INTRODUCTION
Approximately 33.5 million patients worldwide had cardiac arrhythmia almost 10 years ago; the most common arrhythmia is atrial fibrillation [
1,
2]. Arrhythmia is produced by several mechanisms; most arrhythmias are related to ion channels and most anti-arrhythmic agents are ion channel modulators [
3-
5]. Anti-arrhythmic agents are classified into four types based on their ability to block ion channels or cell receptors [
6]. Class I anti-arrhythmic drugs target Na
+ channels and are divided into classes Ia–c according to their degree of inhibition on Na
+ channels [
4]. Encainide is a class Ic anti-arrhythmic drug used since the 1980s [
7]. It is a safe and well-tolerated anti-arrhythmic agent, with some minor side effects such as dizziness, headache, and nausea [
8,
9]. However, the side effects of encainide on other ion channels, particularly the voltage-dependent K
+ (Kv) channels expressed in vascular smooth muscle cells, have not been elucidated.
Smooth muscle cells of the vasculature regulate vascular tone and function. In turn, altered vascular smooth muscle cells are strongly associated with cardiovascular diseases, such as atherosclerosis, diabetes, hypertension, and hypertrophy [
10-
12]. Vascular contractility is regulated by ion channels expressed in smooth muscles [
13]. Several ion channels have been identified on vascular smooth muscle cells. Of these, Kv channels are the most abundant in the membrane and control the vascular tone by changing the resting membrane potential [
14,
15]. Kv channels comprise diverse subtypes that regulate smooth muscle cell functions. Therefore, modulation of Kv subtypes is a potential therapeutic target for cardiovascular diseases. For instance, blockade of Kv1.3 affects the proliferation of smooth muscle cells and is closely related to the pathogenesis of atherosclerosis [
16,
17].
Considering the therapeutic benefit of encainide for cardiac arrhythmia and the pathophysiological importance of vascular Kv channels, it is essential to explore the inhibitory effects of encainide on vascular Kv channels to avoid miscomprehension of vascular function data and to prevent side effects on vasculature. In the present study, we demonstrated the inhibitory effect of encainide on vascular Kv channels using freshly segregated rabbit coronary arterial smooth muscle cells. We found that encainide inhibited vascular Kv channels in a concentration-dependent and use-independent manner. This inhibitory reaction occurs by changing the gating property of the channel.
METHODS
Single smooth muscle cell isolation
Male New Zealand White rabbits (2.2–2.4 kg) were sacrificed by instantaneous injection of heparin (120 U/kg) and sodium pentobarbital (35 mg/kg) into the ear vein. All animal experiments followed the National Institutes of Health guidelines and were approved by the Animal Ethics Committee of the Yangzhou University (approval no.: YZU220180489). The heart was immediately separated and the left descending coronary artery in the heart was segregated for single cell acquisition. Adipose tissues were removed using normal Tyrode’s solution under a stereomicroscope. The endothelial cells were eliminated by injecting air bubbles into the arterial lumen. For single cell acquisition, the arteries were treated using two-step enzymatic procedures. First, the arteries were rinsed in 1 ml of Ca2+-removed normal Tyrode’s solution containing papain (0.8 mg/ml), dithiothreitol (DTT, 1.2 mg/ml), and bovine serum albumin (BSA, 1.1 mg/ml), and incubated for 22 min at 37°C–38°C. Second, the arteries were rinsed into 1 ml of Ca2+-removed normal Tyrode’s solution containing collagenase (2.4 mg/ml), DTT (1.2 mg/ml), and BSA (1.1 mg/ml) for 20–23 min at 37°C–38°C. Fresh cells were separated by mechanical agitation in Kraft–Brühe solution using a heat-polished Pasteur pipette. The freshly separated single cells were kept at 4°C–6°C and used within a day.
Solutions and chemicals
The composition (mM) of normal Tyrode’s solution was KCl, 5.4; NaCl, 135; CaCl2, 1.7; MgCl2, 1.2; NaH2PO4, 0.37; glucose, 15; and HEPES, 5. The pH of the solution was adjusted to 7.4 using NaOH. The composition (mM) of the Kraft–Brühe solution was KOH, 71; KCl, 56; L-glutamate, 53; KH2PO4, 21; taurine, 18; MgCl2, 1.9; glucose, 15; EGTA, 0.5; and HEPES, 10. The pH of the solution was adjusted to 7.3 using KOH. The composition (mM) of the pipette solution for recording of Kv channels was NaCl, 5.5; K-aspartate, 113; KCl, 28; MgCl2, 1.6; Mg-ATP, 4; EGTA, 10; and HEPES, 8. The pH of the solution was adjusted to 7.25 using KOH. Encainide (Sigma-Aldrich) was dissolved in ethanol. Guangxitoxin and DPO-1 (Tocris Cookson) were dissolved in dimethyl sulfoxide. The final solvent contents were below 0.1%, which did not alter the Kv currents.
Electrophysiology and data analysis
Kv currents from single vascular smooth muscle cells were measured using an EPC-10 amplifier (HEKA Elektronik GmbH). Patchpro software was applied for protocol generation and data acquisition. The patch electrodes were pulled using a P-97 microelectrode puller (Sutter Instrument) using a thin glass electrode. The patch electrode had a resistance of 2.5–3.5 MΩ when filled with the pipette solution. The average cell capacitance was 16.22 ± 1.33 pF (n = 15). The perforated-patch clamp technique was applied to record the resting membrane potential. Nystatin (100 µg/ml) was included in the internal solution. The success of the perforated-patch configuration was confirmed by the observation of a slow capacitive current. All experiments were performed at room temperature.
Origin 8.0 software (Microcal Software, Inc.) was used for data analysis. The kinetics of drug–ion channel interaction was described using a first-order blocking scheme. The half (mid)-maximal inhibitory (IC50) value and the Hill coefficient (n) were determined by analysis of concentration-dependent data and fitted to the following Hill equation:
where ƒ is the relative current inhibition (ƒ = 1 – Idrug / Icontrol) at the given potential and [D] is the encainide concentration.
Steady state activation curves were constructed using tail currents, which were produced by returning the voltage to –40 mV after application of short depolarizing voltages (20–50 ms) from –80 to +60 mV in 10-mV increments. The elicited tail currents at each depolarization voltage were normalized to the maximal tail current. The activation curves were fitted using the Boltzmann equation given below:
where V1/2 is the half (mid)-maximal activation voltage, V is the test voltage, and k is the slope value.
The steady-state inactivation curves were constructed by applying the given voltage to +40 mV at a duration of 600 ms after 7-s preconditioning voltages from −80 to +30 mV in 10-mV increments. Steady state inactivation curves were fitted with below Boltzmann equation:
where V1/2 is the half (mid)-maximal inactivation voltage, V is the preconditioning voltage, and k is the slope value.
Statistical analysis
The results are presented as means ± standard errors of the mean. Statistical significance was calculated using the Mann–Whitney U-test. p < 0.05 was considered statistically significant.
DISCUSSION
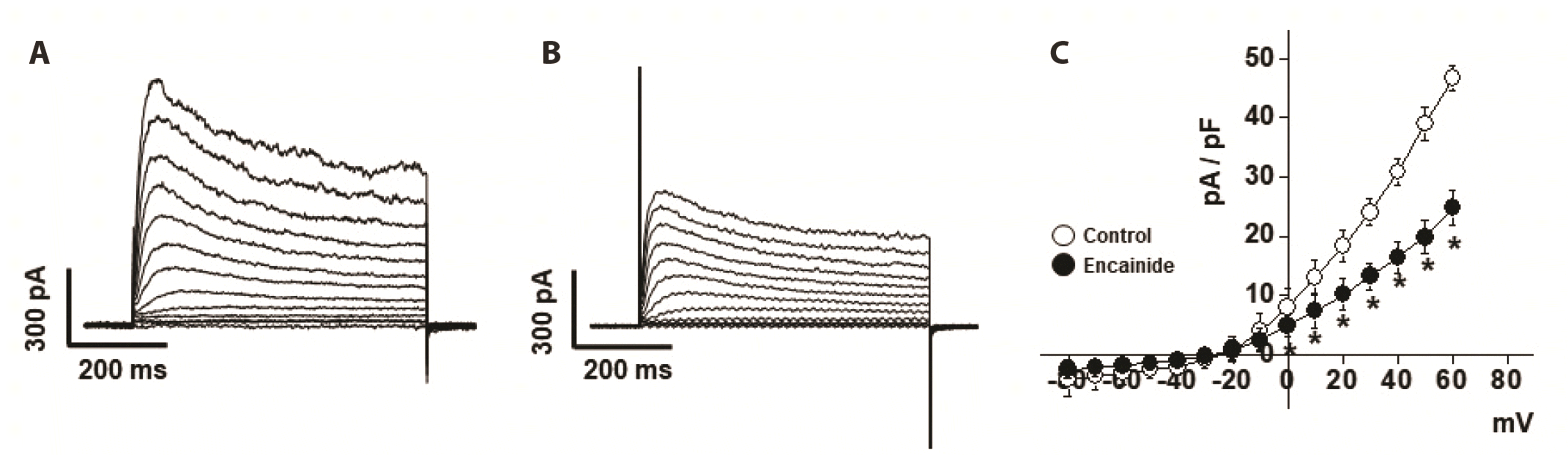
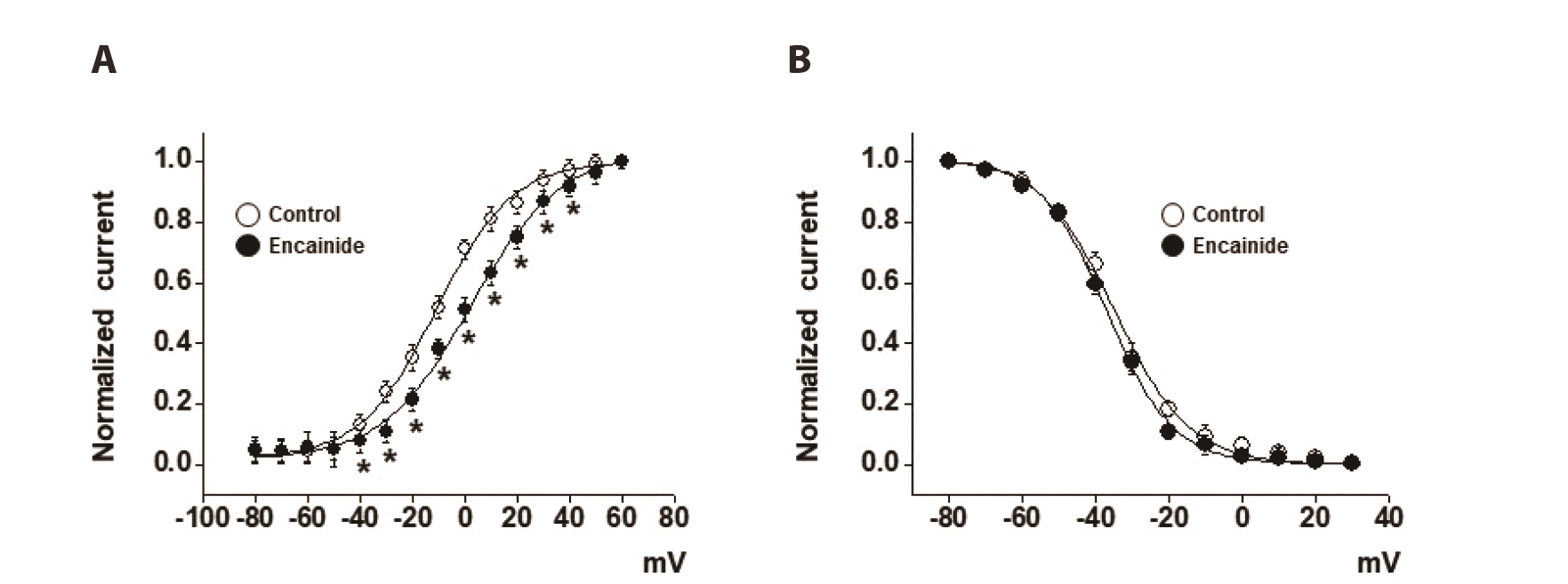
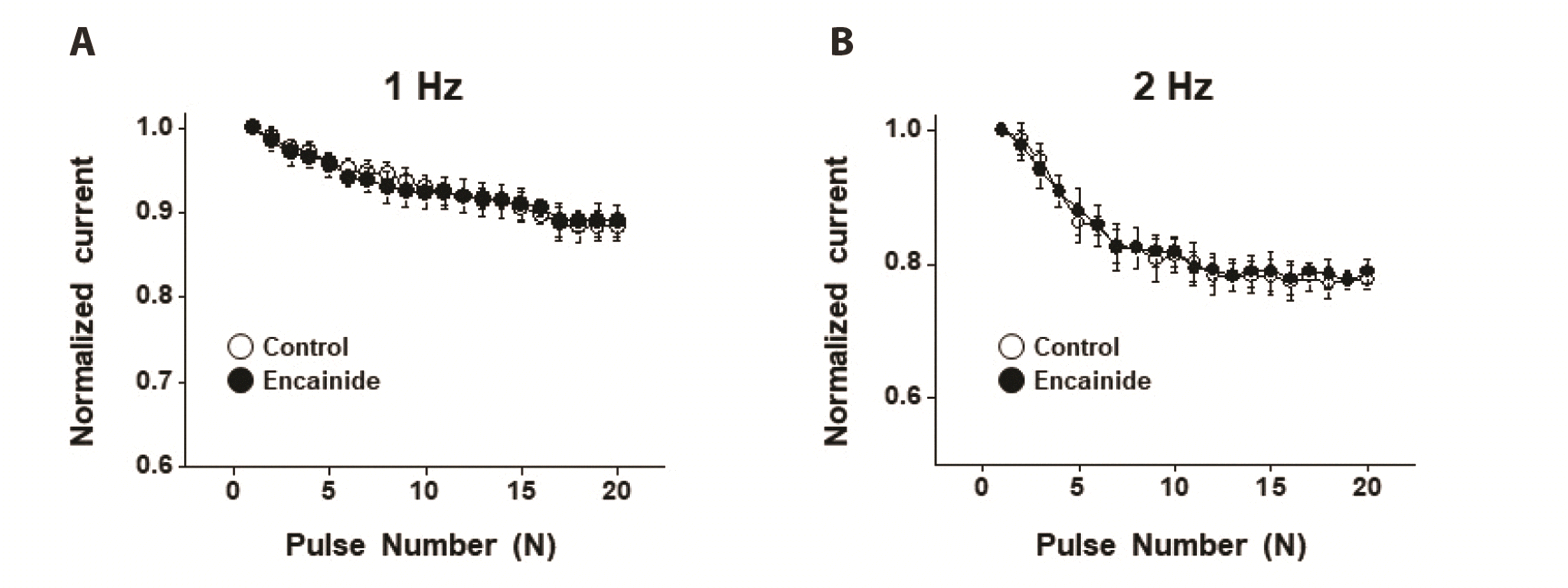
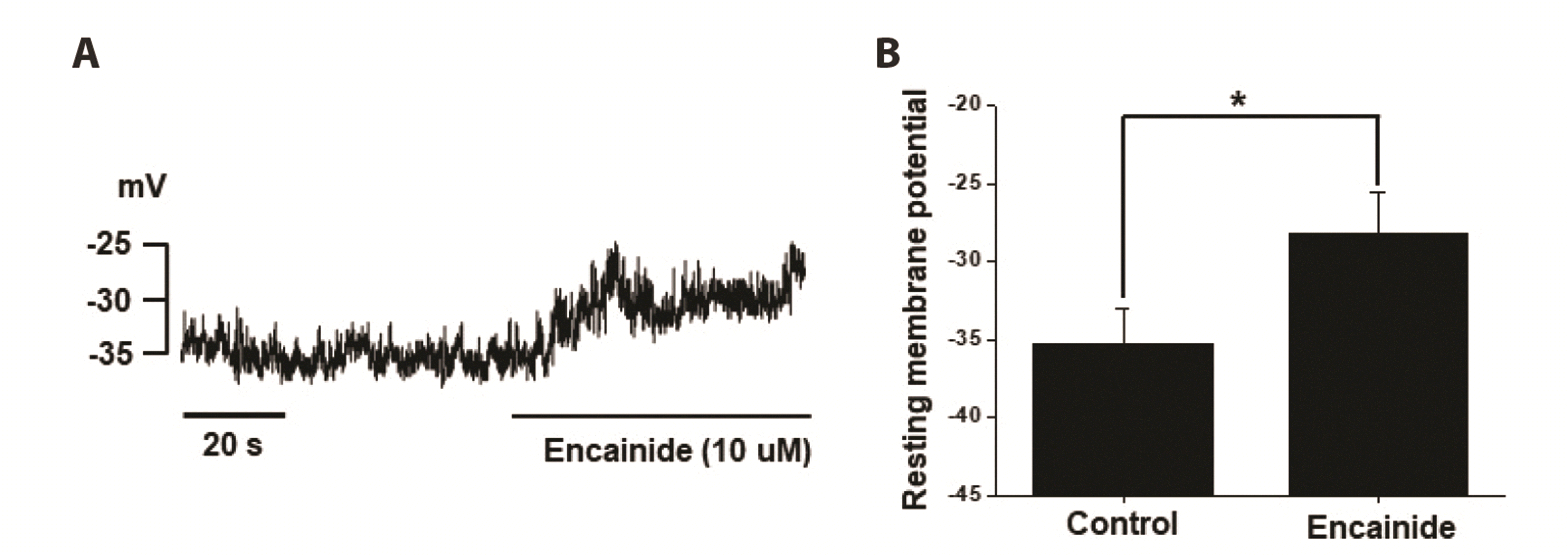
We investigated the inhibitory effect of encainide on Kv channels in smooth muscle cells from rabbit coronary arteries. Our results suggest that encainide inhibits Kv channels by shifting the steady-state activation curve in a dose-dependent and use (state)-independent manner. We found that encainide inhibits Kv channels on vascular smooth muscle regardless of its anti-arrhythmic effect. First, the response time of encainide on inhibition of Kv current is very short (within 1 min), suggesting direct inhibition of Kv channels by encainide. Second, encainide effectively shifted the steady-state activation curve toward a more positive potential (
Fig. 3A), suggesting that encainide inhibited Kv channels by direct interaction with voltage sensors, inducing changes in channel structure. Third, vascular smooth muscle cells, which are non-excitable cells, do not express Na
+ channels. Although the main target of the anti-arrhythmic effect of encainide is the Na
+ channel, the inhibitory effect of encainide on Kv channels is independent of Na
+ channels. Therefore, the inhibitory effect of encainide on Kv currents was not related to Na
+ channel inhibition and was attributed to direct interaction with Kv channels.
Arrhythmia, a common heart disease, is a crucial cause of sudden cardiac death [
1,
2]. In the modern era, insufficient sleep, smoking, and excessive alcohol consumption are closely related to arrhythmias [
1]. Surgical and pharmacological treatments are widely used for arrhythmia treatment. In the 1970s, anti-arrhythmic drugs were categorized into classes I–IV by Vaughan Williams [
6]. The main target of class I antiarrhythmic drugs is the Na
+ channel. These drugs were further classified into classes Ia–c according to their effect on action potential duration. Class Ic drugs do not alter action potential duration and have the strongest effect on the initiation phase 0 of depolarization [
4]. To date, several class Ic anti-arrhythmic drugs, such as flecainide, propafenone, moricizine, lorcainide, and encainide, have been approved for use. These drugs have common noncardiac side effects. Similar to other class Ic antiarrhythmic drugs, encainide has side effects such as nausea, dizziness, headache, and taste disorders [
8,
9]. However, the side effects of encainide due to its effects on other ion channels are unclear. In the present study, we investigated the effects of encainide on Kv channels in coronary arterial smooth muscle cells. We found that encainide inhibits coronary Kv channels and cardiac Na
+ channels. Therefore, the effects of encainide on cardiac and vascular smooth muscle cells should be considered when prescribing encainide as an anti-arrhythmic agent, particularly in patients with hypertension.
Vascular smooth muscle cells have abundant potassium channels, such as BK
Ca, K
ATP, Kir, and Kv channels. Of these, Kv channels, which are the most abundantly expressed K
+ channels in vascular smooth muscle, regulate proliferation, migration, and vascular tone by changing the membrane potential of vascular smooth muscle cells [
14,
15]. Additionally, changes in vascular Kv channels are closely associated with various cardiovascular diseases, such as diabetes, atherosclerosis, hypertension, and abnormal blood pressure [
10-
12]. Therefore, functional recovery of Kv channels is a crucial therapeutic target for cardiovascular diseases. The unexpected effects of certain drugs such as class Ic anti-arrhythmic drugs on vascular Kv channels should be comprehensively explored because of the pathophysiological importance of Kv channels. The class Ic anti-arrhythmic drug propafenone inhibits vascular Kv channels in a dose-dependent manner [
18]. However, unlike encainide, propafenone has no significant effect on the steady-state activation and inactivation curves. Furthermore, propafenone-induced inhibition of Kv currents occurs in a use-dependent manner. Another class Ic anti-arrhythmic drug, flecainide, inhibits vascular Kv channels. Similar to encainide, flecainide inhibits Kv channels in a dose-dependent but not use-dependent manner. Additionally, flecainide shifted the steady-state inactivation curve, but not the activation curve [
19]. Recently, the class Ic anti-arrhythmic drug lorcainide has been shown to have an inhibitory effect. Lorcainide inhibits arterial Kv channels in a concentration- and use-dependent manner by altering their voltage sensor for inactivation gating [
20]. The class Ic anti-arrhythmic drugs have different inhibitory effects on vascular smooth muscle cells. Although the exact reason is unknown, it may be due to structural differences among class Ic anti-arrhythmic drugs. Further studies are needed to confirm this.
To date, various subtypes of Kv channels have been identified in vascular smooth muscle cells, including Kv1.1, Kv1.2, Kv1.4, Kv1.5, Kv2.1, Kv7, and Kv9.3 subtypes [
14,
15]. However, the expression of vascular Kv subtypes differs among species. Most studies of Kv subtype expression have been conducted in rat, mouse, and human arteries [
21]. Therefore, it is unclear which Kv subtypes are expressed in rabbit arteries. However, the Kv subtypes commonly expressed in most species are the vascular Kv1.5 and Kv2.1 subtypes [
22], and specific inhibitors of these subtypes have been developed. Therefore, the roles of Kv1.5 and Kv2.1 subtypes in encainide-induced inhibition of Kv channels were investigated. Our results showed that the application of a Kv1.5 subtype or Kv2.1 subtype inhibitor effectively inhibited the Kv currents, suggesting that these subtypes are expressed in rabbit coronary arterials smooth muscle cells (
Fig. 5). Additionally, we identified a role of the Kv1.5 subtype in encainide-induced inhibition of Kv channels. Due to limitations of native cell experiments, involvement of other Kv subtypes could not be evaluated in the present study. Further studies are needed to determine the roles of specific subtypes in the effects of encainide using an expression system for specific Kv subtypes.
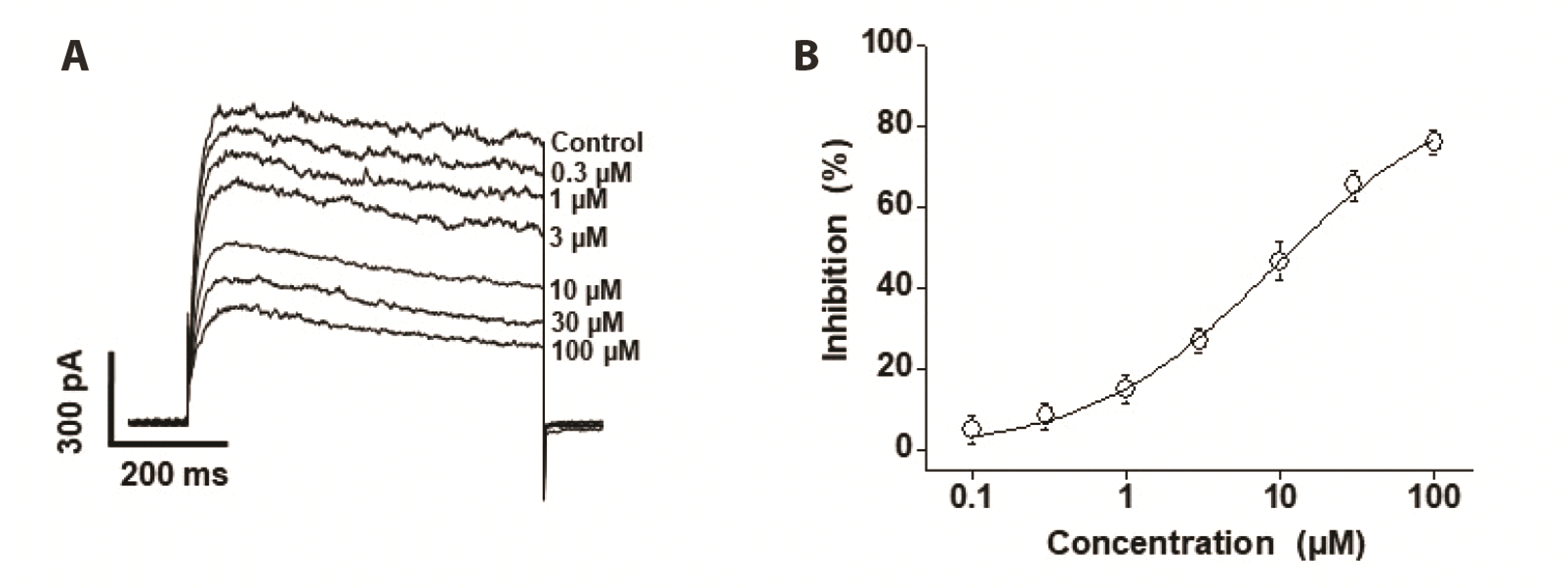
Encainide is administered at a dose of 50 mg/day. After oral administration, encainide is rapidly absorbed and reaches a peak plasma concentration of 511 nM [
23]. Our results show that encainide inhibited Kv channels with an IC
50 value of 8.9 ± 1.75 μM, which was higher than the peak plasma concentration. However, application of 300 nM encainide effectively inhibited the Kv current (
Fig. 2). Considering that arterial smooth muscle cells have high input resistance, small alterations in ionic conductance alter the vascular tone. Furthermore, inadvertent drug use may increase the blood concentration of encainide. Therefore, the dose of encainide should be carefully selected, particularly for patients with multiple cardiovascular diseases.
In summary, our study demonstrated the inhibitory effects of encainide on Kv channels using native rabbit coronary arterial smooth muscle cells. Encainide inhibited vascular Kv channels in a dose-dependent and use (state)-independent manner by altering the steady-state activation curve.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download