TO THE EDITOR: Mixed phenotype acute leukemia (MPAL) is rare, accounting for approximately 1–4% of acute leukemia (AL) cases [1]. Induction therapy for these cases is often based on acute lymphocytic leukemia (ALL) treatment protocols, followed by allogeneic stem cell transplantation (ASCT). However, post-ASCT relapse after ASCT remains the primary cause of treatment failure. The median overall survival (OS) of patients who relapse after ASCT is extremely poor, with a median 3-year survival rate ranging between 9% and 11% [2]. Recent studies have reported better survival rates with a combination of venetoclax (VEN) and hypomethylating agents (HMA). Here, we describe the case of a 36-year-old patient with B/T MPAL who developed medullary relapse after 2 ASCTs and was successfully treated with VEN and azacitidine (VEN-AZA).
CASE
A 36-year-old male was diagnosed with MPAL in 2015. Bone marrow analysis of the blast population revealed heterogeneous blasts (57%). Immunophenotyping by flow cytometry (FC) revealed the expression of B, T, and myeloid markers (CD45 low, HLA-DR+, CD33+, CD13+, CD38+, CD11b+, cCD3 low, CD7+, CD79a+, CD19+, CD71+, and cCD41). According to the scoring system of the European Group for Immunological Classification of Leukemias (EGIL), it was assigned to the B lineage (EGIL=3) and T lineage (EGIL=2.5), but not the myeloid lineage (EGIL=2). Conventional cytogenetic and fluorescence in situ hybridization analyses revealed hyperdiploidy. No specific targeted mutations were found in next-generation sequencing (FLT3-ITD, NPM1, CEBPA, WT1, TP53, IDH1, and IDH2 mutations, and BCR-ABL rearrangements were negative). Clonal rearrangement of the immunoglobulin H locus was negative. Two low-intensity TCR rearrangements were detected in a polyclonal background. Morphological and cytogenetic remission was achieved after induction therapy with daunorubicin, cytarabine, and dexamethasone. After consolidation therapy with cytarabine 9 g/m2, the patient underwent ASCT from an HLA-identical sibling donor. The measurable residual disease (MRD) status, determined by FC before ASCT, was negative (0.2×10-4). The conditioning regimen consisted of busulfan IV (12.8 mg/kg) and cyclophosphamide (120 mg/kg). The donor was the patient’s 44-year-old brother. The graft source was peripheral blood stem cells (PBSC) with minor ABO incompatibility. Graft-versus-host disease (GVHD) prophylaxis was ensured with cyclosporine and a short course of methotrexate. Three months post-ASCT, the patient was still in complete remission (CR). Extensive GVHD occurred on day+136 and was treated with corticosteroids for 10 months, with progressive tapering of cyclosporine levels within 20 months. Late medullary relapse occurred 52 months after the ASCT. Immunophenotyping of the bone marrow blasts using FC revealed positivity for CD19+, CD13+, CD7+, CD3+, CD33+, and CD34+ cells. Karyotype analysis revealed complex abnormalities (chromosomes 1, 5, 11, 12, and 14). The patient was treated according to the ALL protocol (GRAALL 05). The induction therapy was complicated by pulmonary aspergillosis, which was treated with voriconazole for 19 months. A second ASCT was performed using PBSC from the same donor after 4 consolidation cycles. The MRD status before the second ASCT was positive (3×10-4). Conditioning consisted of once daily fractionated total body irradiation with a total dose of 6 Gy (2 Gy per day in 3 fractions) and melphalan (100 mg/m2). GVHD prophylaxis was ensured using cyclosporine and a short course of methotrexate. At day+18, the patient developed grade II acute gut GVHD. Twelve months later, he developed a second medullary relapse with immunophenotyping (CD13+, cMPO+ low, CD14-, CD117, CD20-, CD19+, cCD79a+, cIgM-, cCD3+, CD2-, CD7+, CD1a-, CD4-, and CD8-) and a complex karyotype. Next-generation sequencing revealed mutations in KRAS, SH2B3, and PHF6. The patient initially received 3 cycles of vincristine and methylprednisolone with no morphological remission. VEN-AZA was initiated 2 months after relapse at these doses.
Vidaza 75 mg/m2 was administered subcutaneously once daily (D1 to D5) with VEN 100 mg on day 1, 200 mg on day 2, 300 mg on day 3, and 400 mg once daily (days 4–11); the dose was then reduced to 100 mg once daily with the introduction of voriconazole. Morphologic remission was achieved after 1 cycle with MRD (1.7×10-4) and a normal karyotype. The second cycle was delayed, and the VEN dose was reduced to 50% because of hepatic cytolysis. After improvement, treatment was resumed with a 29-day delay and a reduced dose of VEN. VEN-AZA was discontinued for 10 weeks due to persistent deep thrombocytopenia. During the second cycle, the patient developed a urinary infection due to Klebsiella pneumoniae treated with imipenem. The MRD status was assessed 41 days after the second cycle of AZA and 32 days after the interruption of VEN; it was initially raised (6×10-4) but turned negative (0.1×10-4) on D+61. The third, fourth, fifth, and sixth cycles were administered with the total dose of VEN. Currently, the patient is still receiving VEN-AZA at doses of 75 mg/m2 and 400 mg once daily. The patient is still in CR with a negative MRD status of 10-4 and has no treatment-related complications (Fig. 1).
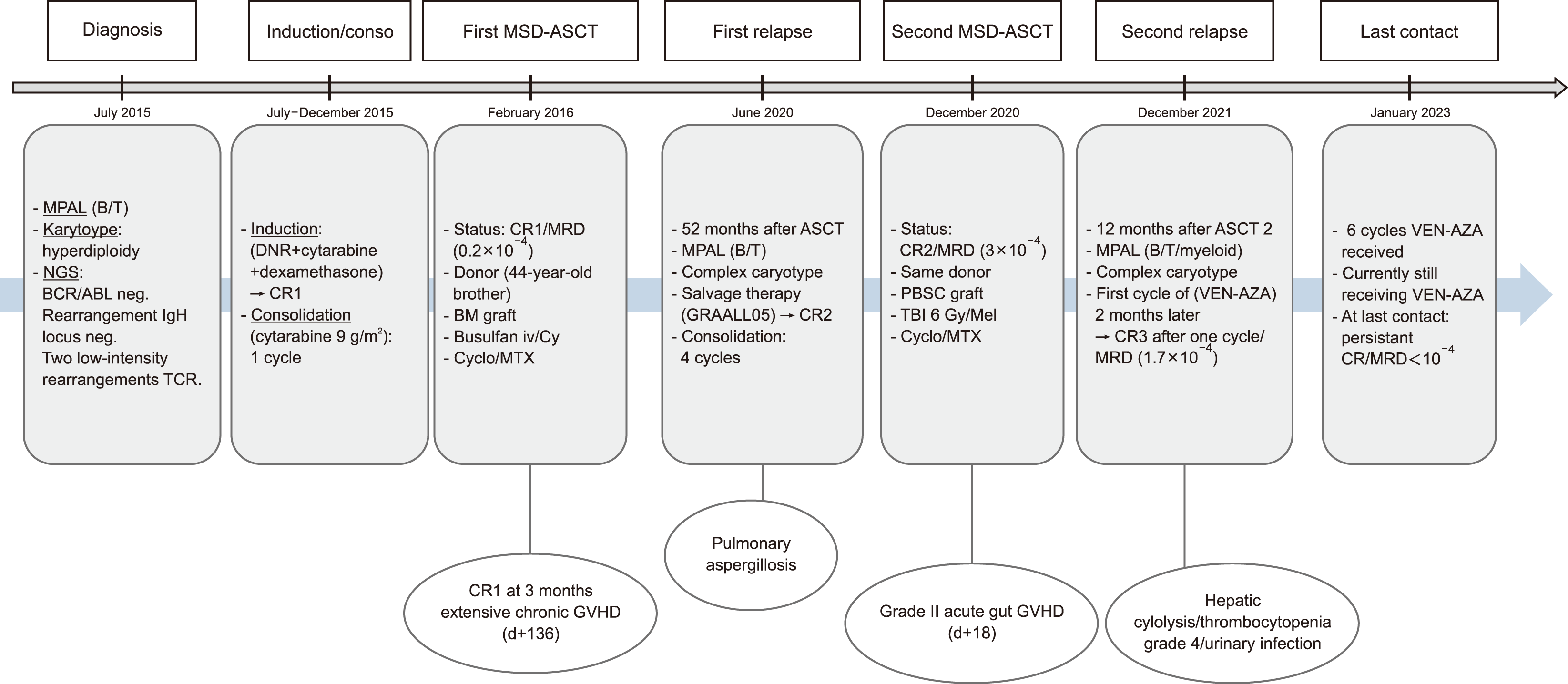 | Fig. 1Summarize of the course of the patient’s treatment.
Abbreviations: ASCT, allogeneic stem cell transplantation; AZA, Azacitidine; BM, bone marrow; Conso, consolidation; CR, complete remission; Cy, cyclophosphamide; Cyclo, cyclosporine; DNR, daunorubicin; GVHD, graft versus host disease; IgH, immunoglobulin heavy; Mel, melphalan; MPAL, mixed phenotype acute leukemia; MRD, measurable residual disease; MSD, matched sibling donor; MTX, methotrexate; NGS, next-generation sequencing; PBSC, peripheral blood stem cells; TBI, total body irradiation; TCR, T-cell receptor; VEN, Venetoclax.
|
DISCUSSION
Relapse is a major factor affecting survival after post-ASCT in patients with MPAL. Here, we have reported the case of a 36-year-old patient with B/T MPAL who was successfully treated with salvage VEN-AZA after relapse 1 year after a second ASCT. The patient was still in CR with a negative MRD at 2 months after 6 cycles of VEN-AZA. Thus, VEN combined with AZA appears to be a highly effective treatment for patients with poor-risk AML [3]. However, the prognosis after salvage therapy with this combination in patients with relapsed AML or MPAL post-ASCT remains poor. Both drugs demonstrate single-target activity against AL, resulting in high CR rates in newly diagnosed and relapsed patients. Schuler et al. [4] reported an overall response rate (ORR) of 47%. The ORR was 86% in the first-salvage patients and 35% in the later-salvage patients. The use of VEN in relapsed undifferentiated leukemia is particularly effective because of the overexpression of BCL-2 in these aggressive forms [5, 6]. Fleischmann et al. [7] reported that VEN-HMA remains an optimal approach for newly diagnosed AML that is not eligible for intensive chemotherapy, regardless of the cytogenetic or molecular risk. In this study, after a median follow-up of 11.5 months (range, 6.1–22.3), the median OS from the start of VEN treatment was 13.3, 5, and 4 months for first-line, subsequent-line treatment, and at relapse post-ASCT, respectively. The ORR was 42.8% after relapsing post-ASCT. Responses are generally quick, with a median time to CR of 1 month. MRD-negative status after treatment predicts better OS [8]. Wang et al. [9] reported 3 patients with refractory MPAL who were successfully treated with VEN as a single agent. Another case report detailed the successful eradication of T/myeloid MPAL relapse after ASCT with VEN plus decitabine. A second ASCT from a second donor was subsequently performed, and the patient remained without evidence of disease for more than 1 year [10]. Many case reports have described the combination of VEN-HMA and MPAL with remarkable responses. In a retrospective study of 26 patients with AML who relapsed after ASCT and were treated with VEN-AZA and donor lymphocyte infusion, the median event-free survival and OS were 120 and 284.5 days, respectively [11]. In terms of toxicity, the most common adverse effects were grade III–IV hematological toxicity and grade III–IV nausea and vomiting as non-hematological toxicities. No major bleeding or serious infections were experienced [12, 13]. No clear recommendations regarding the dose and duration of VEN therapy have been made; however, patients on voriconazole should receive 25% of the VEN dose [14].
CONCLUSION
In this report, we have described the efficacy of VEN-AZA for the treatment of refractory B/T MPAL.
VEN-AZA is a potential therapeutic option for MPAL relapse after ASCT, particularly in patients who are not candidates for targeted therapy.
Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download