Abstract
Non-alcoholic fatty liver disease (NAFLD) is a complex disorder characterized by the accumulation of fat in the liver in the absence of excessive alcohol consumption. It is one of the most common liver diseases worldwide, affecting approximately 25% of the global population. It is closely associated with obesity, type 2 diabetes, and metabolic syndrome. Moreover, NAFLD can progress to non-alcoholic steatohepatitis, which can cause liver cirrhosis, liver failure, and hepatocellular carcinoma. Currently, there are no approved drugs for the treatment of NAFLD. Therefore, the development of effective drugs is essential for NAFLD treatment. In this article, we discuss the experimental models and novel therapeutic targets for NAFLD. Additionally, we propose new strategies for the development of drugs for NAFLD.
Non-alcoholic fatty liver disease (NAFLD) is a metabolic syndrome in chronic liver disease, affecting > 25% of the adult population worldwide [1]. Obesity is a major cause of NAFLD and other metabolic diseases [2]. Various factors contribute to NAFLD development, with obesity and type 2 diabetes mellitus (T2DM) being the most potent factors leading to NAFLD via dysregulation of glucose metabolism and insulin resistance (IR) [3]. Dysfunction in glucose uptake, IR, and hyperlipidemia can induce hepatocellular apoptosis by triggering inflammation or disrupting cellular homeostasis [4]. Although various drugs have been developed to target inflammation, oxidative stress, lipid accumulation, and IR, they exert adverse side effects in patients with NAFLD [5].
Liver disease progression involves hepatic steatosis, non-alcoholic steatohepatitis (NASH), cirrhosis, and hepatocellular carcinoma (HCC) [4]. NAFLD pathogenesis can be explained by the “multiple-hit theory,” which elucidates the progression from normal liver phenotype to HCC based on each stage [6]. Multiple-hit theory suggests that fat accumulation, IR, neoadipogenesis, and hyperglycemia due to excess weight, obesity, or excess calories leads to a change from a normal liver phenotype to NAFLD [6]. Oxidative stress, chronic inflammation, mitochondrial dysfunction, and fibrosis induce the development of NASH [4]. In addition, disruption of the gut microbiota, mitochondrial dysfunction, and endotoxins from lipid peroxidation induce inflammation, oxidative stress, and fibrosis, which are involved in the development of NASH, cirrhosis, and HCC [7].
NAFLD progression is determined by hepatic fat accumulation, inflammation, apoptosis, and liver fibrosis [8]. In a healthy liver, various hepatocytes, including hepatic stellate, Kupffer, and immune cells, regulate hepatocyte homeostasis by inactivating the inflammatory signaling pathways [9]. Hepatocytes are densely packed in a healthy liver [10]; however, normal hepatocyte morphology is altered with increased disruption of cellular homeostasis due to lipid accumulation [11]. In the early stages of NAFLD progression, tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated cell death pathways are promoted in steatotic hepatocytes or activated hepatic stellate cells due to natural killer (NK) cell activation [10]. In addition, activated NK cells secrete interferons, which increase M1 phase pro-inflammatory cytokine release from hepatic macrophages [11]. Activated inflammatory signaling pathway promotes the IR signaling pathway and fibrogenesis, consequently activating the transition from NAFL to NASH [12]. Moreover, the number of statocysts increases, and inflammation and fibrosis are activated to induce cell death [13]. Empty spaces are replaced by cellular fibrosis or abnormal hepatocyte-derived cancer cells, but not hepatocytes, thereby promoting HCC development (Fig. 1) [10].
Dietary model: NASH is mainly caused by the dysregulation of lipid synthesis and hyperglycemia due to obesity [14]. Depending on the experimental purpose, researchers have established various pathogenic animal models via dietary modification, transgenesis, or by injecting chemicals into the animals [14]. A high-fat diet (HFD) is used for establishing NAFLD animal models by increasing the cholesterol and fat content of food [15]. By using an HFD, most pathogenic symptoms, including obesity, T2DM, or liver disease, in NAFLD models were similar to those observed in humans [16]. NAFLD mouse models are mostly established by administering diets rich in fat, cholesterol, and fructose [17]. Depending on the ingredients, HFD can be used to establish animal NAFLD and HCC models in as little as eight weeks [18]. Furthermore, HFD-mediated hepatocyte injury in NAFLD or other metabolic diseases induces typical phenotypic characteristics observed in humans, including weight gain, IR, and increased serum levels of aspartate transaminase and alanine transaminase [19]. Various studies have attempted to establish HCC models using HFD with fructose-mixed water [9]. Animal models of HCC can also be established after 24 weeks using water containing 2.31% fructose, which is a trigger of hyperglycemia [20,21].
Methionine and choline-deficient diet (MCD) is used to establish NAFLD models [22]. In principle, methionine is an amino acid that induces protein and lipid metabolism via DNA and RNA methylation [23]. It functions as a regulator of cellular processes and phospholipid synthesis in hepatocytes and protects against NAFLD progression [23]. Methionine deficiency leads to liver injury, inflammation, and liver fibrosis [24]. In addition, choline deficiency activates macrovascular steatosis in NAFLD models [25]. MCD has been reported to induce hepatic steatosis in a 4–8 weeks dietary model of NAFLD [23]. T helper-17 and -22 cells activate pro-inflammatory cytokine release in the NAFLD model, inducing a cascade of inflammatory signaling pathways, such as the toll-like receptor (TLR)-4-mediated Myd88 or nuclear factor of kappa photopolypeptide enhancer (NF-κB) pathways [26], and activating B cells in an MCD animal model [26]. In addition, MCD inhibits the protein kinase B/mechanistic target of rapamycin kinase (AKT/mTOR)-dependent autophagic cell death and macrophage activation in hepatocytes [27]. MCD model exerts similar effects as the HFD model, such as the induction of hepatic steatosis and liver fibrosis, but it cannot mimic weight gain-mediated T2D and its mediated NAFLD-related signaling pathways [25].
Chemically-induced NAFLD models: Streptozotocin (STZ) induces diabetes by destroying the pancreatic β cells via glucose transporter (GLUT)-2-mediated DNA fragmentation [28]. After inducing cell death with STZ, β-cells do not secrete insulin, resulting in T2DM [28]. STAM model is an NAFLD model in which STZ is injected into 2-day-old mice [28]. Previously, various studies administered STZ to 2-day-old mice without insulin secretion to induce pancreatic β-cell death and dysregulation of insulin-mediated glucose uptake [14]. Four weeks later, STZ-injected mice fed an HFD for up to 8 weeks developed hepatic steatosis [28]. Furthermore, administering HFD over 16–24 weeks induces hepatic steatosis in an HCC model [29]. Consistent progression can be observed in a human HFD-fed NAFLD-HCC model induced by diabetes, which is the main advantage of the STAM model (Table 1) [30].
Inflammation and its mediated signaling pathways are important for NAFLD to NASH progression [11]. Through dysregulation of oxidative stress, endoplasmic reticulum (ER) stress, or lipid metabolism, inflammatory signaling pathways increase the secretion of inflammatory cytokines, including tumor necrosis factor-α (TNF-α), interleukin (IL)-1β, IL-6, and IL-10 [31]. Accumulation of inflammatory cytokines also induces liver cell apoptosis and fibrosis by activating the transforming growth factor-β signaling pathway [32].
NF-κB activation is a key factor in inflammatory cytokine release [33]. Promoter activation of NF-κB induced by inflammatory cytokines or other mechanisms induces cell apoptosis, inflammation, and fibrosis [34]. HFD or MCD induce NF-κB phosphorylation and TNF-α and IL-1β secretion [35,36]. AMP-activated protein kinase (AMPK) is a sensing marker of energy metabolism that is inactivated by HFD-induced NF-κB activation via peroxisome proliferator-activated receptor (PPAR)−γ promoter activation [37]. In addition, palmitic acid (PA), a free fatty acid (FFA), activates NF-κB phosphorylation-mediated TNF-α, IL-1β, and IL-6 production by suppressing AMPKα expression in hepatocytes [38]. TLR4 is a major receptor inducing Myd88-mediated NF-κB p65 phosphorylation and increasing mRNA expression of TNF-α and IL-1β, thereby causing liver damage [39]. HFD induces TLR4 expression and activates the TLR4/NF-κB signaling pathway to induce liver inflammation [40]. In addition, MCD model induces liver inflammation via TLR4 or 9-mediated Myd88/NF-κB activation, which further induces TNF-α, IL-6, and cleaved caspase-1-mediated IL-1β maturation [35]. NF-κB activation caused by HFD activates mitochondrial dysfunction-mediated reactive oxygen species (ROS) production via dysregulation of the mitochondrial respiratory chain reaction [41]. Mitogen-activated protein kinase (MAPK) and signal transducer and activator of transcription (STAT) signaling pathways act as inflammatory signaling pathways upon inflammatory cytokine stimulation [42]. MAPK consists of extracellular signal-regulated kinase-1/2, p38, and c-Jun N-terminal kinase that activates liver inflammation via production of TNF-α, IL-6, and IL-1β [43]. In addition, high levels of ROS increase damage-associated molecular pattern-mediated NLR family pyrin domain-containing 3 (NLRP3) activation, and pro-inflammatory cytokines released by inflammasome expression significantly activate stellate cell fibrosis [44]. Fibrosis in stellate cells increases STAT3-mediated HCC progression [44]. In addition, damage or pathogen-associated molecular pattern-mediated NLRP3 inflammasome increases cleaved caspase-1 activation and IL-1β maturation in hepatocytes [45]. Mature IL-1β is involved in NLRP3 inflammasome internalization into hepatic stellate cells, and its inflammatory signaling pathway increases collagen production-mediated liver fibrosis (Fig. 2A) [45].
MicroRNAs (miRNAs) consist of 20–25 nucleotides and control target gene expression by suppressing mRNA expression [46]. Dysregulated miRNA expression can control NAFLD inflammation by activating the NF-κB, MAPK, and TLR4 signaling pathways, or dysregulation of cellular homeostasis [47]. Inhibition of miR-125b suppresses FFA-treated NF-κB p65 phosphorylation via direct binding with TNF-α-induced protein 3, which is a negative regulator of NAFLD progression in HepG2 cells and animal models [48]. Increased miR-122-5p expression and secretion of inflammatory cytokines, including TNF-α, IL-6, and IL-8, are observed in HFD- and PA-treated HepG2 cells [49]. Mechanistically, HFD or PA-induced miR-122-5p directly inhibits forkhead box-O3 expression and activates the PI3K/AKT/mTOR signaling pathway [49]. In addition, STAT3 phosphorylation caused by the IL-6 signaling pathway increases miR-223-enriched exosome secretion in patients with NAFLD/NASH [50]. Chronic ER stress activation is also involved in NAFLD pathogenesis [51]. Briefly, in HFD-treated cell chronic ER stress signaling pathway, miR-26a overexpression blocks the phosphorylation of eukaryotic translation initiation factor 2 alpha in a negative feedback loop [51]. miR-34a exerts protective effects on HFD-mediated TNF-α release by suppressing TLR4-induced NF-κB activation [52]. Additionally, miR-34a decreases lipid absorption, lipogenesis, and autophagy by activating the sirtuin 1 (SIRT1)-mediated sterol regulatory element-binding transcription factor 1c (SREBP1c) signaling pathway (Fig. 2B) [52,53].
Numerous studies have reported that dysbiosis of the gut microbiota caused by HFD or alcohol affects NAFLD pathogenesis [54]. Dysregulation of gut microbiota caused by bacteria, protists, archaea, fungi, and viruses induces the secretion of inflammatory factors, gut hyperpermeability, ethanol, and LPS stimulation [54]. Accordingly, various secreted factors move into the liver through the portal veins and aggravate the inflammatory signaling pathway, progressing NAFLD fibrosis to HCC by activating oxidative stress, ER stress, lipid accumulation, and hepatocyte cell death [55]. In particular, during the progression of NAFLD or HCC, peripheral blood mononuclear cells secrete inflammatory cytokines, such as IL-6 or IL-10, and their inflammatory signaling pathway increases the number of cytotoxic clusters of differentiation (CD)8+ T cells [56]. Moreover, gut barrier permeability during NASH progression, LPS levels were increased by MCD, and LPS induced liver cell inflammation, oxidative stress, and IR by suppressing glucagon-like peptide 1 (GLP1) and 2 expression [57].
PPAR is a group of fatty acid sensors, and previous studies have indicated that PPARs activation suppresses the disruption of gut microbiota-mediated NAFLD progression [58]. PPARs consist of α, β, δ, and γ, and their components show protective effects on NAFLD inflammation through various signaling pathways [58]. First, PPARα activation can prevent HFD-induced NAFLD steatosis in models by activating the AMPK activation-mediated PPARγ coactivator-1α (PGC-1α) signaling pathway [59]. In addition, treatment with the Shugan Xiaozhi decoction, a component of artemisiae scopariae herb, significantly suppressed the gut-liver axis in NAFLD models by activating PPAR-mediated lipid metabolism [60]. Previous studies have indicated that PPARβ/δ shows high metabolic activity, lipid metabolism, glucose homeostasis, and inflammation in hepatocytes, Kupffer cells, and hepatic stellate cells through AMPK activation-mediated suppression of the inflammatory signaling pathway [58]. In addition, short-chain fatty acid-mediated PPARα and γ expression lead to IL-10 secretion and inducible nitric oxide synthase, which stabilizes gut homeostasis and inhibits colonization of pathogenic bacteria [61] (Fig. 2C).
Apoptosis is a marker of NASH to HCC progression [62]. In patients with NASH, it has been found that terminal deoxynucleotidyl transferase dUTP nick end labeling-positive hepatocytes and cleaved caspase-3 expression are higher than in healthy liver specimen [62]. Additionally, overstimulated inflammatory signaling pathway treated with high glucose and HFD-induced TNF-α secretion induced liver cell apoptosis by activating cleaved caspase-3 and -8 [63]. Another apoptosis marker, cleaved caspase-6 activation, suppressed by AMPK activation, inhibited liver cell apoptosis and steatosis [64]. B-cell lymphoma-associated X (Bax) is one of the markers of the mitochondria-mediated apoptotic signaling pathway; when cell apoptosis occurs, B-cell lymphoma expressed on mitochondria membranes is decreased and Bax expression is increased simultaneously [65]. Hyperglycemia and hyperlipidemia-induced by FFA increased dysregulation of glucose and lipid metabolism in the liver and increased apoptosis through stimulator of interferon gene (STING)/interferon regulatory factor 3 (IRF3)-mediated Bax, cleaved-caspase 3, and cleaved PARP activation [66]. In addition, mitochondrial ROS (mtROS) production, which is one of the reasons for mitochondrial dysfunction [67], can increase the protein expression of STING/IRF3 and its mediated apoptosis signaling pathway [41]. In addition, a complex of death-associated protein kinase-related apoptosis-inducing kinase-2 with serine/arginine-rich splicing factor 6 activation in NAFLD models induces mitochondrial dysfunction by inducing RNA splicing-mediated liver apoptosis [68]. Moreover, mitochondrial dysfunction results in excess production of ROS and calcium, which can cause ER stress-mediated cell apoptosis in patients with NAFLD [68]. In particular, Bax expression is regulated by c/EBP homologous protein (CHOP), a marker of ER stress-mediated apoptosis [67]. NAFLD model estimated by HFD-fed ApoE-/- mice for five weeks show significantly increased inflammatory and ER stress pathways, including protein kinase RNA-like ER kinase/activating transcription factor (ATF)-4, inositol-requiring transmembrane kinase/endoribonuclease 1 (IRE)1/x-box binding 1 (XBP-1), and ATF6-mediated CHOP pathways [69]. CHOP expression results in excessive mTOR-dependent autophagy, inflammation, and apoptosis in hepatocytes via activation of the ER stress signaling pathway in HFD-fed ApoE-deficient mice (Fig. 3) [69].
Lipid metabolism plays an important role in NAFLD progression [70]. There are various reasons for the dysfunction of lipid metabolism, such as (1) overproduction of lipid moved to the liver caused by excessive calorie intake, (2) overproduced FFAs in adipose tissue, (3) activated de novo lipogenesis, (4) suppression of triglyceride (TG) content by very low-density lipoprotein, and (5) inactivation of lipid oxidation in the liver [69]. Accumulation of FFAs in the blood can cause IR and is divided into two signaling pathways depending on the phosphorylation site of insulin receptor substrate (IRS)1 [71]. Normally, phosphorylation at tyrosine 895 on IRS activation leads to PI3K/Akt-mediated GLUT4 uptake in skeletal muscle cells [72]. Moreover, high levels of insulin in the blood increase FFA synthesis by activating acetyl-coenzyme A [72]. Accumulation of FFAs increases NAFLD steatosis and inflammation, thereby inducing HCC progression [72].
Patients with NAFLD exhibit over 2 times higher expression levels of de novo lipogenesis markers than the health controls [73]. Various nuclear receptors or cytoplasmic transcription factors are involved in NAFLD progression by accumulating PPAR, a member of nuclear receptor or SREBP1 and carbohydrate response element (ChREBP), and cytoplasmic transcription factor-mediated de novo lipogenesis [73]. SREBP1 expression is increased by insulin, oxysterol binding protein, or liver X receptor and induced by the IR-mediated Akt signaling pathway [66]. Activation of SREBP1 in de novo lipogenesis stimulates FFA synthesis by stimulating the expression of ACC and FAS [74]. Previous studies have reported that STING/IRF3 activation induces SREBP1-mediated liver cell steatosis and inflammation in HFD-fed NAFLD mouse models [66]. In addition, short-chain fatty acids attenuated MCD-induced SREBP1 and AMPK deactivation-mediated disruption of the antioxidant signaling pathway in hepatocytes [26]. MCD-induced SREBP1 expression increases NF-κB p65 activation and its mediated inflammatory signaling pathway is involved in NASH progression [75]. Suppression of SREBP1 caused by ginsenoside Rg1 treatment significantly increases AMPK expression and suppresses FAS protein expression [38]. Moreover, HFD-mediated SIRT1/AMPK inactivation increases SREBP1-mediated lipogenesis, PGC-1α-induced mitochondrial dysfunction, oxidative stress, and NF-κB-mediated inflammation [36]. A previous report revealed that HFD with high fructose induced TLR4-mediated TNF-α stimulation [76]. At the same time, the de novo lipogenesis pathway was turned on, which increased the protein expression of SREBP1 and FAS [76]. Along with SREBP1, ChREBP-1 also induces de novo lipogenesis and functions as a cytoplasmic transcription factor by inducing pyruvate kinase expression and TG synthesis [73]. High-fructose diet increases ChREBP-1-associated hepatic steatosis by activating de novo lipogenesis and LPS production [77]. Silencing of NF-κB p65 expression using siRNA transfection suppresses IR-mediated ChREBP-1 nuclear translocation, indicating that excessive liver inflammation affects liver steatosis [78]. HFD-mediated increase in ChREBP-1 and SREBP1 expression is suppressed by small heterodimer partner interacting leucine zipper protein, nuclear receptor activation, which is increased by lyophilized maqui berry extract treatment [79].
Lipid oxidation is essential for regulating mitochondrial biogenesis and cellular homeostasis in liver cells [80]. In a healthy liver, mitochondria use FFAs as an energy source [80]. Mechanistically, when FFAs enter the mitochondria, they are converted by acetyl CoA and then conjugated with carnitine, which is located on the outer membrane of the mitochondria [80]. Subsequently, the acetyl CoA-carnitine form was disassociated by carnitine palmitoyl transferase (CPT)-2, and acetyl CoA was degraded by β-oxidation [80]. However, increased influx of FFAs induced mutation of mitochondrial DNA, mitochondrial dysfunction, and increased ROS-mediated inflammatory signaling pathway in hepatocytes [81]. AMPK is a protein kinase that switches lipid synthesis by activating sucrose non-fermenting-1 expression [81]. Based on the function of AMPK in lipid oxidation, PPARα activation is related to β-oxidation by inducing FFA in the mitochondria and CPT-1 activation [82]. Inactivation of AMPK phosphorylation by compound C, an inhibitor of AMPK activation, increases liver cell steatosis by increasing FAS mRNA expression, and HFD-mediated AMPK inactivation causes SIRT1 activation [81]. AMPK-mediated SIRT1 expression suppresses HFD-mediated lipid oxidation by suppressing PGC1-α, SREBP1, SCD-1, and FAS and increasing PPARα expression levels [36]. AMPK phosphorylation increases PPARα-associated fatty acid oxidation and suppresses NF-κB-mediated inflammatory cytokine release [37]. Additionally, the SIRT1 and PGC1α axis activated by leptin or adiponectin-mediated AMPK activation stabilized mitochondrial function and increased fatty acid oxidation in hepatocytes [83]. Moreover, methylation of the controlled J protein, a protein on mitochondria respiratory chain complex 1, is highly expressed in patients with NAFLD, and its overexpression leads to lipid accumulation, suppression β of-oxidation, and hepatocyte damage [84].
Hepatic gluconeogenesis refers to the production of glucose by the GLUT2 signaling pathway under fasting conditions, primarily in response to insulin and glucagon levels [85]. Activation of pyruvate to phosphoenolpyruvate is the first step for phosphoenolpyruvate carboxykinase (PEPCK) and glucose-6-phosphate (G6Pase)-mediated gluconeogenesis [85]. Excessive release of glucose via gluconeogenesis enhances IR-mediated inflammation, steatosis, and de novo lipogenesis in patients with NAFLD [86]. PGC-1α-activated PEPCK expression induced by HFD-mediated inflammation is suppressed by bicyclol, an approved drug for targeting hepatitis, liver fibrosis, and liver injury [86]. Inactivation of the AMPK-mediated SIRT–PGC1 axis is involved in HFD-fed NASH progression [83]. PEPCK and G6Pase activation are modulated by PGC-1α activation and produce glucose in T2DM models [87]. Upstream of PEPCK-mediated gluconeogenesis, glucagon or FFA activates the formation of CREB-regulated transcription coactivator (CRTC)-2 with CEBP, and its complex increases PEPCK gene expression as a transcription factor [86]. HFD-mediated salt-induced kinase 1 suppression leads to CRTC2-mediated PEPCK and G6Pase gene expression and increases hyperglycemia and liver steatosis [88]. ER stress-mediated inhibition of AMPK phosphorylation increases the phosphorylation of CREB and PEPCK proteins [89].
NASH is a liver disease characterized by the buildup of fat in the liver, which causes inflammation and damage to liver cells, leading to fibrosis (scarring) and cirrhosis [90]. Several drugs are being developed for the treatment of NASH by exerting anti-fibrotic effects on the liver [91]. Liver fibrosis occurs when there is chronic damage to the liver, such as inflammation and oxidative stress, which leads to the accumulation of scar tissue. This scar tissue can impair liver function and, if left unchecked, progress to cirrhosis, liver failure, and HCC [90]. Therefore, anti-fibrotic drugs can help slow or even reverse liver fibrosis by inhibiting the production of collagen fibers, promoting the breakdown of scar tissue, and reducing inflammation in the liver [91]. These drugs can potentially reduce the risk of liver-related complications and improve the overall prognosis of patients with NASH. Several different classes of drugs have been studied for their anti-fibrotic effects in NASH, including peroxisome PPAR agonists, farnesoid X receptor (FXR) agonists, GLP-1 receptor agonists, and C–C chemokine receptor (CCR) 2/5 inhibitors [92,93]. PPAR agonists, including pioglitazone and elafibranor, regulate lipid metabolism and reduce inflammation in the liver [92]. Recent study suggested that anti-diabetic drugs with a potential anti-inflammatory effect can ameliorate the manifestations of NAFLD, and thus it can be a therapeutic option for such a condition came from metabolic syndrome [92]. In addition to their anti-fibrotic effects, many NASH drugs have other beneficial effects on the liver, such as reducing hepatic steatosis (fat accumulation), improving insulin sensitivity, and reducing inflammation [94]. For example, some drugs that target FXR can also improve lipid metabolism and reduce hepatic inflammation, in addition to their anti-fibrotic effects [94]. Furthermore, in clinical trials, CCR2/5 inhibitors have shown promise in improving liver fibrosis and inflammation in patients with NASH [95]. In another study, patients with NASH receiving maraviroc, another CCR2/5 inhibitor, exhibited a significant reduction in liver inflammation compared to those receiving placebo [96]. Therefore, anti-fibrotic drugs are important components of the multidisciplinary approach to treat NASH. Further research is needed to identify new and effective therapies for treating NAFLD/NASH.
Accumulating evidence indicates that GLP-1 and glucose-dependent insulinotropic polypeptide (GIP) agonists can improve NAFLD. These drugs have been shown to reduce liver fat content, improve liver enzymes, and decrease markers of inflammation in patients with NAFLD [97-99]. Various GLP-1 agonists, such as liraglutide, semaglutide, and exenatide, have significantly reduced the liver fat content and improved the liver enzymes in clinical trials [100]. GIP agonists have also shown promising results in reducing liver fat content and improving insulin sensitivity [100]. In addition to their effects on liver fat, GLP-1 and GIP agonists have been shown to improve cardiovascular risk factors, such as blood pressure, lipid levels, and markers of inflammation [98,101]. These drugs may also have a protective effect on the pancreas, reducing the risk of pancreatic fat accumulation and pancreatitis [98,101]. Tirzepatide is a novel dual GIP and GLP-1 receptor agonist [98]. It is currently being evaluated in clinical trials as a potential therapeutic for type 2 diabetes and obesity [98]. There is limited data available on the effects of tirzepatide on NAFLD, as most clinical trials have focused on its effects on glycemic control and weight loss [98,102]. However, some studies have investigated the potential benefits of GLP-1 and GIP agonists, including tirzepatide, in NAFLD [97,98]. One randomized control trial evaluated the effects of semaglutide, a GLP-1 agonist, in patients with NASH, a more severe form of NAFLD [103]. The trial found that semaglutide significantly improves liver histology, with 59% of patients showing improvement in fibrosis and 43% showing resolution of NASH [103]. Overall, GLP-1 and GIP agonists appear to be promising therapeutic agents for NAFLD treatment. However, further studies are needed to determine the optimal dosing and duration of treatment as well as the long-term safety and efficacy of these drugs in patients with NAFLD.
Despite several efforts to develop treatments for NAFLD, the unmet need for effective drug therapies remains significant. The complexity of the disease, lack of understanding of its pathogenesis, and limited availability of relevant preclinical models increase the difficulty of drug development for NAFLD. One potential strategy is to focus on the identification of NAFLD-specific novel therapeutic targets via a comprehensive understanding of the underlying molecular mechanisms and identification of key pathways dysregulated in NAFLD. Another approach is to develop preclinical models that accurately recapitulate the features of human NAFLD. Development of such models will enable more accurate evaluation of the efficacy and safety of potential NAFLD therapeutics. For example, humanized mouse models, in which human liver cells are engrafted into immunodeficient mice, or 3D liver organoids, for study of liver function and disease in vitro, can be used for future studies.
In this review, we have outlined the inflammation, apoptosis, lipid metabolism, and gluconeogenesis mechanisms involved in NAFLD progression. Various pathways, such as PPAR, NF-κB, MAPK, STAT, and AMPK signaling pathways, are associated with and accelerate the development of liver steatosis and fibrosis. Several studies suggest targeting the PPAR signaling pathway to treat NAFLD progression. PPARs act as transcriptional targets and are highly associated with inflammation, liver steatosis, IR, and liver fibrosis. Diverse natural products exert pharmacological effects in suppressing liver fibrosis, ROS production, and lipid metabolism. However, the detailed molecular mechanisms and functions of organelles involved in NAFLD progression remain unknown and require further investigation. Additionally, natural products should be modified with effective drugs by targeting specific molecules to prevent NAFLD progression. In summary, determination of the underlying mechanisms and modification of natural compounds for drug development are potential strategies for the treatment of NAFLD and other metabolic diseases, such as T2DM and cardiovascular disease.
REFERENCES
1. Gray ME, Bae S, Ramachandran R, Baldwin N, VanWagner LB, Jacobs DR Jr, Terry JG, Shikany JM. 2022; Dietary patterns and prevalent NAFLD at year 25 from the coronary artery risk development in young adults (CARDIA) study. Nutrients. 14:854. DOI: 10.3390/nu14040854. PMID: 35215504. PMCID: PMC8878386. PMID: 4c852c79ea4c437aac7c4088fabc4aa6.

2. Polyzos SA, Kountouras J, Mantzoros CS. 2019; Obesity and nonalcoholic fatty liver disease: from pathophysiology to therapeutics. Metabolism. 92:82–97. DOI: 10.1016/j.metabol.2018.11.014. PMID: 30502373.

3. Ter Horst KW, Serlie MJ. 2017; Fructose consumption, lipogenesis, and non-alcoholic fatty liver disease. Nutrients. 9:981. DOI: 10.3390/nu9090981. PMID: 28878197. PMCID: PMC5622741. PMID: 68026d13ef864dd8a97f831d2ab271a0.

4. Kumar S, Duan Q, Wu R, Harris EN, Su Q. 2021; Pathophysiological communication between hepatocytes and non-parenchymal cells in liver injury from NAFLD to liver fibrosis. Adv Drug Deliv Rev. 176:113869. DOI: 10.1016/j.addr.2021.113869. PMID: 34280515.

5. Tanase DM, Gosav EM, Costea CF, Ciocoiu M, Lacatusu CM, Maranduca MA, Ouatu A, Floria M. 2020; The intricate relationship between type 2 diabetes mellitus (T2DM), insulin resistance (IR), and nonalcoholic fatty liver disease (NAFLD). J Diabetes Res. 2020:3920196. DOI: 10.1155/2020/3920196. PMID: 32832560. PMCID: PMC7424491. PMID: 5534b2bacae74a2dba72bf5cc5ba90ef.

6. Ioannou GN. 2021; Epidemiology and risk-stratification of NAFLD-associated HCC. J Hepatol. 75:1476–1484. DOI: 10.1016/j.jhep.2021.08.012. PMID: 34453963.

7. Shabalala SC, Dludla PV, Mabasa L, Kappo AP, Basson AK, Pheiffer C, Johnson R. 2020; The effect of adiponectin in the pathogenesis of non-alcoholic fatty liver disease (NAFLD) and the potential role of polyphenols in the modulation of adiponectin signaling. Biomed Pharmacother. 131:110785. DOI: 10.1016/j.biopha.2020.110785. PMID: 33152943.

8. Abdel-Bakky MS, Helal GK, El-Sayed EM, Amin E, Alqasoumi A, Alhowail A, Abdelmoti ESS, Saad AS. 2021; Loss of RAR-α and RXR-α and enhanced caspase-3-dependent apoptosis in N-acetyl-p-aminophenol-induced liver injury in mice is tissue factor dependent. Korean J Physiol Pharmacol. 25:385–393. DOI: 10.4196/kjpp.2021.25.5.385. PMID: 34448456. PMCID: PMC8405435.

9. Gong G, Zhao R, Zhu Y, Yu J, Wei B, Xu Y, Cui Z, Liang G. 2021; Gastroprotective effect of cirsilineol against hydrochloric acid/ethanol-induced gastric ulcer in rats. Korean J Physiol Pharmacol. 25:403–411. DOI: 10.4196/kjpp.2021.25.5.403. PMID: 34448458. PMCID: PMC8405436.

10. Martínez-Chantar ML, Delgado TC, Beraza N. 2021; Revisiting the role of natural killer cells in non-alcoholic fatty liver disease. Front Immunol. 12:640869. DOI: 10.3389/fimmu.2021.640869. PMID: 33679803. PMCID: PMC7930075. PMID: f20ecdd332db4413bf44156c12a70c46.

11. Hundertmark J, Krenkel O, Tacke F. 2018; Adapted immune responses of myeloid-derived cells in fatty liver disease. Front Immunol. 9:2418. DOI: 10.3389/fimmu.2018.02418. PMID: 30405618. PMCID: PMC6200865. PMID: a7dbc75d5dd54e3b85c2fdbf219d55a7.

12. Puengel T, Liu H, Guillot A, Heymann F, Tacke F, Peiseler M. 2022; Nuclear receptors linking metabolism, inflammation, and fibrosis in nonalcoholic fatty liver disease. Int J Mol Sci. 23:2668. DOI: 10.3390/ijms23052668. PMID: 35269812. PMCID: PMC8910763. PMID: 3bd012ba53de463bb8d3570ead664810.

13. Peng C, Stewart AG, Woodman OL, Ritchie RH, Qin CX. 2020; Non-alcoholic steatohepatitis: a review of its mechanism, models and medical treatments. Front Pharmacol. 11:603926. DOI: 10.3389/fphar.2020.603926. PMID: 33343375. PMCID: PMC7745178. PMID: 2dc08ed0b09742598ac9467466fe05ab.

14. Márquez-Quiroga LV, Arellanes-Robledo J, Vásquez-Garzón VR, Villa-Treviño S, Muriel P. 2022; Models of nonalcoholic steatohepatitis potentiated by chemical inducers leading to hepatocellular carcinoma. Biochem Pharmacol. 195:114845. DOI: 10.1016/j.bcp.2021.114845. PMID: 34801522.

15. Feng M, Liu F, Xing J, Zhong Y, Zhou X. 2021; Anemarrhena saponins attenuate insulin resistance in rats with high-fat diet-induced obesity via the IRS-1/PI3K/AKT pathway. J Ethnopharmacol. 277:114251. DOI: 10.1016/j.jep.2021.114251. PMID: 34052350.

16. Marengo A, Rosso C, Bugianesi E. 2016; Liver cancer: connections with obesity, fatty liver, and cirrhosis. Annu Rev Med. 67:103–117. DOI: 10.1146/annurev-med-090514-013832. PMID: 26473416.

17. Chyau CC, Wang HF, Zhang WJ, Chen CC, Huang SH, Chang CC, Peng RY. 2020; Antrodan alleviates high-fat and high-fructose diet-induced fatty liver disease in C57BL/6 mice model via AMPK/Sirt1/SREBP-1c/PPARγ pathway. Int J Mol Sci. 21:360. DOI: 10.3390/ijms21010360. PMID: 31935815. PMCID: PMC6981486. PMID: 4f4399ffc2aa42f9af8d7a805e316974.

18. Zheng T, Yang X, Li W, Wang Q, Chen L, Wu D, Bian F, Xing S, Jin S. 2018; Salidroside attenuates high-fat diet-induced nonalcoholic fatty liver disease via AMPK-dependent TXNIP/NLRP3 pathway. Oxid Med Cell Longev. 2018:8597897. DOI: 10.1155/2018/8597897. PMID: 30140371. PMCID: PMC6081551.

19. Softic S, Stanhope KL, Boucher J, Divanovic S, Lanaspa MA, Johnson RJ, Kahn CR. 2020; Fructose and hepatic insulin resistance. Crit Rev Clin Lab Sci. 57:308–322. DOI: 10.1080/10408363.2019.1711360. PMID: 31935149. PMCID: PMC7774304.

20. Tsuchida T, Lee YA, Fujiwara N, Ybanez M, Allen B, Martins S, Fiel MI, Goossens N, Chou HI, Hoshida Y, Friedman SL. 2018; A simple diet- and chemical-induced murine NASH model with rapid progression of steatohepatitis, fibrosis and liver cancer. J Hepatol. 69:385–395. Erratum in: J Hepatol. 2018;69:988. DOI: 10.1016/j.jhep.2018.07.010. PMID: 30082074.

21. Im YR, Hunter H, de Gracia Hahn D, Duret A, Cheah Q, Dong J, Fairey M, Hjalmarsson C, Li A, Lim HK, McKeown L, Mitrofan CG, Rao R, Utukuri M, Rowe IA, Mann JP. 2021; A systematic review of animal models of NAFLD finds high-fat, high-fructose diets most closely resemble human NAFLD. Hepatology. 74:1884–1901. DOI: 10.1002/hep.31897. PMID: 33973269.

22. Segal-Salto M, Barashi N, Katav A, Edelshtein V, Aharon A, Hashmueli S, George J, Maor Y, Pinzani M, Haberman D, Hall A, Friedman S, Mor A. 2020; A blocking monoclonal antibody to CCL24 alleviates liver fibrosis and inflammation in experimental models of liver damage. JHEP Rep. 2:100064. DOI: 10.1016/j.jhepr.2019.100064. PMID: 32039405. PMCID: PMC7005554.

23. Parlati L, Régnier M, Guillou H, Postic C. 2021; New targets for NAFLD. JHEP Rep. 3:100346. DOI: 10.1016/j.jhepr.2021.100346. PMID: 34667947. PMCID: PMC8507191.

24. Li X, Wang TX, Huang X, Li Y, Sun T, Zang S, Guan KL, Xiong Y, Liu J, Yuan HX. 2020; Targeting ferroptosis alleviates methionine-choline deficient (MCD)-diet induced NASH by suppressing liver lipotoxicity. Liver Int. 40:1378–1394. DOI: 10.1111/liv.14428. PMID: 32145145.

25. Stephenson K, Kennedy L, Hargrove L, Demieville J, Thomson J, Alpini G, Francis H. 2018; Updates on dietary models of nonalcoholic fatty liver disease: current studies and insights. Gene Expr. 18:5–17. DOI: 10.3727/105221617X15093707969658. PMID: 29096730. PMCID: PMC5860971.

26. Deng M, Qu F, Chen L, Liu C, Zhang M, Ren F, Guo H, Zhang H, Ge S, Wu C, Zhao L. 2020; SCFAs alleviated steatosis and inflammation in mice with NASH induced by MCD. J Endocrinol. 245:425–437. DOI: 10.1530/JOE-20-0018. PMID: 32302970.

27. Veskovic M, Mladenovic D, Milenkovic M, Tosic J, Borozan S, Gopcevic K, Labudovic-Borovic M, Dragutinovic V, Vucevic D, Jorgacevic B, Isakovic A, Trajkovic V, Radosavljevic T. 2019; Betaine modulates oxidative stress, inflammation, apoptosis, autophagy, and Akt/mTOR signaling in methionine-choline deficiency-induced fatty liver disease. Eur J Pharmacol. 848:39–48. DOI: 10.1016/j.ejphar.2019.01.043. PMID: 30689995.

28. Zhao W, He C, Jiang J, Zhao Z, Yuan H, Wang F, Shen B. 2022; The role of discoid domain receptor 1 on renal tubular epithelial pyroptosis in diabetic nephropathy. Korean J Physiol Pharmacol. 26:427–438. DOI: 10.4196/kjpp.2022.26.6.427. PMID: 36302618. PMCID: PMC9614395.

29. Morishita A, Tadokoro T, Fujihara S, Iwama H, Oura K, Fujita K, Tani J, Takuma K, Nakahara M, Shi T, Haba R, Okano K, Nishiyama A, Ono M, Himoto T, Masaki T. 2022; Ipragliflozin attenuates non-alcoholic steatohepatitis development in an animal model. PLoS One. 17:e0261310. DOI: 10.1371/journal.pone.0261310. PMID: 35192632. PMCID: PMC8863244. PMID: bf48577133404d54ac87805d0b6d9c74.

30. Yoshimine Y, Uto H, Kumagai K, Mawatari S, Arima S, Ibusuki R, Mera K, Nosaki T, Kanmura S, Numata M, Tamai T, Moriuchi A, Tsubouchi H, Ido A. 2015; Hepatic expression of the Sptlc3 subunit of serine palmitoyltransferase is associated with the development of hepatocellular carcinoma in a mouse model of nonalcoholic steatohepatitis. Oncol Rep. 33:1657–1666. DOI: 10.3892/or.2015.3745. PMID: 25607821.

31. Jin Y, Nguyen TLL, Myung CS, Heo KS. 2022; Ginsenoside Rh1 protects human endothelial cells against lipopolysaccharide-induced inflammatory injury through inhibiting TLR2/4-mediated STAT3, NF-κB, and ER stress signaling pathways. Life Sci. 309:120973. DOI: 10.1016/j.lfs.2022.120973. PMID: 36150463.

32. Pawlak M, Lefebvre P, Staels B. 2015; Molecular mechanism of PPARα action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J Hepatol. 62:720–733. DOI: 10.1016/j.jhep.2014.10.039. PMID: 25450203.

33. Van Nguyen D, Nguyen TLL, Jin Y, Kim L, Myung CS, Heo KS. 2022; 6'-Sialylactose abolished lipopolysaccharide-induced inflammation and hyper-permeability in endothelial cells. Arch Pharm Res. 45:836–848. DOI: 10.1007/s12272-022-01415-0. PMID: 36401777.

34. Chen Z, Yu R, Xiong Y, Du F, Zhu S. 2017; A vicious circle between insulin resistance and inflammation in nonalcoholic fatty liver disease. Lipids Health Dis. 16:203. Erratum in: Lipids Health Dis. 2018; 17:33. DOI: 10.1186/s12944-017-0572-9. PMID: 29037210. PMCID: PMC5644081. PMID: affc31d5c7b746ebbc0d17caf6f1e363.

35. Yan T, Wang H, Cao L, Wang Q, Takahashi S, Yagai T, Li G, Krausz KW, Wang G, Gonzalez FJ, Hao H. 2018; Glycyrrhizin alleviates nonalcoholic steatohepatitis via modulating bile acids and meta-inflammation. Drug Metab Dispos. 46:1310–1319. DOI: 10.1124/dmd.118.082008. PMID: 29959134. PMCID: PMC6081736.

36. Li CX, Gao JG, Wan XY, Chen Y, Xu CF, Feng ZM, Zeng H, Lin YM, Ma H, Xu P, Yu CH, Li YM. 2019; Allyl isothiocyanate ameliorates lipid accumulation and inflammation in nonalcoholic fatty liver disease via the Sirt1/AMPK and NF-κB signaling pathways. World J Gastroenterol. 25:5120–5133. DOI: 10.3748/wjg.v25.i34.5120. PMID: 31558861. PMCID: PMC6747284.

37. Diniz TA, de Lima Junior EA, Teixeira AA, Biondo LA, da Rocha LAF, Valadão IC, Silveira LS, Cabral-Santos C, de Souza CO, Rosa Neto JC. 2021; Aerobic training improves NAFLD markers and insulin resistance through AMPK-PPAR-α signaling in obese mice. Life Sci. 266:118868. DOI: 10.1016/j.lfs.2020.118868. PMID: 33310034.

38. Xiao Q, Zhang S, Yang C, Du R, Zhao J, Li J, Xu Y, Qin Y, Gao Y, Huang W. 2019; Ginsenoside Rg1 ameliorates palmitic acid-induced hepatic steatosis and inflammation in HepG2 cells via the AMPK/NF-κB pathway. Int J Endocrinol. 2019:7514802. DOI: 10.1155/2019/7514802. PMID: 31467529. PMCID: PMC6699274. PMID: 81c42df073804a37833110c45bb3fe67.
39. Nguyen TLL, Huynh DTN, Jin Y, Jeon H, Heo KS. 2021; Protective effects of ginsenoside-Rg2 and -Rh1 on liver function through inhibiting TAK1 and STAT3-mediated inflammatory activity and Nrf2/ARE-mediated antioxidant signaling pathway. Arch Pharm Res. 44:241–252. DOI: 10.1007/s12272-020-01304-4. PMID: 33537886.

40. Zhu W, Sahar NE, Javaid HMA, Pak ES, Liang G, Wang Y, Ha H, Huh JY. 2021; Exercise-induced irisin decreases inflammation and improves NAFLD by competitive binding with MD2. Cells. 10:3306. DOI: 10.3390/cells10123306. PMID: 34943814. PMCID: PMC8699279. PMID: 72948fe9874b44a18ece0156762b6e01.

41. Bai J, Liu F. 2019; The cGAS-cGAMP-STING pathway: a molecular link between immunity and metabolism. Diabetes. 68:1099–1108. DOI: 10.2337/dbi18-0052. PMID: 31109939. PMCID: PMC6610018.

42. Chen L, Deng H, Cui H, Fang J, Zuo Z, Deng J, Li Y, Wang X, Zhao L. 2017; Inflammatory responses and inflammation-associated diseases in organs. Oncotarget. 9:7204–7218. DOI: 10.18632/oncotarget.23208. PMID: 29467962. PMCID: PMC5805548.

43. Jin Y, Tangchang W, Kwon OS, Lee JY, Heo KS, Son HY. 2023; Ginsenoside Rh1 ameliorates the asthma and allergic inflammation via inhibiting Akt, MAPK, and NF-κB signaling pathways in vitro and in vivo. Life Sci. 321:121607. DOI: 10.1016/j.lfs.2023.121607. PMID: 36958436.

44. Mohammed S, Nicklas EH, Thadathil N, Selvarani R, Royce GH, Kinter M, Richardson A, Deepa SS. 2021; Role of necroptosis in chronic hepatic inflammation and fibrosis in a mouse model of increased oxidative stress. Free Radic Biol Med. 164:315–328. DOI: 10.1016/j.freeradbiomed.2020.12.449. PMID: 33429022. PMCID: PMC8845573.

45. Gaul S, Leszczynska A, Alegre F, Kaufmann B, Johnson CD, Adams LA, Wree A, Damm G, Seehofer D, Calvente CJ, Povero D, Kisseleva T, Eguchi A, McGeough MD, Hoffman HM, Pelegrin P, Laufs U, Feldstein AE. 2021; Hepatocyte pyroptosis and release of inflammasome particles induce stellate cell activation and liver fibrosis. J Hepatol. 74:156–167. DOI: 10.1016/j.jhep.2020.07.041. PMID: 32763266. PMCID: PMC7749849.

46. Jin Y, Huynh DTN, Nguyen TLL, Jeon H, Heo KS. 2020; Therapeutic effects of ginsenosides on breast cancer growth and metastasis. Arch Pharm Res. 43:773–787. DOI: 10.1007/s12272-020-01265-8. PMID: 32839835.

47. Zhang Z, Moon R, Thorne JL, Moore JB. 2023; NAFLD and vitamin D: evidence for intersection of microRNA-regulated pathways. Nutr Res Rev. 36:120–139. DOI: 10.1017/S095442242100038X. PMID: 35109946.

48. Zhang Q, Yu K, Cao Y, Luo Y, Liu Y, Zhao C. 2021; miR-125b promotes the NF-κB-mediated inflammatory response in NAFLD via directly targeting TNFAIP3. Life Sci. 270:119071. DOI: 10.1016/j.lfs.2021.119071. PMID: 33515562.

49. Hu Y, Peng X, Du G, Zhang Z, Zhai Y, Xiong X, Luo X. 2022; MicroRNA-122-5p inhibition improves inflammation and oxidative stress damage in dietary-induced non-alcoholic fatty liver disease through targeting FOXO3. Front Physiol. 13:803445. DOI: 10.3389/fphys.2022.803445. PMID: 35222075. PMCID: PMC8874326. PMID: 8efcb73afd2f4735b6882b87c625e44f.

50. Hou X, Yin S, Ren R, Liu S, Yong L, Liu Y, Li Y, Zheng MH, Kunos G, Gao B, Wang H. 2021; Myeloid-cell-specific IL-6 signaling promotes MicroRNA-223-enriched exosome production to attenuate NAFLD-associated fibrosis. Hepatology. 74:116–132. DOI: 10.1002/hep.31658. PMID: 33236445. PMCID: PMC8141545.

51. Xu H, Tian Y, Tang D, Zou S, Liu G, Song J, Zhang G, Du X, Huang W, He B, Lin W, Jin L, Huang W, Yang J, Fu X. 2021; An endoplasmic reticulum stress-MicroRNA-26a feedback circuit in NAFLD. Hepatology. 73:1327–1345. DOI: 10.1002/hep.31428. PMID: 32567701.

52. Torres LF, Cogliati B, Otton R. 2019; Green tea prevents NAFLD by modulation of miR-34a and miR-194 expression in a high-fat diet mouse model. Oxid Med Cell Longev. 2019:4168380. DOI: 10.1155/2019/4168380. PMID: 31885789. PMCID: PMC6914886.

53. Zhao J, Wu Q, Yang T, Nie L, Liu S, Zhou J, Chen J, Jiang Z, Xiao T, Yang J, Chu C. 2022; Gaseous signal molecule SO2 regulates autophagy through PI3K/AKT pathway inhibits cardiomyocyte apoptosis and improves myocardial fibrosis in rats with type II diabetes. Korean J Physiol Pharmacol. 26:541–556. DOI: 10.4196/kjpp.2022.26.6.541. PMID: 36302628. PMCID: PMC9614393.

54. Bashiardes S, Shapiro H, Rozin S, Shibolet O, Elinav E. 2016; Non-alcoholic fatty liver and the gut microbiota. Mol Metab. 5:782–794. DOI: 10.1016/j.molmet.2016.06.003. PMID: 27617201. PMCID: PMC5004228. PMID: 829f3bf9256c4ba982f5e506775f3123.

55. Zhang X, Coker OO, Chu ES, Fu K, Lau HCH, Wang YX, Chan AWH, Wei H, Yang X, Sung JJY, Yu J. 2021; Dietary cholesterol drives fatty liver-associated liver cancer by modulating gut microbiota and metabolites. Gut. 70:761–774. DOI: 10.1136/gutjnl-2019-319664. PMID: 32694178. PMCID: PMC7948195.

56. Behary J, Amorim N, Jiang XT, Raposo A, Gong L, McGovern E, Ibrahim R, Chu F, Stephens C, Jebeili H, Fragomeli V, Koay YC, Jackson M, O'Sullivan J, Weltman M, McCaughan G, El-Omar E, Zekry A. 2021; Gut microbiota impact on the peripheral immune response in non-alcoholic fatty liver disease related hepatocellular carcinoma. Nat Commun. 12:187. DOI: 10.1038/s41467-020-20422-7. PMID: 33420074. PMCID: PMC7794332. PMID: b2728d3b48ed45bd99530aeb278cdc20.

57. Rubio C, Puerto M, García-Rodríquez JJ, Lu VB, García-Martínez I, Alén R, Sanmartín-Salinas P, Toledo-Lobo MV, Saiz J, Ruperez J, Barbas C, Menchén L, Gribble FM, Reimann F, Guijarro LG, Carrascosa JM, Valverde ÁM. 2020; Impact of global PTP1B deficiency on the gut barrier permeability during NASH in mice. Mol Metab. 35:100954. DOI: 10.1016/j.molmet.2020.01.018. PMID: 32244182. PMCID: PMC7082558.

58. Wu L, Li J, Feng J, Ji J, Yu Q, Li Y, Zheng Y, Dai W, Wu J, Guo C. 2021; Crosstalk between PPARs and gut microbiota in NAFLD. Biomed Pharmacother. 136:111255. DOI: 10.1016/j.biopha.2021.111255. PMID: 33485064.

59. Li DD, Ma JM, Li MJ, Gao LL, Fan YN, Zhang YN, Tao XJ, Yang JJ. 2022; Supplementation of Lycium barbarum polysaccharide combined with aerobic exercise ameliorates high-fat-induced nonalcoholic steatohepatitis via AMPK/PPARα/PGC-1α pathway. Nutrients. 14:3247. DOI: 10.3390/nu14153247. PMID: 35956423. PMCID: PMC9370707. PMID: c18581c6400246a5a032860682c13c9e.

60. Yang H, Feng L, Xu L, Jiang D, Zhai F, Tong G, Xing Y. 2022; Intervention of Shugan Xiaozhi decoction on nonalcoholic fatty liver disease via mediating gut-liver axis. Biomed Res Int. 2022:4801695. DOI: 10.1155/2022/4801695. PMID: 35837380. PMCID: PMC9276511.

61. Hasan AU, Rahman A, Kobori H. 2019; Interactions between host PPARs and gut microbiota in health and disease. Int J Mol Sci. 20:387. DOI: 10.3390/ijms20020387. PMID: 30658440. PMCID: PMC6359605. PMID: 6d20714c548246c983c10fa37922c936.

62. Kanda T, Matsuoka S, Yamazaki M, Shibata T, Nirei K, Takahashi H, Kaneko T, Fujisawa M, Higuchi T, Nakamura H, Matsumoto N, Yamagami H, Ogawa M, Imazu H, Kuroda K, Moriyama M. 2018; Apoptosis and non-alcoholic fatty liver diseases. World J Gastroenterol. 24:2661–2672. DOI: 10.3748/wjg.v24.i25.2661. PMID: 29991872. PMCID: PMC6034146.

63. Chang XM, Xiao F, Pan Q, Wang XX, Guo LX. 2021; Sitagliptin attenuates endothelial dysfunction independent of its blood glucose controlling effect. Korean J Physiol Pharmacol. 25:425–437. DOI: 10.4196/kjpp.2021.25.5.425. PMID: 34448460. PMCID: PMC8405439.

64. Zhao P, Sun X, Chaggan C, Liao Z, In Wong K, He F, Singh S, Loomba R, Karin M, Witztum JL, Saltiel AR. 2020; An AMPK-caspase-6 axis controls liver damage in nonalcoholic steatohepatitis. Science. 367:652–660. DOI: 10.1126/science.aay0542. PMID: 32029622. PMCID: PMC8012106.

65. Jeon H, Jin Y, Myung CS, Heo KS. 2021; Ginsenoside-Rg2 exerts anti-cancer effects through ROS-mediated AMPK activation associated mitochondrial damage and oxidation in MCF-7 cells. Arch Pharm Res. 44:702–712. DOI: 10.1007/s12272-021-01345-3. PMID: 34302638.

66. Qiao JT, Cui C, Qing L, Wang LS, He TY, Yan F, Liu FQ, Shen YH, Hou XG, Chen L. 2018; Activation of the STING-IRF3 pathway promotes hepatocyte inflammation, apoptosis and induces metabolic disorders in nonalcoholic fatty liver disease. Metabolism. 81:13–24. DOI: 10.1016/j.metabol.2017.09.010. PMID: 29106945.

67. Jin Y, Huynh DTN, Heo KS. 2022; Ginsenoside Rh1 inhibits tumor growth in MDA-MB-231 breast cancer cells via mitochondrial ROS and ER stress-mediated signaling pathway. Arch Pharm Res. 45:174–184. DOI: 10.1007/s12272-022-01377-3. PMID: 35325393.

68. Li Y, Xu J, Lu Y, Bian H, Yang L, Wu H, Zhang X, Zhang B, Xiong M, Chang Y, Tang J, Yang F, Zhao L, Li J, Gao X, Xia M, Tan M, Li J. 2021; DRAK2 aggravates nonalcoholic fatty liver disease progression through SRSF6-associated RNA alternative splicing. Cell Metab. 33:2004–2020.e9. DOI: 10.1016/j.cmet.2021.09.008. PMID: 34614409.

69. Nasiri-Ansari N, Nikolopoulou C, Papoutsi K, Kyrou I, Mantzoros CS, Kyriakopoulos G, Chatzigeorgiou A, Kalotychou V, Randeva MS, Chatha K, Kontzoglou K, Kaltsas G, Papavassiliou AG, Randeva HS, Kassi E. 2021; Empagliflozin attenuates non-alcoholic fatty liver disease (NAFLD) in high fat diet fed ApoE(-/-) mice by activating autophagy and reducing ER stress and apoptosis. Int J Mol Sci. 22:818. DOI: 10.3390/ijms22020818. PMID: 33467546. PMCID: PMC7829901. PMID: f967148ee656483cac3dae9949ce40ff.

70. Pei K, Gui T, Kan D, Feng H, Jin Y, Yang Y, Zhang Q, Du Z, Gai Z, Wu J, Li Y. 2020; An overview of lipid metabolism and nonalcoholic fatty liver disease. Biomed Res Int. 2020:4020249. DOI: 10.1155/2020/4020249. PMID: 32733940. PMCID: PMC7383338.

71. Prasomthong J, Limpeanchob N, Daodee S, Chonpathompikunlert P, Tunsophon S. 2022; Hibiscus sabdariffa extract improves hepatic steatosis, partially through IRS-1/Akt and Nrf2 signaling pathways in rats fed a high fat diet. Sci Rep. 12:7022. DOI: 10.1038/s41598-022-11027-9. PMID: 35487948. PMCID: PMC9054782. PMID: 82f7aaf0fccd429a97edf775230404b2.

72. Li F, Ye J, Sun Y, Lin Y, Wu T, Shao C, Ma Q, Liao X, Feng S, Zhong B. 2021; Distinct dose-dependent association of free fatty acids with diabetes development in nonalcoholic fatty liver disease patients. Diabetes Metab J. 45:417–429. DOI: 10.4093/dmj.2020.0039. PMID: 33705650. PMCID: PMC8164943. PMID: 8bbe0bc385c949c8aabd39577c08e02b.

73. Wang S, Jung S, Ko KS. 2022; Effects of amino acids supplementation on lipid and glucose metabolism in HepG2 cells. Nutrients. 14:3050. DOI: 10.3390/nu14153050. PMID: 35893906. PMCID: PMC9332103. PMID: 9f6073fa2e634f949e14d06940ce2a8f.

74. Li R, Li Y, Yang X, Hu Y, Yu H, Li Y. 2022; Reducing VEGFB accelerates NAFLD and insulin resistance in mice via inhibiting AMPK signaling pathway. J Transl Med. 20:341. DOI: 10.1186/s12967-022-03540-2. PMID: 35907871. PMCID: PMC9338666. PMID: 147f4ab94a114d7490bbc87e88dc85d9.

75. Xu B, Jiang M, Chu Y, Wang W, Chen D, Li X, Zhang Z, Zhang D, Fan D, Nie Y, Shao F, Wu K, Liang J. 2018; Gasdermin D plays a key role as a pyroptosis executor of non-alcoholic steatohepatitis in humans and mice. J Hepatol. 68:773–782. DOI: 10.1016/j.jhep.2017.11.040. PMID: 29273476.

76. Todoric J, Di Caro G, Reibe S, Henstridge DC, Green CR, Vrbanac A, Ceteci F, Conche C, McNulty R, Shalapour S, Taniguchi K, Meikle PJ, Watrous JD, Moranchel R, Najhawan M, Jain M, Liu X, Kisseleva T, Diaz-Meco MT, Moscat J, et al. 2020; Fructose stimulated de novo lipogenesis is promoted by inflammation. Nat Metab. 2:1034–1045. DOI: 10.1038/s42255-020-0261-2. PMID: 32839596. PMCID: PMC8018782.

77. Vasques-Monteiro IML, Silva-Veiga FM, Miranda CS, de Andrade Gonçalves ÉCB, Daleprane JB, Souza-Mello V. 2021; A rise in Proteobacteria is an indicator of gut-liver axis-mediated nonalcoholic fatty liver disease in high-fructose-fed adult mice. Nutr Res. 91:26–35. DOI: 10.1016/j.nutres.2021.04.008. PMID: 34130208.

78. Daniel PV, Dogra S, Rawat P, Choubey A, Khan AS, Rajak S, Kamthan M, Mondal P. 2021; NF-κB p65 regulates hepatic lipogenesis by promoting nuclear entry of ChREBP in response to a high carbohydrate diet. . J Biol Chem. 296:100714. DOI: 10.1016/j.jbc.2021.100714. PMID: 33930463. PMCID: PMC8144664.

79. Sandoval V, Sanz-Lamora H, Marrero PF, Relat J, Haro D. 2021; Lyophilized maqui (Aristotelia chilensis) berry administration suppresses high-fat diet-induced liver lipogenesis through the induction of the nuclear corepressor SMILE. Antioxidants (Basel). 10:637. DOI: 10.3390/antiox10050637. PMID: 33919415. PMCID: PMC8143281. PMID: fa5892aacf3e44c5ab21df0fdf85b37f.

80. Luo G, Xiang L, Xiao L. 2022; Acetyl-CoA deficiency is involved in the regulation of iron overload on lipid metabolism in apolipoprotein E knockout mice. Molecules. 27:4966. DOI: 10.3390/molecules27154966. PMID: 35956917. PMCID: PMC9370536. PMID: b088f247d86d468280f0be391f80b844.

81. Hardie DG. 2007; AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nat Rev Mol Cell Biol. 8:774–785. DOI: 10.1038/nrm2249. PMID: 17712357.

82. Hsu MC, Guo BC, Chen CH, Hu PA, Lee TS. 2021; Apigenin ameliorates hepatic lipid accumulation by activating the autophagy-mitochondria pathway. J Food Drug Anal. 29:240–254. DOI: 10.38212/2224-6614.3269. PMID: 35696209. PMCID: PMC9261827.

83. Shih PH, Shiue SJ, Chen CN, Cheng SW, Lin HY, Wu LW, Wu MS. 2021; Fucoidan and fucoxanthin attenuate hepatic steatosis and inflammation of NAFLD through modulation of leptin/adiponectin axis. Mar Drugs. 19:148. DOI: 10.3390/md19030148. PMID: 33809062. PMCID: PMC8001566. PMID: 53f4c3f331c041e385653fb91ea1a0e9.

84. Barbier-Torres L, Fortner KA, Iruzubieta P, Delgado TC, Giddings E, Chen Y, Champagne D, Fernández-Ramos D, Mestre D, Gomez-Santos B, Varela-Rey M, de Juan VG, Fernández-Tussy P, Zubiete-Franco I, García-Monzón C, González-Rodríguez Á, Oza D, Valença-Pereira F, Fang Q, Crespo J, et al. 2020; Silencing hepatic MCJ attenuates non-alcoholic fatty liver disease (NAFLD) by increasing mitochondrial fatty acid oxidation. Nat Commun. 11:3360. DOI: 10.1038/s41467-020-16991-2. PMID: 32620763. PMCID: PMC7334216. PMID: f4585c15ec8641c2ad4a105f86c1a9ca.

85. Lu Q, Tian X, Wu H, Huang J, Li M, Mei Z, Zhou L, Xie H, Zheng S. 2021; Metabolic changes of hepatocytes in NAFLD. Front Physiol. 12:710420. DOI: 10.3389/fphys.2021.710420. PMID: 34526911. PMCID: PMC8437340. PMID: 34d4a99eb3544a13a215b9f7ae621c2d.

86. Li H, Xu Q, Xu C, Hu Y, Yu X, Zhao K, Li M, Li M, Xu J, Kuang H. 2021; Bicyclol regulates hepatic gluconeogenesis in rats with type 2 diabetes and non-alcoholic fatty liver disease by inhibiting inflammation. Front Pharmacol. 12:644129. DOI: 10.3389/fphar.2021.644129. PMID: 34093184. PMCID: PMC8175979. PMID: 72ec72ee182a4c92b90af230041a565d.

87. Im SS, Kim MY, Kwon SK, Kim TH, Bae JS, Kim H, Kim KS, Oh GT, Ahn YH. 2011; Peroxisome proliferator-activated receptor {alpha} is responsible for the up-regulation of hepatic glucose-6-phosphatase gene expression in fasting and db/db mice. J Biol Chem. 286:1157–1164. DOI: 10.1074/jbc.M110.157875. PMID: 21081500. PMCID: PMC3020722.

88. Song D, Yin L, Wang C, Wen X. 2020; Zhenqing recipe attenuates non-alcoholic fatty liver disease by regulating the SIK1/CRTC2 signaling in experimental diabetic rats. BMC Complement Med Ther. 20:27. DOI: 10.1186/s12906-019-2811-2. PMID: 32020874. PMCID: PMC7076741. PMID: 1471a750307546c5b6034c75bc2bf4fb.

89. Choudhury M, Qadri I, Rahman SM, Schroeder-Gloeckler J, Janssen RC, Friedman JE. 2011; C/EBPβ is AMP kinase sensitive and up-regulates PEPCK in response to ER stress in hepatoma cells. Mol Cell Endocrinol. 331:102–108. DOI: 10.1016/j.mce.2010.08.014. PMID: 20797423. PMCID: PMC2981635.

90. Phung HH, Lee CH. 2022; Mouse models of nonalcoholic steatohepatitis and their application to new drug development. Arch Pharm Res. 45:761–794. DOI: 10.1007/s12272-022-01410-5. PMID: 36318445.

91. Khan MS, Lee C, Kim SG. 2022; Non-alcoholic fatty liver disease and liver secretome. Arch Pharm Res. 45:938–963. DOI: 10.1007/s12272-022-01419-w. PMID: 36441472. PMCID: PMC9703441.

92. Shaaban HH, Alzaim I, El-Mallah A, Aly RG, El-Yazbi AF, Wahid A. 2022; Metformin, pioglitazone, dapagliflozin and their combinations ameliorate manifestations associated with NAFLD in rats via anti-inflammatory, anti-fibrotic, anti-oxidant and anti-apoptotic mechanisms. Life Sci. 308:120956. DOI: 10.1016/j.lfs.2022.120956. PMID: 36103959.

93. Liu B, Xiang L, Ji J, Liu W, Chen Y, Xia M, Liu Y, Liu W, Zhu P, Jin Y, Han Y, Lu J, Li X, Zheng M, Lu Y. 2021; Sparcl1 promotes nonalcoholic steatohepatitis progression in mice through upregulation of CCL2. J Clin Invest. 131:e144801. DOI: 10.1172/JCI144801. PMID: 34651580. PMCID: PMC8516465.

94. Huang S, Wu Y, Zhao Z, Wu B, Sun K, Wang H, Qin L, Bai F, Leng Y, Tang W. 2021; A new mechanism of obeticholic acid on NASH treatment by inhibiting NLRP3 inflammasome activation in macrophage. Metabolism. 120:154797. DOI: 10.1016/j.metabol.2021.154797. PMID: 33984334.
95. Puengel T, Lefere S, Hundertmark J, Kohlhepp M, Penners C, Van de Velde F, Lapauw B, Hoorens A, Devisscher L, Geerts A, Boehm S, Zhao Q, Krupinski J, Charles ED, Zinker B, Tacke F. 2022; Combined therapy with a CCR2/CCR5 antagonist and FGF21 analogue synergizes in ameliorating steatohepatitis and fibrosis. Int J Mol Sci. 23:6696. DOI: 10.3390/ijms23126696. PMID: 35743140. PMCID: PMC9224277. PMID: 8267c70907c64c32a767d42485b7e4e4.

96. Crengle S. 2020; Comment on: Jaine et al Cost-effectiveness of a low-dose computed tomography screening programme for lung cancer in New Zealand. Lung Cancer 2018, 124, 233-240. 2018/10/01. DOI: 10.1016/j.lungcan.2018.08.004. Lung Cancer. 145:219–220. DOI: 10.1016/j.lungcan.2018.08.004. PMID: 30268467.

97. Ala M, Eftekhar SP. 2023; Weight loss breaks the bond between nonalcoholic fatty liver disease and cardiovascular diseases: a clinical and epidemiological perspective. Obes Rev. 24:e13563. DOI: 10.1111/obr.13563. PMID: 36951144.

98. Ma Z, Jin K, Yue M, Chen X, Chen J. 2023; Research progress on the GIP/GLP-1 receptor coagonist tirzepatide, a rising star in type 2 diabetes. J Diabetes Res. 2023:5891532. DOI: 10.1155/2023/5891532. PMID: 37096236. PMCID: PMC10122586. PMID: ebf346f2c12c4f8c8785124c0ae0cc3d.

99. Valenzuela-Vallejo L, Guatibonza-García V, Mantzoros CS. 2022; Recent guidelines for non-alcoholic fatty liver disease (NAFLD)/fatty liver disease (FLD): are they already outdated and in need of supplementation? Metabolism. 136:155248. DOI: 10.1016/j.metabol.2022.155248. PMID: 35803320.
100. Giugliano D, Scappaticcio L, Longo M, Caruso P, Maiorino MI, Bellastella G, Ceriello A, Chiodini P, Esposito K. 2021; GLP-1 receptor agonists and cardiorenal outcomes in type 2 diabetes: an updated meta-analysis of eight CVOTs. Cardiovasc Diabetol. 20:189. DOI: 10.1186/s12933-021-01366-8. PMID: 34526024. PMCID: PMC8442438. PMID: 350ec22390e74c53b7c53d0cd7a32144.

101. Greenan J. 1984; Cardiac dysrhythmias and heart rate changes at induction of anaesthesia: a comparison of two intravenous anticholinergics. Acta Anaesthesiol Scand. 28:182–184. DOI: 10.1111/j.1399-6576.1984.tb02037.x. PMID: 6375235.

102. Tacke F, Puengel T, Loomba R, Friedman SL. 2023; An integrated view of anti-inflammatory and antifibrotic targets for the treatment of NASH. J Hepatol. S0168-8278(23)00218-0. DOI: 10.1016/j.jhep.2023.03.038. PMID: 37061196.

103. Pennisi G, Celsa C, Enea M, Vaccaro M, Di Marco V, Ciccioli C, Infantino G, La Mantia C, Parisi S, Vernuccio F, Craxì A, Cammà C, Petta S. 2022; Effect of pharmacological interventions and placebo on liver histology in nonalcoholic steatohepatitis: a network meta-analysis. Nutr Metab Cardiovasc Dis. 32:2279–2288. DOI: 10.1016/j.numecd.2022.07.001. PMID: 35970684.

Fig. 1
Pathogenesis of non-alcoholic fatty liver disease (NAFLD) progression.
Various factors including hyperglycemia, hyperlipidemia, and insulin resistance signaling pathways are involved in the NAFL and NASH progression by accelerating the inflammatory signaling pathway and its mediated apoptosis. Additionally, continuous activities NAFLD involved into accumulating of liver fibrosis and genetic exchange. Finally, hepatocarcinoma was activated and patients need to surgical treatment. NAFL, non-alcoholic fatty liver; NASH, non-alcoholic steatohepatitis.
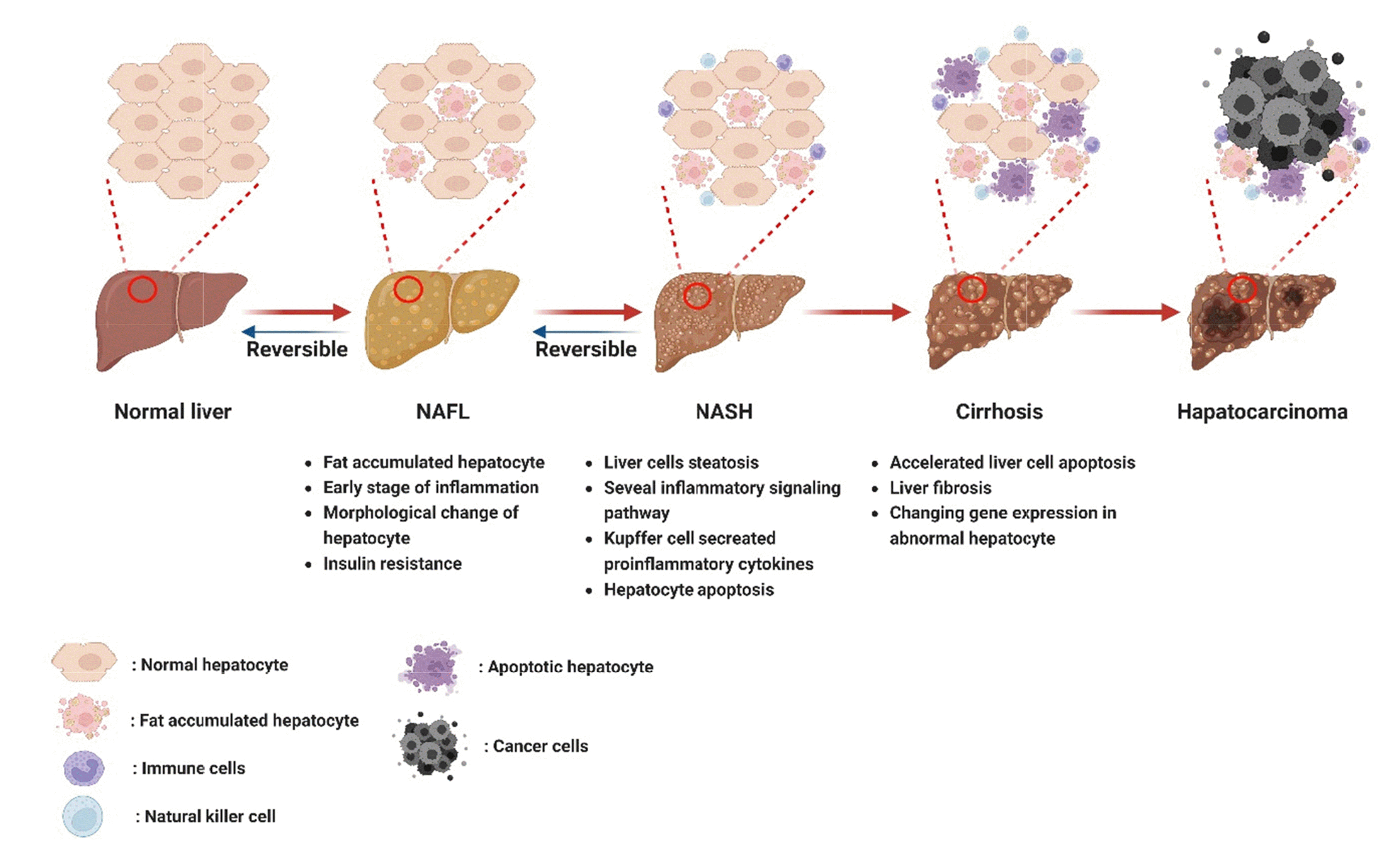
Fig. 2
Mechanisms of inflammation on NAFLD progression.
(A) TNF-α or LPS activates each receptor, such as TNFR and LPS4 or 9, leading to increase NF-κB phosphorylation through the activation of MAPK, STAT3, JNK, or AMPK expression. Additionally, the production of reactive oxygen species (ROS) contributes to mitochondrial dysfunction and inflammasome activation, which in turn accelerate IL-1β maturation and secretion. (B) miR-34a inhibited MAPK-mediated NF-κB phosphorylation via activation of TLR4, which is triggered by LPS or high-glucose levels. Besides, NF-κB-mediated TNF-α induction activates its receptor and activated inflammatory signaling pathway. However, this signaling pathway is inactivated by miR-125b/TRNAIP3-mediated TNF-α receptor ubiquitination. In addition, IL-6 secretion by NF-κB activation increased phosphorylation of STAT3 and activated miR-233-enriched exosome which was source of IL-6 over production. Accelerated inflammatory signaling pathway induced PERK-mediated eIF2α phosphorylation suppressed by miR-26a and miR-26a suppressed eIF2α-activated NF-κB p65 translocation into nucleus. Moreover, high-glucose activated IRS-1/AKT signaling pathway and FOXO3 that suppressed by miR-122-5p activation. (C) The environment of the intestine and liver is closely linked to the disruption of gut microbiota, which can be induced by dysregulation of diets. Briefly, LPS secreted by disruption of gut microbiota increased gut inflammation through inactivating AMPK/PPARβ/δ mechanisms. Moreover, dephosphorylation of AMPK-mediated PPARα suppression caused by disruption of gut microbiota increased PGC1α-mediated lipid metabolisms and induced liver steatosis. In addition, activation of PPARγ stabilized cellular homeostasis through induction of IL-10 and iNOS mRNA expression. Furthermore, LPS comes from dysfunction of gut microbiota activated TLR4-mediated NF-κB signaling pathway in hepatocyte through blood flow in portal vein. NAFLD, non-alcoholic fatty liver disease; TNF-α, tumor necrosis factor-α; NF-κB, nuclear factor of kappa photopolypeptide enhancer; MAPK, mitogen-activated protein kinase; STAT3, signal transducer and activator of transcription 3; AMPK, AMP-activated protein kinase; IL, interleukin; TLR, toll-like receptor; IRS-1, insulin receptor substrate 1; PPAR, peroxisome proliferator-activated receptor; PGC1α, PPARγ coactivator-1α; iNOS, inducible nitric oxide synthase; ER, endoplasmic reticulum; HFD, high-fat diet; FFA, free fatty acid.
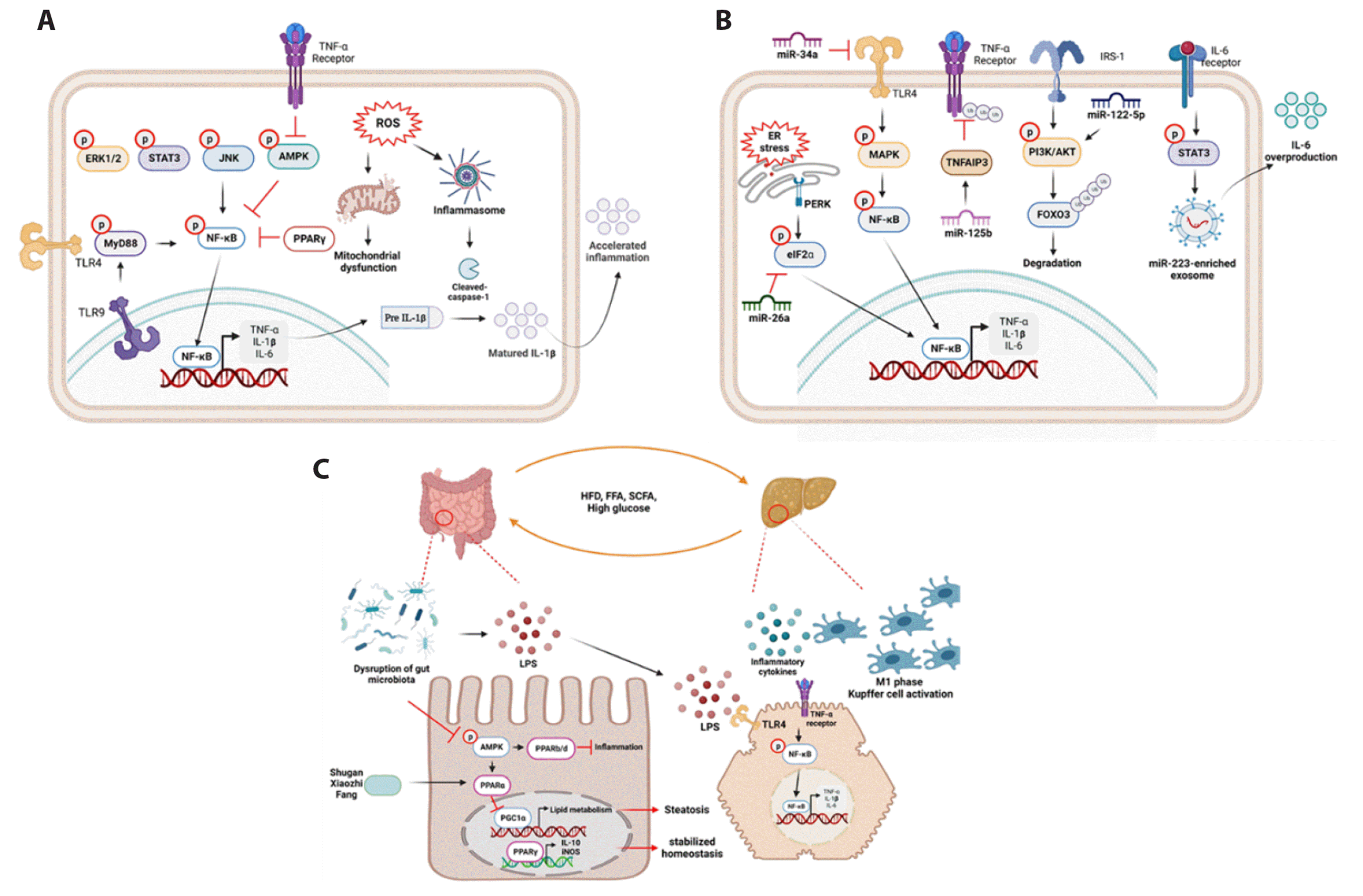
Fig. 3
Mechanisms of apoptosis in NAFLD patient.
Liver apoptosis plays several roles in NAFLD progression. Factors such as high glucose levels or cellular dysfunction, including reactive oxygen species (ROS) production, ROS-mediated mitochondrial dysfunction, and endoplasmic reticulum (ER) stress signaling pathways, contribute to the expression of Bax. Mechanistically, PERK-mediated CHOP expression induced Bax mRNA expression, which is one of inducers on mitochondrial dysfunction-mediated apoptosis signaling pathway. After mitochondrial dysfunction occurs cytochrome C releasement required caspase-3 and cleaved PARP ex-pression. In addition, damaged mitochondria produce mtROS and mtROS activated STING/IRF3 phosphorylation-mediated BAX mRNA expression. In addition, complex with SRSF6 and DRAK2 induced abnormal mRNA production of Polg2, Rnasel, and Nme4 and its abnormal form induced mitochondrial dysfunction-mediated hepatocyte apoptosis. Moreover, AMPK activation sup-pressed cleaved caspase-6-mediated apoptosis signaling pathway. NAFLD, non-alcoholic fatty liver disease; CHOP, c/EBP homologous protein; mtROS, mitochondrial ROS; STING, stimulator of interferon gene; IRF3, interferon regulatory factor 3; AMPK, AMP-activated protein kinase.
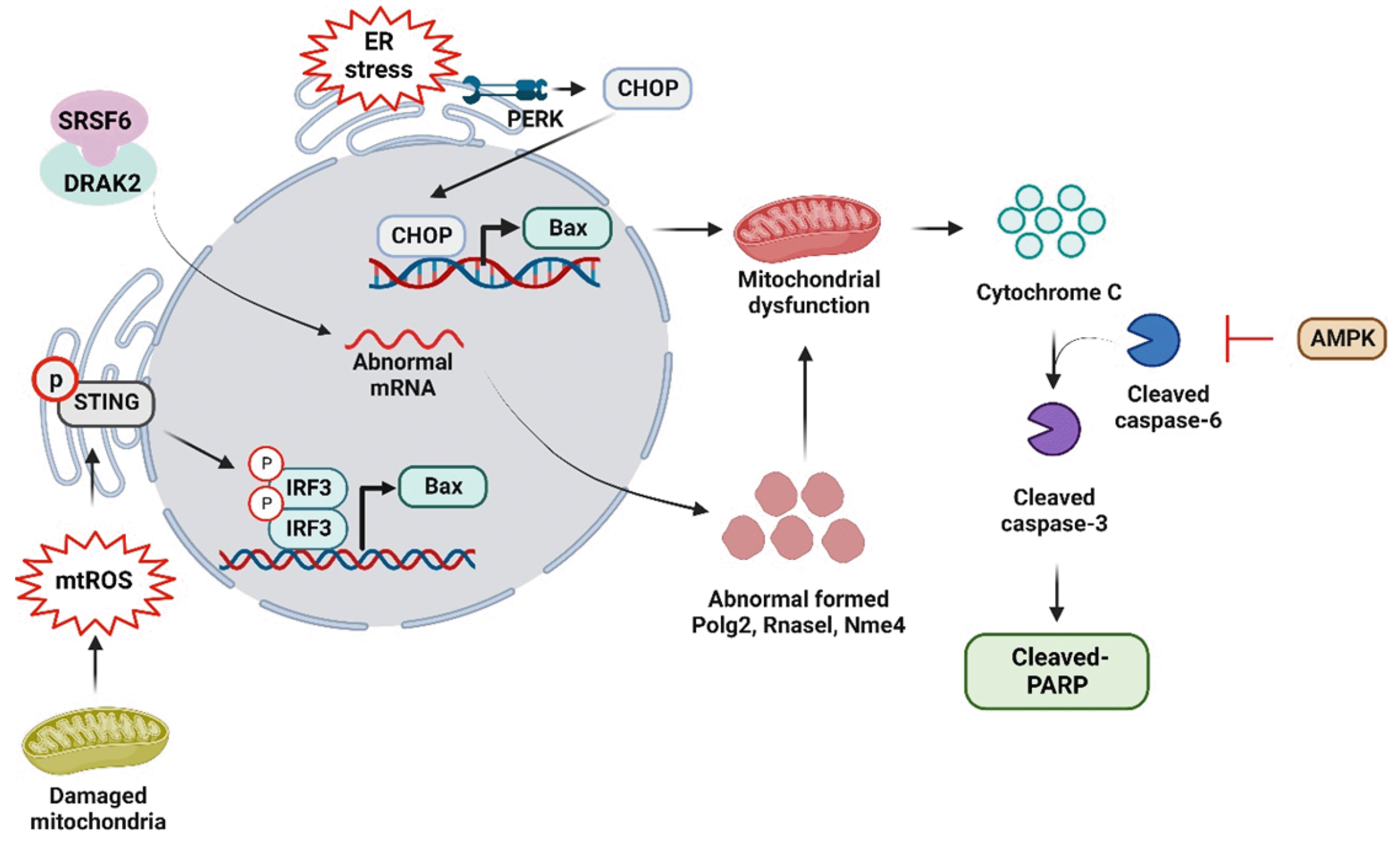
Table 1
Dietary or chemical-mediated animal model for inducing NAFLD progression
| Animal models | Composition of diet | Feeding date (wk) |
Characters of liver |
Indicators | References |
|---|---|---|---|---|---|
| Dietary model | 40% cholesterol with 22% glucose | 15 | NASH | High level of TG, glucose, AST, ALT, and uric acid in plasma | [17] |
| HFD | 8 | NASH | Liver inflammation and steatosis | [18] | |
| HFD | 14 | NASH | AST, ALT increase | [19] | |
| HFD with 2.31% fructose in water | 24 | HCC progression | High level of AST, ALT, glucose, and total cholesterol | [20] | |
| MCD diet | 6 | NASH | High level of AST, ALT, and bilirubin | [22] | |
| 2–4 | NASH | DNA and RNA methylation | [23] | ||
| 2–4 | NASH | Liver fibrosis and inflammation | [24] | ||
| 8 | NASH | Steatosis | [25] | ||
| 8 | NASH | T helper 17 and 22 cell-mediated inflammatory cytokines | [26,35] | ||
| 6 | NASH | AKT/mTOR-dependent autophagic apoptosis in hepatocyte | [27] | ||
| STAM model | HFD | 4 | NASH | GLUT2-mediated DNA fragmentation & liver steatosis | [29] |
| HFD | 4 | NASH | Insulin-mediated glucose intake | [14] | |
| HFD | 16 | HCC progression | High expression of Sptlc3 expression lead to HCC progression | [30] |
NAFLD, non-alcoholic fatty liver disease; MCD, methionine and choline-deficient; HFD, high-fat diet; NASH, non-alcoholic steatohepatitis; HCC, hepatocellular carcinoma; TG, triglyceride; AST, aspartate transaminase; ALT, alanine transaminase; AKT/mTOR, protein kinase B/mechanistic target of rapamycin kinase.




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download