Abstract
α1-adrenoceptors link via the G-protein Gq/G11 to both Ca2+ entry and release from stores, but may also activate Rho kinase, which causes calcium sensitization. This study aimed to identify the subtype(s) of α1-adrenoceptor involved in Rho kinase-mediated responses in both rat aorta and mouse spleen, tissues in which contractions involve multiple subtypes of α1-adrenoceptor. Tissues were contracted with cumulative concentrations of noradrenaline (NA) in 0.5 log unit increments, before and in the presence of an antagonist or vehicle. Contractions produced by NA in rat aorta are entirely α1-adrenoceptor mediated as they are competitively blocked by prazosin. The α1A-adrenoceptor antagonist RS100329 had low potency in rat aorta. The α1D-adrenoceptor antagonist BMY7378 antagonized contractions in rat aorta in a biphasic manner: low concentrations blocking α1D-adrenoceptors and high concentrations blocking α1B-adrenoceptors. The Rho kinase inhibitor fasudil (10 µM) significantly reduced aortic contractions in terms of maximum response, suggesting inhibition of α1B-adrenoceptor mediated responses. In the mouse spleen, a tissue in which all 3 subtypes of α1-adrenoceptor are involved in contractions to NA, fasudil (3 µM) significantly reduced both early and late components to the NA contraction, the early component involving α1B- and α1D-adrenoceptors, and the late component involving α1B- and α1A-adrenoceptors. This suggests that fasudil inhibits α1B-adrenoceptor mediated responses. It is concluded that α1D- and α1B-adrenoceptors interact in rat aorta and α1D-, α1A- and α1B-adrenoceptors interact in the mouse spleen to produce contractions and these interactions suggest that one of the receptors preferentially activates Rho kinase, most likely the α1B-adrenoceptor.
α1-adrenoceptors link via the G-protein Gq/G11 to both Ca2+ entry and release of Ca2+ from stores [1,2], but may also activate the small GTP binding protein RhoA and thus Rho kinase, which causes calcium sensitization by phosphorylating myosin light-chain phosphatase, reducing its function and so withdrawing an inhibitory input [3-5].
Although calcium sensitization involving Rho kinase is involved in α1-adrenoceptor mediated contractions in at least some tissues in the rat [6,7], the overall role of RhoA/Rho kinase in α1-adrenoceptor mediated contractions has not been established [8]. In rat aorta, contractions to both phenylephrine [9] and angiotensin II [10] involve Rho kinase. In rat tail artery, cooling increases contractions to the α1-adrenoceptor agonist phenylephrine at least partly via Rho kinase [11]. Conversely, in rat aorta, cooling decreases contractions to phenylephrine, involving inhibition of Rho kinase [12]. However, in mouse aorta, contractions to the thromboxane mimetic U46619, but not to phenylephrine involve Rho kinase, so that not all α1-adrenoceptor subtypes are linked to Rho kinase [13]. There is no strong evidence as yet to suggest that a particular α1-adrenoceptor subtype is preferentially linked to Rho kinase in vascular contractions, and whether phasic or tonic contractions rely particularly on this mechanism.
The rat aorta has been widely studied in adrenergic pharmacology as a model blood vessel in investigations of contractile responses, but, surprisingly, there is still uncertainly as to the α-adrenoceptor subtype or subtypes involved: contractions have been reported to involve predominantly α1D-adrenoceptors and possibly also α1B-adrenoceptors [8]. In mouse (and, indeed, rat) spleen, contractions to noradrenaline (NA) involve all subtypes of α1-adrenoceptor in addition to α2-adrenoceptors, and we have recently delineated the various components of this complex response [14,15]. In both rat aorta and mouse spleen, with multiple receptor subtypes present, there is likely to be at least one subtype of α1-adrenoceptor that acts primarily through Rho kinase.
Therefore, the aims of this study were to identify the subtype(s) of α1-adrenoceptor involved in Rho kinase mediated responses in rat aorta and mouse spleen. We first confirmed the biphasic nature, involving two receptor subtypes, of the antagonism by the α1D-adrenoceptor antagonist BMY7378 of contractions to NA in rat aorta, and have studied the role of Rho kinase in α1-adrenoceptor mediated contractions in both rat aorta and mouse spleen. Since both of these tissues contain multiple subtypes of α1-adrenoceptor involved in smooth muscle contractions, we wished to identify a specific subtype of α1-adrenoceptor preferentially linked to Rho Kinase and calcium sensitization. Until now, no specific subtype of α1-adrenoceptor has been preferentially linked to Rho kinase.
Male Wistar rats (230–300 g) and male C57-BL6 mice (18–25 g) were obtained from Envigo. The studies were approved by the Department of Health and Health Products Regulatory Agency (HPRA) in Ireland and by the RCSI Research Ethics Committee, as required. The animals were housed in a controlled environment with a 12-h light, 12-h dark cycle and were fed a standard rat diet. The studies were approved by the Department of Health and Health Products Regulatory Agency (HPRA) (B100/762 and AE 19127/1185) in Ireland and by the RCSI Research Ethics Committee (REC 1188 and 1284).
Animals were killed by overdose of CO2, cervical dislocation and exsanguination. For removal of aorta, the rib cage was opened, the heart and lungs removed, and the vena cava lifted and removed. The descending aorta was then carefully lifted by holding the surrounding connective tissue with forceps, and the connections with the spinal column were cut. The aorta was transferred to a petri dish in Krebs-Henseleit solution (see below), and aortic rings of approximately 3 mm in length were cut with a scalpel. The rings were carefully lifted using fine forceps touching only the connective tissue, and a threaded hook was then placed through the lumen for bath suspension of aortic rings. Aortic rings were mounted between a fine fixed rod, and a transducer (Grass FT03) to which the thread on the hook was attached under 1 g tension in organ baths at 37°C in Krebs-Henseleit solution of the following composition: (mM): NaCl 119; NaHCO3 25; D-glucose 11.1; KCl 4.7; CaCl2 2.5; KH2PO4 1.2; MgSO4 1.0. Cocaine (3 µM) was additionally present to block the NA transporter (NET).
Animals were killed by overdose of CO2, cervical dislocation and exsanguination. The abdomen was opened and whole spleen was manipulated with a probe, gently freed of connections with fine scissors and placed in a petri dish containing Krebs-Henseleit solution of the same composition as above. Using a needle, threads were placed through top and bottom (long thread to top and open knot to bottom) of the spleen and tied loosely in a loop to avoid damage to spleen. Spleens were mounted between a fixed rod and a hook attached to a transducer (Grass FT03) under 0.5 g tension in organ baths at 37°C in Krebs-Henseleit solution as described above. Cocaine (3 µM) was additionally present to block the NA transporter (NET).
Bathing fluid was changed every 15 min, except during concentration response curves. Following 30–45 min equilibration, and 15 min after changing bathing fluid, tissues were contracted with NA (10 µM), and washed. Bathing fluid was again changed every 15 min for the next hour. Tissues were then contracted, beginning 15 min after last changing bathing fluid, with cumulative concentrations of NA in 0.5 log unit increments, beginning with 1 nM (rat aorta) or 10 nM (mouse spleen) up to a maximum of 100 µM. This first, or control, concentration response curve confirmed the responsiveness of tissues, but was not used in calculations. Following a 1 h washout, and 1 h in the presence of antagonist concentration or vehicle, changing bathing fluid every 15 min, concentration response curves to NA were repeated, beginning 15 min after last changing bathing fluid. This second, or test, concentration response curve was used in calculations, comparing the response in the presence of an antagonist concentration with the response in the presence of vehicle. In all studies, a single concentration of antagonist or of vehicle was administered per experiment. For rat aorta, experiments were carried out in 2 groups, resulting in 2 sets of vehicle. Most experiments were carried out utilising vehicle 1, but fasudil experiments were carried out with vehicle 2.
Contractions are expressed as the cumulative maximum obtained to increasing concentrations of agonist. Concentration-response curves were plotted as a % of the maximum response to agonist or as absolute tension (g). NA potency was expressed as a pD2 (pEC50: –log EC50), a pEC25 and a pEC75 (concentrations producing 50%, 25%, or 75% of maximum response, respectively), from data plotted as a % of maximum by non-linear regression, constraining curves to between 0% and 100%, using GraphPad Prism 5.0 for MacIntosh (GraphPad Software).
Antagonist potency was calculated only where the potency of NA was significantly shifted as compared to NA potency in vehicle experiments. Antagonist potency was expressed as an apparent dissociation constant (pKB) from effects of a single antagonist concentration, using the equation pKB = log(DR – 1) – log [B], where [B] is the concentration (M) of antagonist and DR is the NA dose ratio produced by the antagonist, or as a pA2 from the effects of a range of antagonist concentrations. The PA2 was calculated as the x intercept of the Schild plot, plotting antagonist log(DR – 1) for each antagonist concentration, using Graphpad Prism for Macintosh.
BMY7378 (8-[2-(4-(2-methoxyphenyl) piperazin-1-yl)ethyl]-8-azaspiro[4,5]decane-7,9-dione) (Tocris Bioscience); cocaine hydrochloride (Sigma Aldrich); fasudil hydrochloride (Aobious); (-)-noradrenaline bitartrate (Sigma Aldrich); prazosin hydrochloride (Sigma Aldrich); RS100329 (5-methyl-3-[3-[4-[2-(2,2,2,-trifluoroethoxy)phenyl]-1-piperazinyl]propyl]-2,4-(1H)-pyrimidinedione) (Tocris Bioscience). Drugs were dissolved in distilled water.
Values are expressed as mean ± standard error of mean (SEM) from n experiments in brackets. The minimum level for statistical significance was p < 0.05. Differences between groups and vehicle in NA potency or maximum contractile response were compared using the GraphPad Prism programme by Student’s t-test for two groups, or by one way Anova for multiple groups, and, only when Anova showed significance of p < 0.05, with Dunnett test for comparison of anatagonist effects with effects of vehicle or Bonferroni test for comparison of all groups.
NA potently produced contractions of rat aorta in vehicle experiments with maximum contraction of 0.79 ± 0.07 g and pD2 (–logEC50) of 7.08 ± 0.13 (n = 7) (Table 1).
Antagonists and inhibitors did not significantly affect contractions to NA in terms of maximum contraction, except for fasudil (10–5 M), which significantly reduced the maximum contraction (Table 1).
Prazosin (3 × 10–9 M) produced a parallel shift in the potency of NA, with a pKB value of 9.14 (Fig. 1, Table 2). The α1A-adrenoceptor antagonist RS100329 (10–7 M) produced an approximately parallel shift in the response to NA but exhibited low potency in rat aorta, with a pKB of 7.66 (Fig. 1, Table 2), consistent with antagonist actions at α1B- or α1D-adrenoceptors.
The α1D-adrenoceptor selective antagonist BMY7378 had a complex interaction with NA. BMY7378 (10–8 M) showed high potency against contractions to NA, with a pKB of 8.73 for 10–8 M (Fig. 2, Table 2). However no further shift in NA potency was obtained until a concentration of 10–6 M (Fig. 2, Table 2). A Schild plot was constructed and a pA2 of 8.45 and a slope of –0.69 was obtained for BMY7378 (all concentrations), the shallow slope indicating a non-competitive interaction or more than one receptor present. Hence, BMY7378 had two separate potency values, suggesting actions at 2 receptors, high potency at α1D-adrenoceptors (pKB 8.73) and low potency at another adrenoceptor. Since BMY7378 produced log (DR – 1) values of 0.73, 0.75, and 0.63 for 10–8 M, 3 × 10–8 M and 10–7 M, there was an average shift of approximately 0.75 log units by action at α1D-adrenoceptors. However, when only BMY7378 in concentrations of 10–7 M to 10–5 M were considered, and taking intercept as y = 0.75 (to eliminate the α1D-adrenoceptor mediated component), a pA2 of 6.81 with slope of –1.12 was obtained at the second, low affinity, receptor (Fig. 3), and, based on the low potency of RS100329, this is an α1B-adrenoceptor.
Fasudil (3 × 10–6 M) did not significantly reduce the maximum response to NA, but fasudil (10–5 M) markedly reduced the maximum response to NA (Fig. 4). Potency of NA at the pEC50 level was not significantly altered in the presence of fasudil (Table 1). However, fasudil (10–5 M) but not fasudil (3 × 10–6M) significantly shifted the potency of NA at the pEC75 level (vehicle: 6.14 ± 0.22; fasudil 3 × 10–6 M: 5.95 ± 0.18, non-significant; fasudil 10–5 M: 5.35 ± 0.18, p < 0.05; all n = 5).
NA produced contractions of mouse spleen in vehicle experiments with a maximum contraction of 0.113 ± 0.006 g and pD2 (–logEC50) of 7.06 ± 0.16 (n = 4) (Fig. 5). Fasudil (3 × 10–6 M) did not significantly affect the maximum response to NA, with a maximum of 0.107 ± 0.016 g (n = 6) (Fig. 5).
Fasudil (3 × 10–6 M) shifted NA potency in a biphasic manner, with shifts in potency at both low (NA pEC25 values: vehicle, 7.64 ± 0.15, n = 4; fasudil, 7.18 ± 0.12, n = 6; p < 0.05) and high (NA pEC75 values: vehicle, 6.51 ± 0.17, n = 4; fasudil, 5.75 ± 0.22, n = 6; p < 0.05) concentrations of NA (Fig. 5).
The main objectives of this study were to identify components of contractions to NA involving Rho kinase in both rat aorta and mouse spleen and the subtype(s) of α1-adrenoceptor involved. We have recently published a study of the α1-adrenoceptor subtypes involved in components of contractions to NA in mouse spleen [14]. Hence, in the present study, firstly, the components of contractions to NA were investigated in rat aorta. Other authors have reported complex interactions between antagonists and components of the contractions to NA in rat aorta.
It is necessary to consider the selectivities of antagonists used in the present study. BMY7378 has high potency at α1D-adrenoceptors (published results: average of 8.60, –log M), but low potency at α1A- and α1B-adrenoceptors (average of 6.55 and 7.07, respectively) [8]. RS100329 has high potency (average of 9.40) at α1A-adrenoceptors, but low potency (average of around 8.15) at other α1-adrenoceptor subtypes [8]. While relatively non-selective, prazosin has higher potency at the α1B- and α1D-adrenoceptors than the α1A-adrenoceptor [8].
Prazosin has high potency in rat aorta with a pKB of 9.14 in the present study, and high potency in a range of published studies [8]. Since prazosin in increasing concentrations produces concentration-dependent parallel shifts in NA potency with no evidence of a resistant component [8], this suggests that all receptors involved in contractions to NA in rat aorta are α1-adrenoceptors. Moreover, this also suggests that prazosin has similar potency at all receptors present, and that these receptors must therefore be α1B- or α1D-adrenoceptors, or both [8].
BMY7378 in low concentrations (10–8 M) produced shifts in the potency of NA in rat aorta with a pKB value of 8.73, but it took concentrations of 10–6 M and above to produce further shifts in NA potency. Since low concentrations of BMY7378 produced a shift in NA potency of approximately 0.75 log units, a baseline of 0.75 units was taken on the y-axis, and a second potency level with a pA2 of 6.81 was obtained for high concentrations of BMY7378. BMY7378 also showed two potency levels against the agonist phenylephrine in rat aorta [16]. Hence, it is clear that BMY7378 acts with high affinity at an α1D-adrenoceptor and with low affinity at a second receptor, and this is most likely to be an α1B-adrenoceptor since the low potency of RS100329 (pKB of 7.66) would rule out an α1A-adrenoceptor.
In rat aorta, chloroethylclonidine, an agent that alkylates particularly α1B-adrenoceptors, makes isometric contractions to NA biphasic [17]. This effect also suggests that α1B-adrenoceptors mediate a component of contractions to NA in rat aorta.
In rat aorta, the Rho kinase inhibitor fasudil greatly reduced the contractile response to NA, reducing the maximum response with little effect on NA potency, demonstrating a largely non-competitive interaction, except that the highest concentration of fasudil shifted the pEC75 for NA. Fasudil has previously been shown to relax contractions to the Rho kinase activator NaF, as well as phenylephrine, in rat aorta [9]. These marked actions of fasudil suggest that the receptor linked to Rho kinase is probably the α1B-adrenoceptor, since the relatively small α1D-adrenoceptor mediated component to the response occurs predominantly to low concentrations of NA.
In mouse spleen, contractile responses to low concentrations of NA involve α1B-, α1D-, and α2A-adrenoceptors, and to high concentrations of NA, responses involve α1B- and α2A-adrenoceptors and an α1A-adrenoceptor mediated component [14]. The responses to both low and high concentrations of NA were significantly inhibited by fasudil. In contrast, the α1D-adrenoceptor antagonist BMY7378 shifted potency of NA only at low NA concentrations and the α1A-adrenoceptor antagonist RS100329 shifted potency of NA only at high NA concentrations [14]. Hence, fasudil appears to block both the α1A- and α1D-adrenoceptor mediated components, or to block the α1B-adrenoceptor mediated component which occurs at both low and high concentrations of NA. In rat portal vein, contractions are mediated by both α1A- and α1B-adrenoceptors, with no evidence for α1D-adrenoceptors, and the mainly α1B-adrenoceptor mediated tonic contraction involves Rho kinase [18]. The evidence links particularly α1B-adrenoceptors to Rho kinase, with α1D-, and possibly α1A-adrenoceptor mediated responses, being affected by interactions at the post-receptor level.
Rat aorta expresses all 3 α1-adrenoceptors (proportion of mRNA: α1D 79.1%; α1B 5.6%; α1A 15.3%) [19], and similar expression was true for mouse aorta (proportion of mRNA: α1D 72.9%; α1B 19.8%; α1A 7.3%) [20] and human aorta (proportion of mRNA: α1D 59%; α1B 30%; α1A 11%) [21]. Mouse spleen and human spleen express all 3 subtypes [21-23]. Hence, both rat aorta and mouse spleen express all 3 subtypes of α1-adrenoceptor, but the proportion of each subtype expressed would be unlikely to match the components of the contractile response, due to uncertainly as to function of expressed receptors, differences in coupling to contraction and even affinity of NA for the receptor.
In a recent review of α1-adrenoceptor subtypes, it was suggested that a specific α1-adrenoceptor subtype may be linked to Rho kinase, and although the α1B-adrenoceptor looked the most likely suspect, the evidence was not sufficient to identify the α1B-adrenoceptor as linked to Rho kinase [8]. Studies in whole blood vessels or organs are much more problematic that those in cell lines or membranes, so that, to our knowledge, this is the first study that identifies the α1-adrenoceptor preferentially linked to Rho kinase mediated contractions in intact smooth muscle. Furthermore, this study suggests that the involvement of multiple subtypes of α1-adrenoceptor in smooth muscle contractions may be more widespread than previously thought.
In conclusion, in the mouse spleen and rat aorta, multiple subtypes of α1-adrenoceptor are involved in contractions to NA (spleen: α1A-, α1B-, and α1D-adrenoceptors; aorta: α1B- and α1D-adrenoceptors). In both these tissues and in rat portal vein (α1A- and α1B-adrenoceptors) [18], a component of contractions to NA is sensitive to fasudil and so involves Rho kinase. Combining all these findings and comparing effects of fasudil with effects of subtype selective α1-adrenoceptor antagonists, it would seem most likely that α1B-adrenoceptors are preferentially involved in Rho kinase mediated responses. Further work is necessary to confirm this novel suggestion that α1B-adrenoceptors are particularly linked to Rho kinase in smooth muscle.
REFERENCES
1. Harraz OF, Jensen LJ. 2021; Vascular calcium signalling and ageing. J Physiol. 599:5361–5377. DOI: 10.1113/JP280950. PMID: 34705288. PMCID: PMC9002240.

2. Fan G, Cui Y, Gollasch M, Kassmann M. 2019; Elementary calcium signaling in arterial smooth muscle. Channels (Austin). 13:505–519. DOI: 10.1080/19336950.2019.1688910. PMID: 31797713. PMCID: PMC6930021.

3. Shimokawa H, Sunamura S, Satoh K. 2016; RhoA/Rho-kinase in the cardiovascular system. Circ Res. 118:352–366. DOI: 10.1161/CIRCRESAHA.115.306532. PMID: 26838319.

4. Szasz T, Webb RC. 2017; Rho-mancing to sensitize calcium signaling for contraction in the vasculature: role of Rho kinase. Adv Pharmacol. 78:303–322. DOI: 10.1016/bs.apha.2016.09.001. PMID: 28212799.
5. Nunes KP, Webb RC. 2021; New insights into RhoA/Rho-kinase signaling: a key regulator of vascular contraction. Small GTPases. 12:458–469. DOI: 10.1080/21541248.2020.1822721. PMID: 32970516. PMCID: PMC8583239.

6. Ergul M, Turgut NH, Sarac B, Altun A, Yildirim Ş, Bagcivan I. 2016; Investigating the effects of the Rho-kinase enzyme inhibitors AS1892802 and fasudil hydrochloride on the contractions of isolated pregnant rat myometrium. Eur J Obstet Gynecol Reprod Biol. 202:45–50. DOI: 10.1016/j.ejogrb.2016.04.031. PMID: 27160814.

7. Mochalov SV, Tarasova NV, Kudryashova TV, Gaynullina DK, Kalenchuk VU, Borovik AS, Vorotnikov AV, Tarasova OS, Schubert R. 2018; Higher Ca2+ -sensitivity of arterial contraction in 1-week-old rats is due to a greater Rho-kinase activity. Acta Physiol (Oxf). 223:e13044. DOI: 10.1111/apha.13044. PMID: 29383848.
8. Docherty JR. 2019; The pharmacology of α1-adrenoceptor subtypes. Eur J Pharmacol. 855:305–320. DOI: 10.1016/j.ejphar.2019.04.047. PMID: 31067439.
9. Ahmed A, Fusi F, Valoti M. 2022; Perivascular adipose tissue modulates the effects of flavonoids on rat aorta rings: role of superoxide anion and β3 receptors. Pharmacol Res. 180:106231. DOI: 10.1016/j.phrs.2022.106231. PMID: 35462011.
10. Terada Y, Yayama K. 2021; Angiotensin II-induced vasoconstriction via Rho kinase activation in pressure-overloaded rat thoracic aortas. Biomolecules. 11:1076. DOI: 10.3390/biom11081076. PMID: 34439742. PMCID: PMC8391281. PMID: 34165df9e2394e6d9c78fa28973d958a.

11. Ishida H, Saito SY, Hishinuma E, Kitayama T, Ishikawa T. 2018; Differential contribution of calcium channels to α1-adrenoceptor-mediated contraction is responsible for diverse responses to cooling between rat tail and iliac arteries. Eur J Pharmacol. 826:9–16. DOI: 10.1016/j.ejphar.2018.02.023. PMID: 29458039.

12. Chung YH, Oh KW, Kim ST, Park ES, Je HD, Yoon HJ, Sohn UD, Jeong JH, La HO. 2018; Hypothermia inhibits endothelium-independent vascular contractility via Rho-kinase inhibition. Biomol Ther (Seoul). 26:139–145. DOI: 10.4062/biomolther.2016.233. PMID: 28208012. PMCID: PMC5839492.

13. Malacarne PF, Bezzenberger J, Lopez M, Warwick T, Müller N, Brandes RP, Rezende F. 2022; Epoxyeicosatrienoic acid and prostanoid crosstalk at the receptor and intracellular signaling levels to maintain vascular tone. Int J Mol Sci. 23:5939. DOI: 10.3390/ijms23115939. PMID: 35682616. PMCID: PMC9180422. PMID: 8c36df0c07b74a5e9bb249e648c8008b.

14. Alsufyani HA, Daly C, Docherty JR. 2021; Interaction between α1B - and other α1 - and α2 -adrenoceptors in producing contractions of mouse spleen. Basic Clin Pharmacol Toxicol. 129:416–426. DOI: 10.1111/bcpt.13639. PMID: 34383990.

15. Alsufyani HA, McCormick PA, Docherty JR. 2021; Both α1B- and α1A-adrenoceptor subtypes are involved in contractions of rat spleen. Pharmacol Rep. 73:255–260. DOI: 10.1007/s43440-020-00118-x. PMID: 32860192.

16. Hussain MB, Marshall I. 1997; Characterization of α1-adrenoceptor subtypes mediating contractions to phenylephrine in rat thoracic aorta, mesenteric artery and pulmonary artery. Br J Pharmacol. 122:849–858. DOI: 10.1038/sj.bjp.0701461. PMID: 9384500. PMCID: PMC1565016.

17. O’Rourke M, Gavin K, Docherty JR. 1997; Further investigation of the alpha-adrenoceptor-mediated actions of chloroethylclonidine in rat aorta. Eur J Pharmacol. 336:37–42. DOI: 10.1016/S0014-2999(97)01257-0. PMID: 9384252.
18. Alsufyani HA, Docherty JR. 2021; Involvement of G proteins and Rho kinase in α1-adrenoceptor mediated contractions of the rat portal vein. Can J Physiol Pharmacol. 99:654–659. DOI: 10.1139/cjpp-2020-0347. PMID: 33096009.

19. Martí D, Miquel R, Ziani K, Gisbert R, Ivorra MD, Anselmi E, Moreno L, Villagrasa V, Barettino D, D’Ocon P. 2005; Correlation between mRNA levels and functional role of α1-adrenoceptor subtypes in arteries: evidence of alpha1L as a functional isoform of the α1A-adrenoceptor. Am J Physiol Heart Circ Physiol. 289:H1923–H1932. DOI: 10.1152/ajpheart.00288.2005. PMID: 15951348.
20. Hosoda C, Tanoue A, Shibano M, Tanaka Y, Hiroyama M, Koshimizu TA, Cotecchia S, Kitamura T, Tsujimoto G, Koike K. 2005; Correlation between vasoconstrictor roles and mRNA expression of α1-adrenoceptor subtypes in blood vessels of genetically engineered mice. Br J Pharmacol. 146:456–466. DOI: 10.1038/sj.bjp.0706325. PMID: 16113694. PMCID: PMC1576278.

21. Weinberg DH, Trivedi P, Tan CP, Mitra S, Perkins-Barrow A, Borkowski D, Strader CD, Bayne M. 1994; Cloning, expression and characterization of human alpha adrenergic receptors alpha 1a, α1B and α1c. Biochem Biophys Res Commun. 201:1296–1304. DOI: 10.1006/bbrc.1994.1845. PMID: 8024574.

22. Cavalli A, Lattion AL, Hummler E, Nenniger M, Pedrazzini T, Aubert JF, Michel MC, Yang M, Lembo G, Vecchione C, Mostardini M, Schmidt A, Beermann F, Cotecchia S. 1997; Decreased blood pressure response in mice deficient of the α1B-adrenergic receptor. Proc Natl Acad Sci U S A. 94:11589–11594. DOI: 10.1073/pnas.94.21.11589. PMID: 9326654. PMCID: PMC23548.

23. Tanoue A, Nasa Y, Koshimizu T, Shinoura H, Oshikawa S, Kawai T, Sunada S, Takeo S, Tsujimoto G. 2002; The α1D-adrenergic receptor directly regulates arterial blood pressure via vasoconstriction. J Clin Invest. 109:765–775. DOI: 10.1172/JCI200214001. PMID: 11901185. PMCID: PMC150908.

Fig. 1
Concentration-response curves to noradrenaline (NA) obtained in rat aorta in the presence of vehicle, the relatively non-selective antagonist prazosin (3 × 10–9 M), the α1D-adrenoceptor antagonist BMY7378 (10–8 M) and the α1A-adrenoceptor antagonist RS100329 (10–7 M).
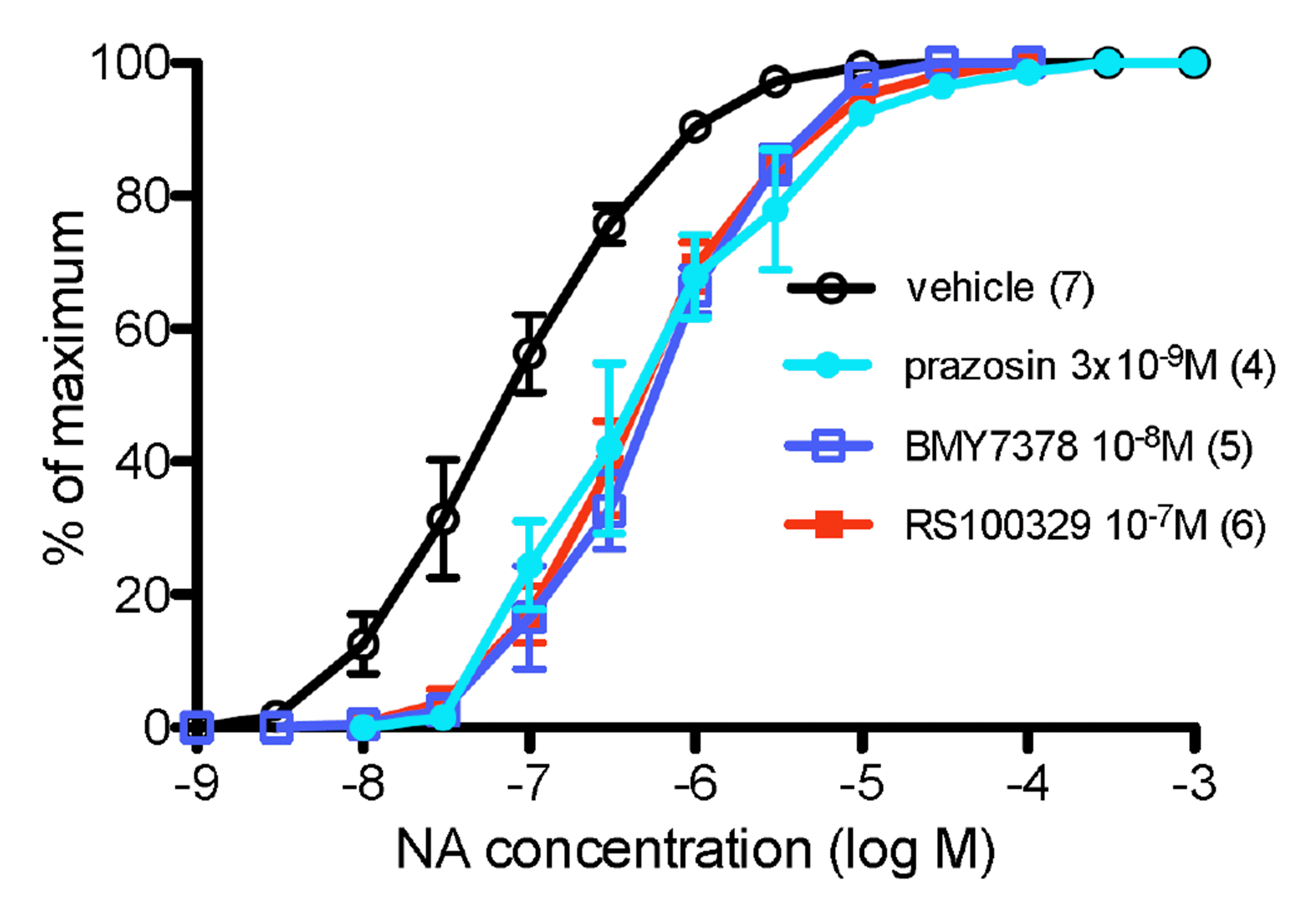
Fig. 2
Concentration-response curves to noradrenaline (NA) obtained in rat aorta in the presence of vehicle and a range of concentrations of the α1D-adrenoceptor antagonist BMY7378: 10–8 M, 3 × 10–8 M, 10–7 M, 3 × 10–7 M, 10–6 M, 10–5 M.
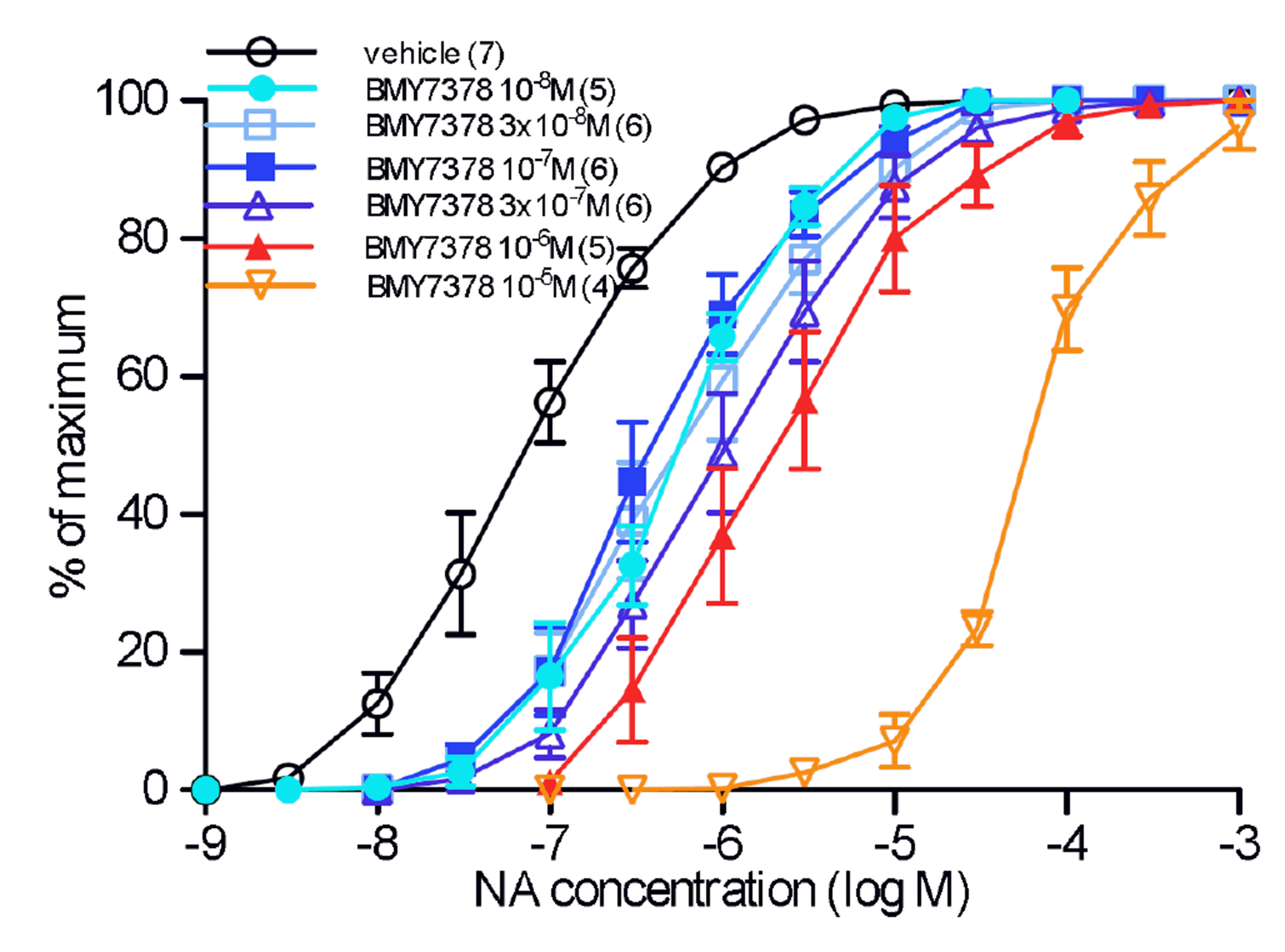
Fig. 3
Schild plots obtained for the α1D-adrenoceptor antagonist BMY7378 against contractions to noradrenaline (NA) in rat aorta.
The pA2 value is the concentration of antagonist at y = 0 (utilising all data points, both black and red; text in black). A second apparent pA2 value is shown for the concentration of BMY7378 at y = 0.75 (utilising only red data points; text in red).
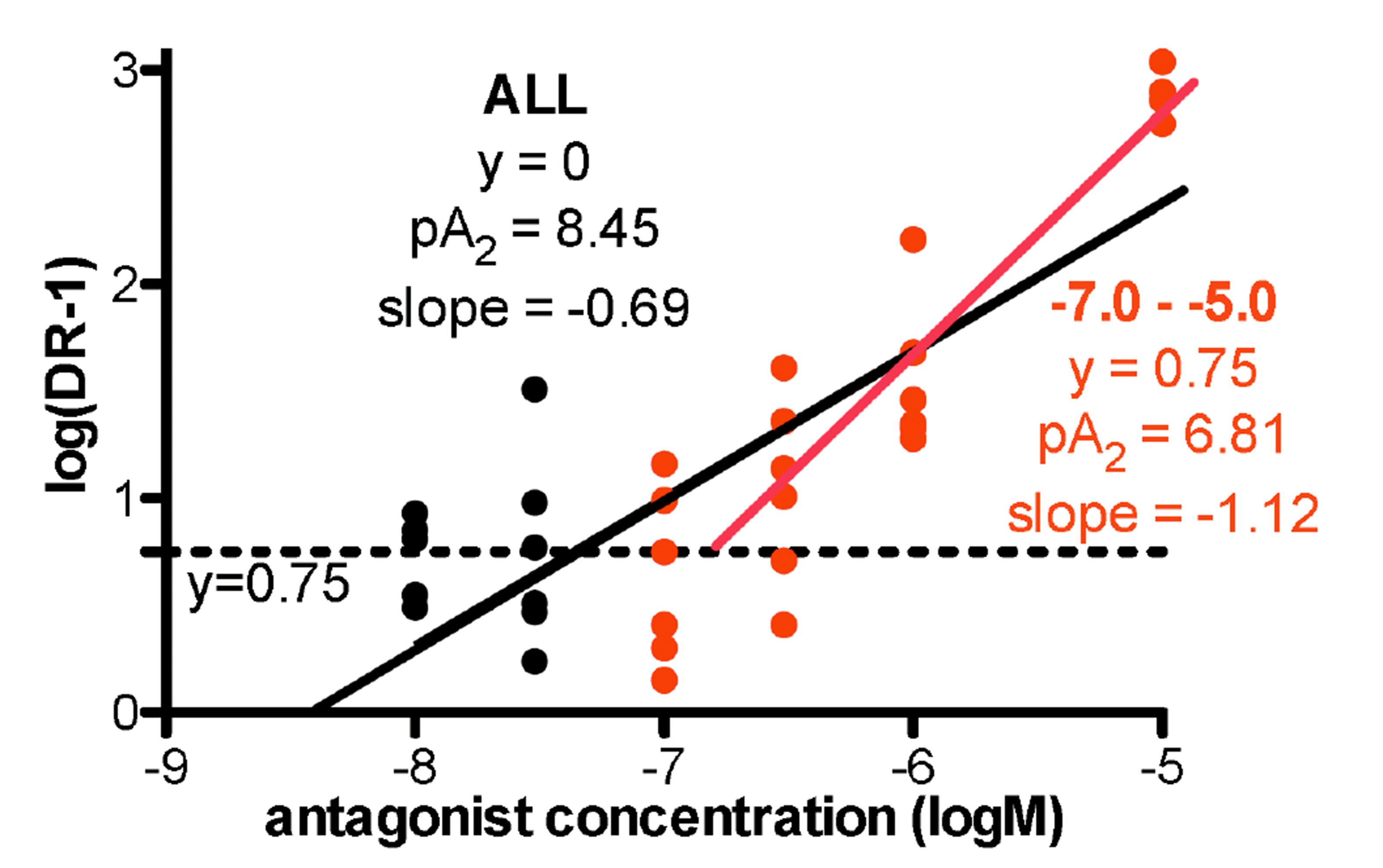
Fig. 4
Concentration-response curves to noradrenaline (NA) obtained in rat aorta in the presence of vehicle and the Rho kinase inhibitor fasudil (3 × 10–6 M and 10–5 M).
Values are mean ± SEM from 5 experiments. For NA potency calculations, see Table 1.
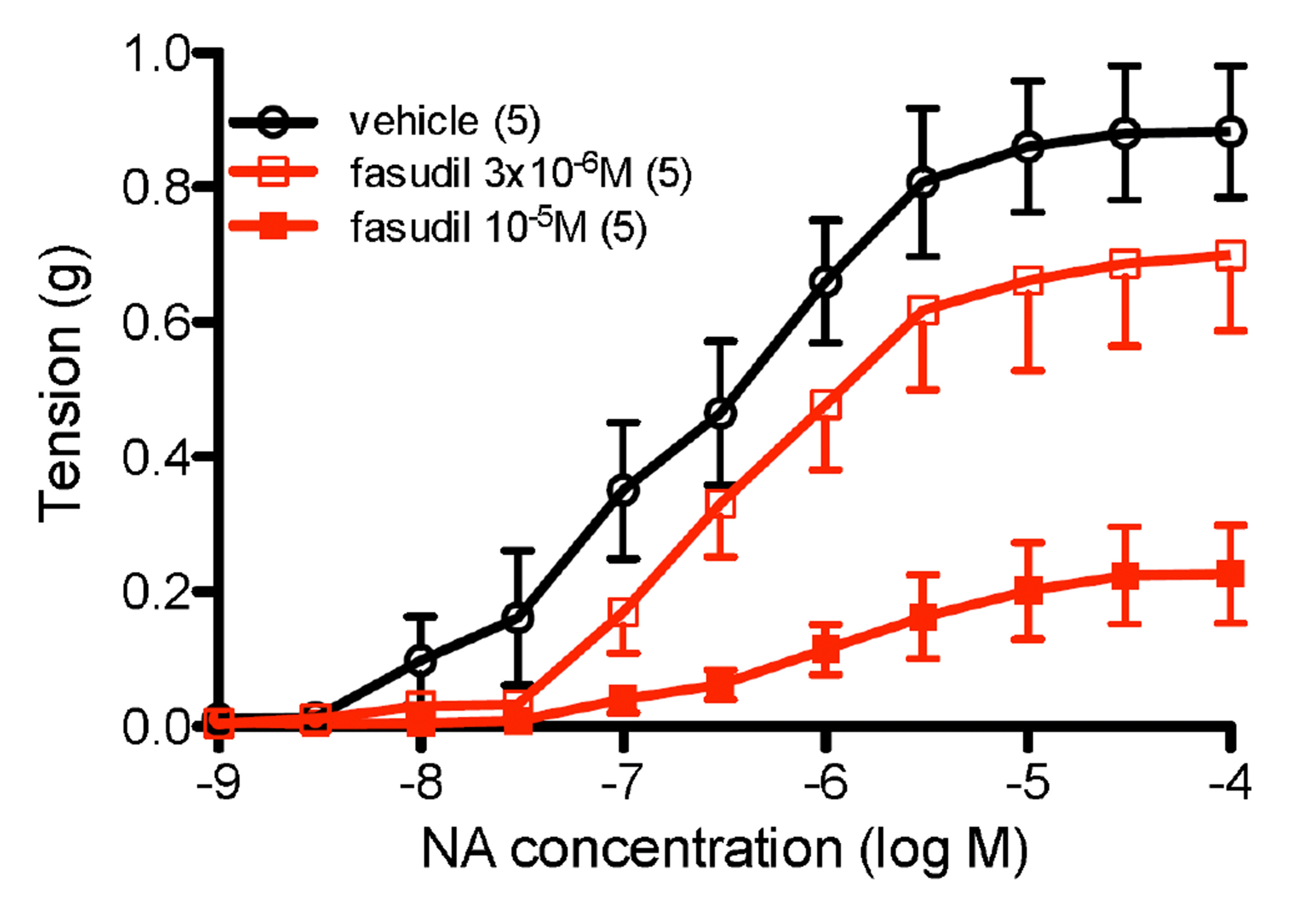
Fig. 5
Concentration-response curves to noradrenaline (NA) obtained in mouse spleen in the presence of vehicle and the Rho kinase inhibitor fasudil (3 × 10–6 M).
Values are mean ± SEM from 4–6 experiments. For NA potency calculations, see Results.
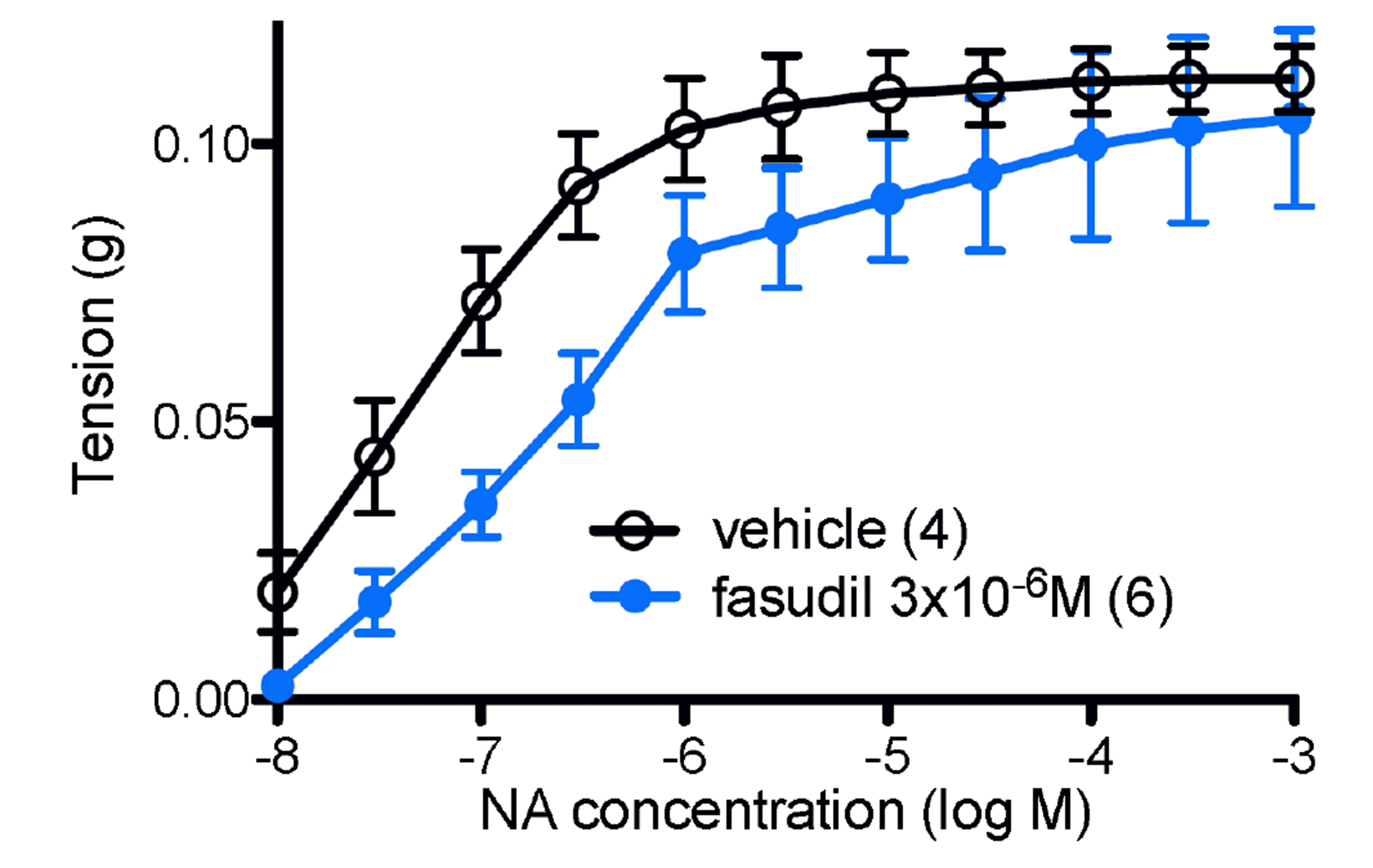
Table 1
Effects of antagonists on concentration response curves to noradrenaline (NA) in rat aorta in terms of maximum contraction (g) (NA T [g]) and NA potency (NA pEC50, –log M)
Table 2
Antagonist pKB (–log M) values by antagonist concentration in rat aorta




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download