TO THE EDITOR: Allogeneic hematopoietic stem cell transplantation (allo-HSCT) is an effective and curative treatment for hematologic diseases. However, graft-versus-host disease (GvHD) is a major barrier to the success of allo-HSCT. Gastrointestinal (GI) tract is one of the frequently diagnosed GvHD target organs. Patients with GI-GvHD show poor prognosis and response rates to first-line corticosteroid treatment compared to those without GI-GvHD, indicating that GI-GvHD may be a major cause of non-relapse mortality [1]. Numerous studies demonstrated that GI-GvHD is associated with a significant change in the intestinal microbiota and antimicrobial functions of immune cells against pathogenic microorganisms after allo-HSCT [2-5]. In addition, a clinical study reported that patients undergoing donor fecal microbiota transplantation improved GvHD symptoms and survival rate at week 24 after transplantation by increasing intestinal microbial diversity, especially a microbial metabolite butyrate-producing Clostridiales [2]. Likewise, a non-absorbed broad-spectrum oral antibiotic, rifaximin, is associated with a decrease in Enterococcus species, which accompanies the domination of Clostridiales, improving the overall survival of patients after allo-HSCT [3]. Furthermore, in vivo administration of butyrate or high-butyrate-producing bacteria Clostridium cluster IV (Clostridium leptum group) decreased GI-GvHD while increasing the survival of allo-HSCT recipients [4]. In a mouse model of GvHD, Lactobacillales elimination from flora before allo-HSCT aggravated GvHD, whereas Lactobacillus-colonization showed significant protection of GvHD [5]. Since lactose availability is important for enterococcus growth, lactose-free diet showed inhibition of their growth, thereby reducing GvHD in mice [6]. In addition, allo-HSCT patients with lactose-nonabsorber genotypes had higher enterococcus domination compared to those with lactose-absorber genotypes due to increased luminal availability of lactose as a growth substrate, showing a trend toward increased cumulative incidence of acute GvHD grade 2 to 4 [6]. All of these studies suggest that alteration of the intestinal microbial composition is involved in the pathogenesis of GI GvHD.
Our previous studies demonstrated that the blockade of interferon-gamma receptor (IFNGR) signaling in donor T cells reduced GvHD while preserving graft-versus-leukemia (GvL) effects [7-9]. In addition, we recently demonstrated for the first time that S100A9 is upregulated by IFNGR signaling blockade in both murine and human T cells and functions as a novel GvHD-suppressor effector molecule without compromising GvL effects of donor T cells [10]. We observed that mice transplanted with S100a9-overexpressing T cells resulted in a significant improvement in overall survival and suppression of GvHD as seen in the recipient mice transplanted with Ifngr1-/- T cells [7, 10]. Of note, in vivo oral administration of recombinant murine S100A9 recapitulated the GvHD prevention effect of S100a9-overexpressing T cells and Ifngr1-/- T cells [10]. Although S100A9 is known to be an antimicrobial peptide, the effect of overexpression of S100A9 in donor T cells on the modulation of microbiota after allo-HSCT has not been investigated. Therefore, we examine whether Ifngr1-/- T cells reduce GvHD through the alteration of intestinal microbiota by overexpressing S100a9.
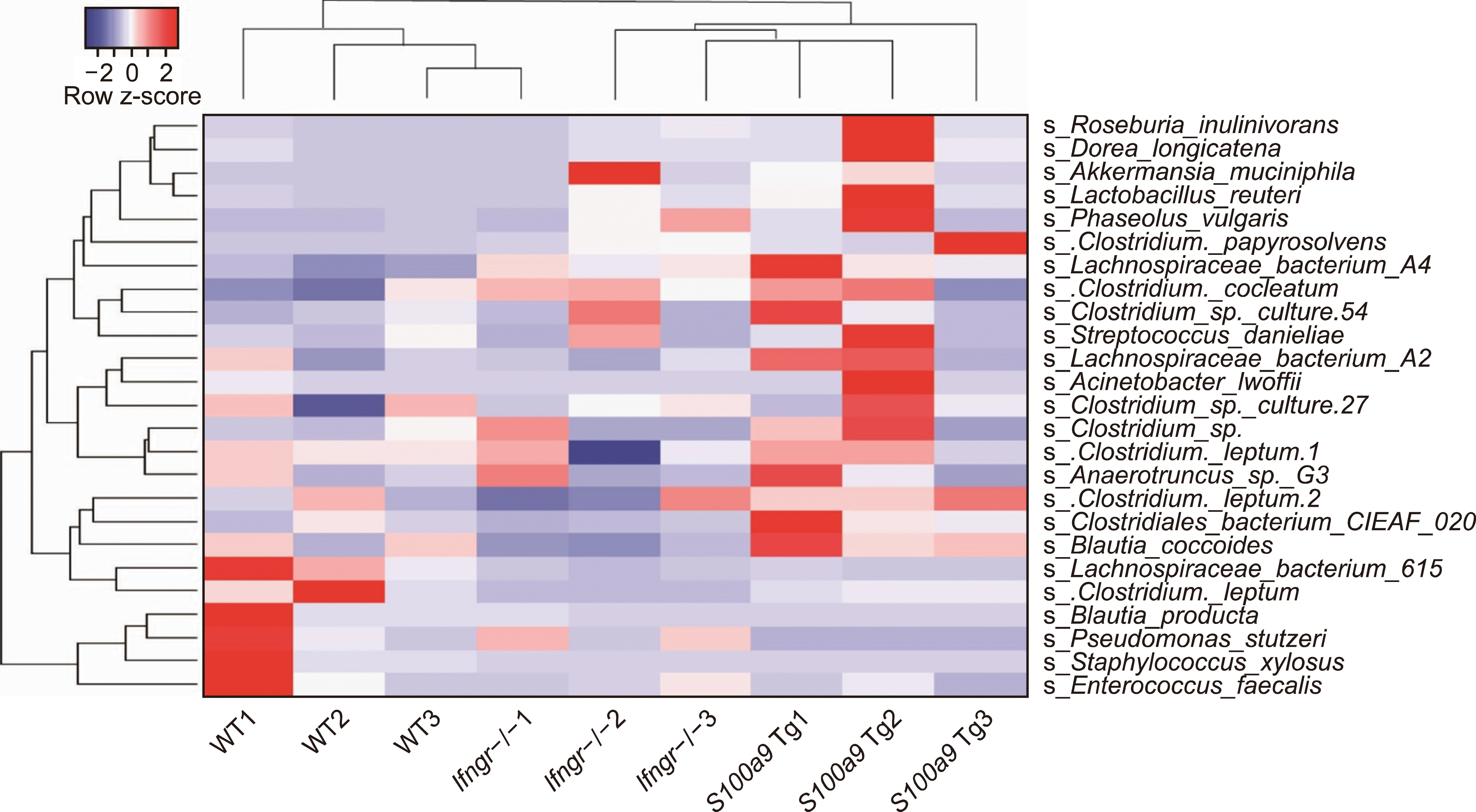
Verrucomicrobia has been shown to increase FOXP3, the master transcription factor of regulatory T cells (Tregs) in the ileal compartment of horses [11], implying the potential immune suppressive effect of Verrucomicrobia on GvHD. In addition, severe GvHD is associated with a decrease in butyrate levels or butyrate-producing bacteria such as Ruminococcaceae and Eryperlotrichaceae families [4, 12]. A high abundance of Ruminococcaceae is also positively correlated with an increased ratio of Treg/Th17 in allo-HSCT recipients [13]. Erysipelatoclostridium genus in the Erysipelotrichaceae family can serve as a negative prognostic biomarker of GvHD as seen in the anticorrelation between its abundance and degree of GvHD in patients [4]. Akkermansia muciniphila species is recently introduced as a next-generation beneficial microbe in the gut, enhancing intestinal barrier function through their outer membrane protein Amuc-1100-induced TLR2 activation of intestinal epithelial cells in obese and diabetic mice [14]. In contrast, the domination of Enterococcus genus and Enterococcus faecalis species has been shown to be associated with a high risk of multidrug-resistant infections and severe GvHD in preclinical and clinical studies [4, 6]. Interestingly, Blautia was increased in mice transplanted with WT T cells compared to those with Ifngr1-/- and S100a9-overexpressing T cells (Fig. 1C) despite its negative association with lethal GvHD. Given that multiple species of butyrate-producing probiotics such as Clostridium difficile and Clostridium butyricum have a negative correlation with the abundant of multiple species of Blautia [15], it is conceivable that the abundance of Blautia genus might be affected by butyrate-producing bacteria Ruminococcaceae and Eryperlotrichaceae families that are highly increased in mice transplanted with Ifngr1-/- and S100a9-overexpressing T cells (Fig. 1C). Furthermore, mice transplanted with WT T cells showed an increase in Eubacterium coprostanoligenes (Fig. 1C), a beneficial bacteria known to reduce cholesterol. Even though Eubacterium coprostanoligenes are associated with healthy aging, there has been no correlation reported between these bacteria and GvHD. However, the overall microbial composition found in mice transplanted with Ifngr1-/- and S100a9-overexpressing T cells (Fig. 1) has been shown to be associated with less GvHD in preclinical and clinical studies [2-4, 6, 11-13, 15]. In addition, we found that the mice transplanted with Ifngr1-/- T cells share a similar intestinal bacterial community at the species level with those transplanted with S100a9-overexpressing T cells but not the mice receiving WT T cells (Fig. 2).
We recently demonstrated that oral administration of murine recombinant S100A9 homodimers (mrS100A9) or S100A8/A9 heterodimers (mrS100A8/A9), both of which are functional forms of S100A9, suppresses GvHD in our mouse model of allo-HSCT [10]. Furthermore, S100A9 upregulation by in vivo administration of anti-human IFNGR antibodies significantly improves the overall survival of immune-deficient NOD/SCID/γcnull (NSG) mice transplanted with human peripheral blood mononuclear cells (PBMCs) [10]. In the current study, we examined if systemic administration of the recombinant proteins via intraperitoneal (i.p.) injection, which is a preferred route for therapeutic agents to avoid the GI tract, can also mitigate GvHD. Two independent allo-HSCT models were used; B6 to Balb/c murine GvHD model with mrS100A8/A9 proteins (Supplementary Fig. 2A) and human PBMCs to NSG mice with human recombinant S100A8/A9 proteins (hrS100A8/A9) (Supplementary Fig. 2B). We found that systemic administration (i.p.) of S100A8/A9 recombinant proteins failed to reduce GvHD in both GvHD models (Supplementary Fig. 2). These results further suggest that maximizing bioavailability or local effect of S100A9 in the GI tract is essential for GvHD reduction by modulating intestinal microbiota.
In this study, we report that mice transplanted with Ifngr1-/- or S100a9-overexpressing T cells have a similar intestinal microbiota composition after allo-HSCT. Our studies suggest that S100A9 released by Ifngr1-/- or S100a9-overexpressing T cells contributes to GvHD prevention by modulating intestinal microbiota reconstitution after allo-HSCT. Our findings provide not only significant insights into the mechanism by which blocking IFNGR signaling modulates intestinal microbiota and reduces GvHD but also a potential novel therapeutic strategy with recombinant S100A9 proteins for the prevention or treatment of GvHD in patients undergoing allo-HSCT.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download