of VAS stiffness scores by time in all patients (pretreatment, during treatment, 4 weeks posttreatment). The slope was significantly decreased from weeks 4 to 8. Values are shown as the mean±standard error of the mean. LED: light-emitting diode, VAS: visual analog scale. *p<0.05, **p<0.01.
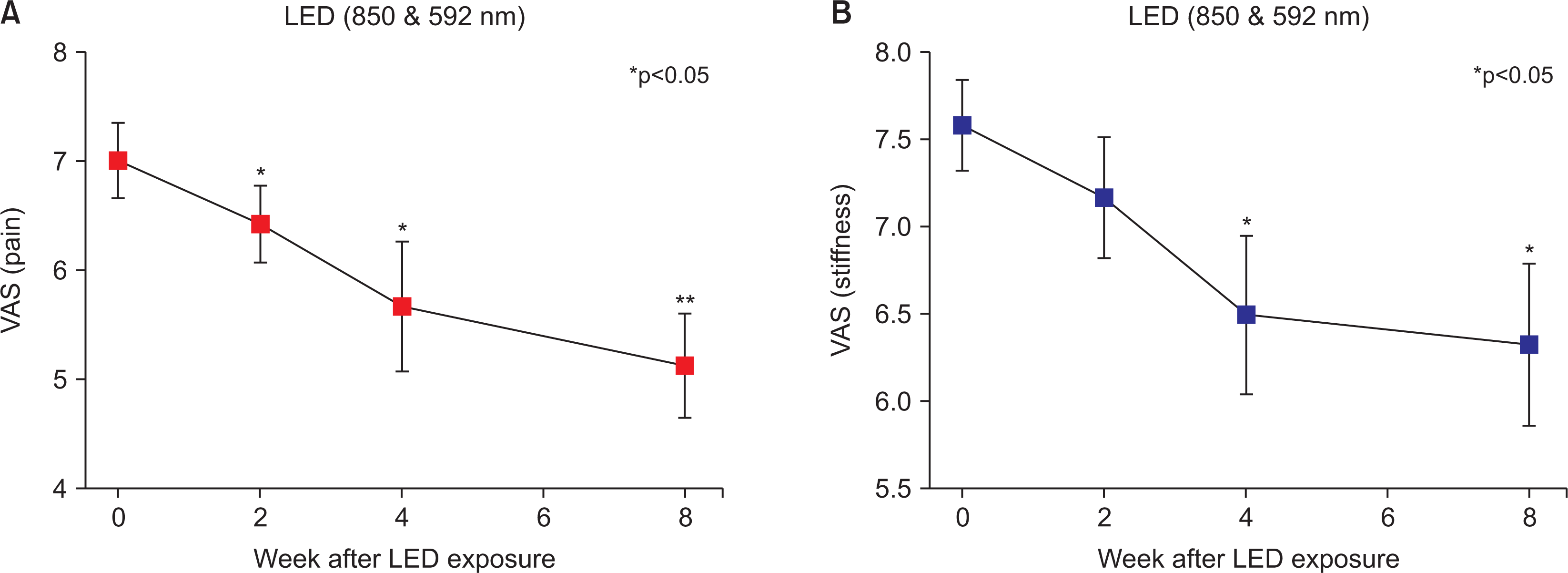
Fig. 2
Comparison between LEDT (–) and LEDT (+) group in levels of type III collagen. Tissue samples were collected from the supraspinatus tendon undergoing arthroscopic rotator cuff repair. Then, tenoctyes were isolated and cultured according to the protocol. All the cells used were at passages 3 or 4. To determine the effect of LEDT, tenocytes were stimulated by lipopolysaccharide with or without LEDT. After stimulation, the levels of RNA expression of collagen type III in each tenocyte were measured by quantitative polymerase chain reaction. LEDT: light-emitting diodes therapy. *p<0.05.
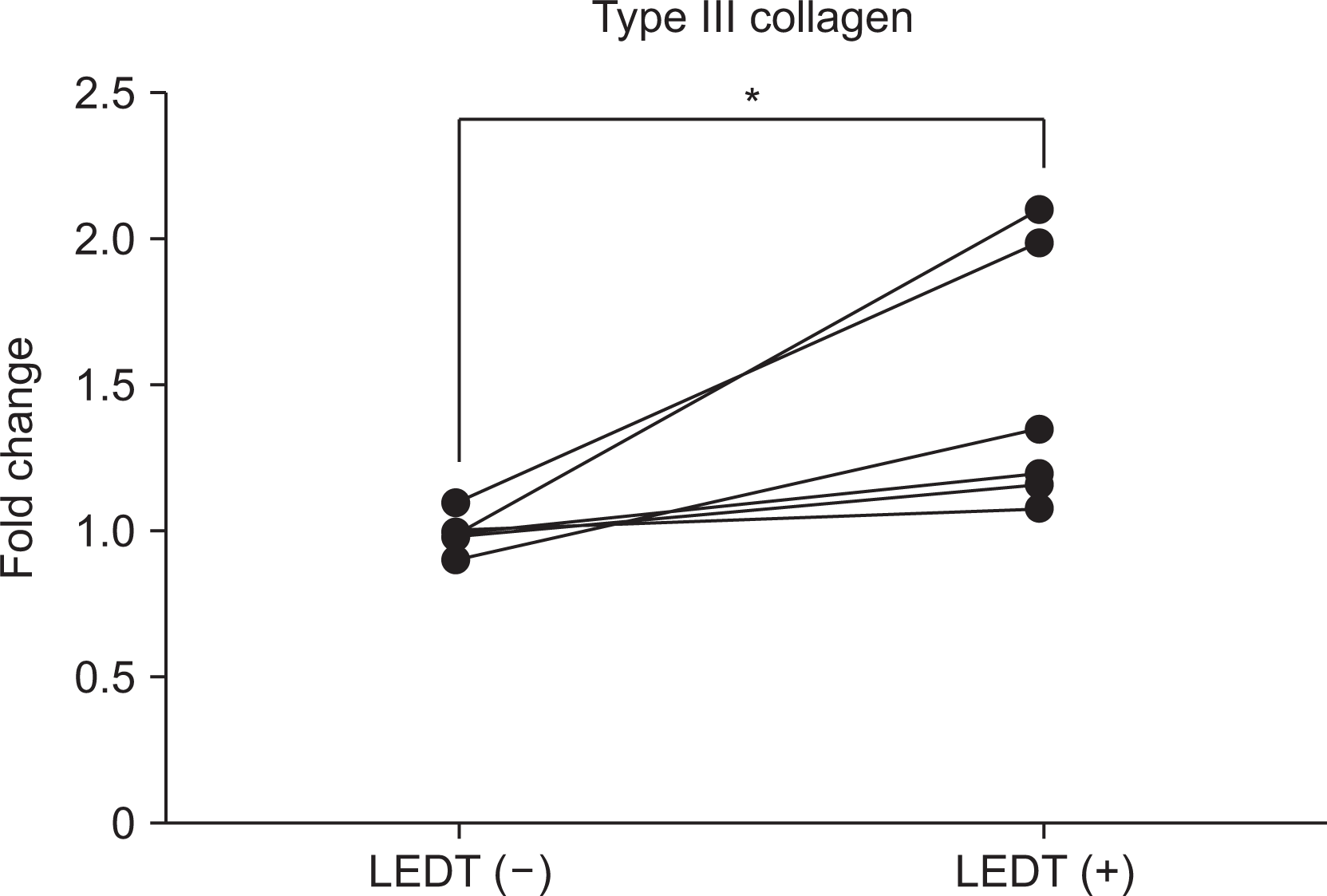
Table 1
Baseline characteristics of 12 patients with hand tenosynovitis
Table 2
Follow up of outcome measures from baseline to each time point




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download