TO THE EDITOR: An eight-month-old child presented with a fever for one month with no localizing features. Upon examination, hepatosplenomegaly and lymphadenopathy were observed. A complete blood count revealed anemia (hemoglobin, 75 g/L), thrombocytopenia (10×109/L), and leukocytosis (312×109/L). A peripheral blood smear revealed 46% blasts and 25% monocytes (Fig. 1A, B). Cytochemistry revealed that the blasts were negative for myeloperoxidase (MPO) staining. By 10-color flow cytometry (Beckman Coulter Navios Ex, USA; all antibodies from Beckman Coulter), 45% of the viable cells were observed in the precursor region. These cells were positive (cutoff of 20% for surface markers and 10% for cytoplasmic markers) for CD19, CD38, cytoplasmicCD79a, CD81, and HLA-DR. They were negative for CD10, CD20, CD34, myeloid (CD13, CD15, MPO), and T cell (CD2, cytoplasmicCD3, surfaceCD3, CD4, CD5, CD7, and CD8) markers. Nearly 40% of the cells were observed in the monocyte window of the CD45 versus the side scatter plot. These cells were positive for CD7 (dim), CD14, CD13 (dim to negative), CD33, CD36, CD56 (subset), CD64, CD86, and HLA-DR and negative for CD34, B cells, and other T cell markers (Fig. 2, only selected markers are shown in the image).
The co-occurrence of B-lineage blasts with abnormal monocytes (indicated by abnormal immunophenotype – dim CD13, dim CD7, and CD56 in the subset) suggested mixed phenotype acute leukemia (MPAL, B/monocytic). However, the possibility of B-lineage acute lymphoblastic leukemia with reactive/nonclonal monocytosis (due to infection or alternate medications) could not be completely excluded. Fluorescence in situ hybridization (FISH) for BCR::ABL1, ETV6::RUNX1 translocations and ZNF384, TCF3, and KMT2A rearrangements (Metaystems, Germany or Zytovision, Germany) revealed KMT2A rearrangement in nearly 90% of the cells. To confirm the involvement of both B cells and monocytes in the leukemic process, immunomagnetic sorting of bone marrow aspirate samples was performed using anti-CD19 antibodies (Stem Cell Technology, Canada). Both positively and negatively selected samples were fixed and tested again by FISH using KMT2A break-apart probes. Rearrangement of KMT2A was observed in both CD19 positive (90%) and CD19 negative (90%) populations, establishing a diagnosis of bilineage MPAL-B/monocytes with KMT2A rearrangement (Fig. 1C, D). Induction-phase chemotherapy with prophase oral prednisolone was initiated. However, the child developed severe pneumonia and pulmonary hemorrhage, and succumbed to his illness.
MPAL belongs to a heterogeneous group of rare leukemias, accounting for 1–3% of leukemia cases worldwide [1]. Diagnosis is based on the combined expression of lymphoid, B- or T-, and myeloid markers, potentially showing B/myeloid, T/myeloid, and rarely B/T or B/T/myeloid commitment [2]. Various sets of diagnostic criteria have been established, including the European Group for the Immunological Characterization of Leukemias (EGIL) and the World Health Organization (WHO) 2022 classification system, which are increasingly being utilized for MPAL diagnosis [3]. Currently, immunophenotyping using flow cytometry is the central pillar of MPAL diagnosis. Although this helps in most cases, diagnostic difficulties are encountered in a subset of cases.
The B/monocytic type of MPAL is rarely encountered among all acute leukemias of ambiguous lineage, with only case reports and small series in the literature [4]. Currently, its diagnosis requires the demonstration of either bright CD19 and one or more B lineage antigens to define the B lineage, that is, CD10, cytoplasmicCD22, CD22, CD79a, or dim CD19, and at least two of the other B antigens along with more than one monocyte-associated antigen (CD11c, CD14, CD36, CD64, or lysozyme), or a diffuse positivity for non-specific esterase/NSE in cytochemistry analysis [2, 3]. The patterns of immunophenotype in monocytes in the literature include CD11c, CD14, CD33, the immaturity marker CD34, and B lymphoid-associated markers, namely CD19 and cytoplasmic CD79a [5-7].
Diagnosis is not difficult if monocytes co-express B-lineage or immature markers. The presence of abnormal maturation patterns also helps confirm that they are part of the leukemic process [5]. However, a dilemma arises when the percentage of monocytes is low or immunophenotypically normal, or when limited flow cytometry panels do not allow for detailed immunophenotypic characterization of monocytes. The current diagnostic criteria do not consider the possibility of reactive monocytosis in B or T acute lymphoblastic leukemia (ALL) [8, 9]. The absence of definite cut-offs in the criteria for the percentage of the monocyte population adds to this problem. CD10 negativity in B-lineage blasts with monocytosis suggests the possibility of an underlying KMT2A rearrangement but fails to distinguish between B-ALL with KMT2A rearrangement and B-monocytic MPAL with KMT2A rearrangement [7].
Here, we report a case of bilineage MPAL-B/monocytes, confirmed by the presence of KMT2A-r in B cell and non-B cell populations. Since MPALs have poorer outcomes than pure B or T acute lymphoblastic leukemias/ALLs, it is important to use the available tools efficiently to separate B or T ALLs from MPAL. Distinguishing between B- or T-ALLs and MPAL is also important for assessing minimal residual disease during follow-up [10]. Our approach may be attempted in suspected cases of bilineage/multilineage acute leukemia with well-defined genomic driver lesions. In suspected acute leukemia of ambiguous lineage, the demonstration of KMT2A, BCR::ABL1, ZNF384, BCL11B translocations, or genetic abnormalities in immunosorted cell populations can be used as an alternative efficient method to support the diagnosis of MPAL.
REFERENCES
1. Batra S, Ross AJ. 2021; Pediatric mixed-phenotype acute leukemia: what's new? Cancers (Basel). 13:4658. DOI: 10.3390/cancers13184658. PMID: 34572885. PMCID: PMC8469808. PMID: c218fe799bd64544a9f2b444b811bb79.

2. Hammond WA, Advani P, Ketterling RP, Van Dyke D, Foran JM, Jiang L. 2018; Biphenotypic acute leukemia versus myeloid antigen-positive ALL: clinical relevance of WHO criteria for mixed phenotype acute leukemia. Case Rep Hematol. 2018:7456378. DOI: 10.1155/2018/7456378. PMID: 30140473. PMCID: PMC6081595. PMID: 23995debc8a248129a8fcd32a98a821d.

3. Khoury JD, Solary E, Abla O, et al. 2022; The 5th edition of the World Health Organization Classification of haematolymphoid tumours: myeloid and histiocytic/dendritic neoplasms. Leukemia. 36:1703–19. DOI: 10.1038/s41375-022-01613-1. PMID: 35732831. PMCID: PMC9252913.

4. Kim HN, Hur M, Kim H, et al. 2016; First case of biphenotypic/bilineal (B/myeloid, B/monocytic) mixed phenotype acute leukemia with t(9;22)(q34;q11.2);BCR-ABL1. Ann Clin Lab Sci. 46:435–8.
5. Szpecht D, Skalska-Sadowska J, Michniewicz B, et al. 2018; A newborn with congenital mixed phenotype acute leukemia with complex translocation t(10;11)(p12;q23) with KMT2A/MLLT10 rearranged - a report of an extremely rare case. Cent Eur J Immunol. 43:346–52. DOI: 10.5114/ceji.2018.80056. PMID: adfa5830fb64411eac8c6fd6fa23eaf9.

6. Seetharam S, Thankamony P, Gopakumar KG, et al. 2021; Outcomes of pediatric mixed phenotype acute leukemia treated with lymphoid directed therapy: analysis of an institutional series from India. Pediatr Hematol Oncol. 38:358–66. DOI: 10.1080/08880018.2020.1871453. PMID: 33635170.

7. Lou Z, Zhang CC, Tirado CA, et al. 2010; Infantile mixed phenotype acute leukemia (bilineal and biphenotypic) with t(10;11) (p12;q23);MLL-MLLT10. Leuk Res. 34:1107–9. DOI: 10.1016/j.leukres.2010.02.029. PMID: 20299091.
8. Khera R, Gautam P, Gupta S, Arora P, Singh T, Gupta N. 2014; Adult T lymphoblastic leukemia (pro-T ALL) with reactive monocytosis: a case report. Indian J Hematol Blood Transfus. 30:29–33. DOI: 10.1007/s12288-012-0179-2. PMID: 24554818. PMCID: PMC3921325.

9. Lee Y, Chittezhath M, André V, et al. 2012; Protumoral role of monocytes in human B-cell precursor acute lymphoblastic leukemia: involvement of the chemokine CXCL10. Blood. 119:227–37. DOI: 10.1182/blood-2011-06-357442. PMID: 22058116.

10. Alexander TB, Orgel E. 2021; Mixed phenotype acute leukemia: current approaches to diagnosis and treatment. Curr Oncol Rep. 23:22. DOI: 10.1007/s11912-020-01010-w. PMID: 33544265.

Fig. 1
(A) Peripheral blood smear showing leucocytosis (May-Grünwald-Giemsa stain, ×200). (B) A peri-pheral blood smear showing leukemic blasts (black arrow) and mono-cytosis (blue arrow) (May-Grünwald-Giemsa stain, ×1,000). (C) Fluore-scent in situ hybridization from CD19 negative immune-sorted population reveals KMT2A re-arrangement based on the XL LSI KMT2A dual-color break-apart probe in 90% of cells. (D) Fluorescent in situ hybridization of CD19 positive immune-sorted population revealed KMT2A rearrangement using the XL LSI KMT2A dual-color break-apart probe in 90% of cells.
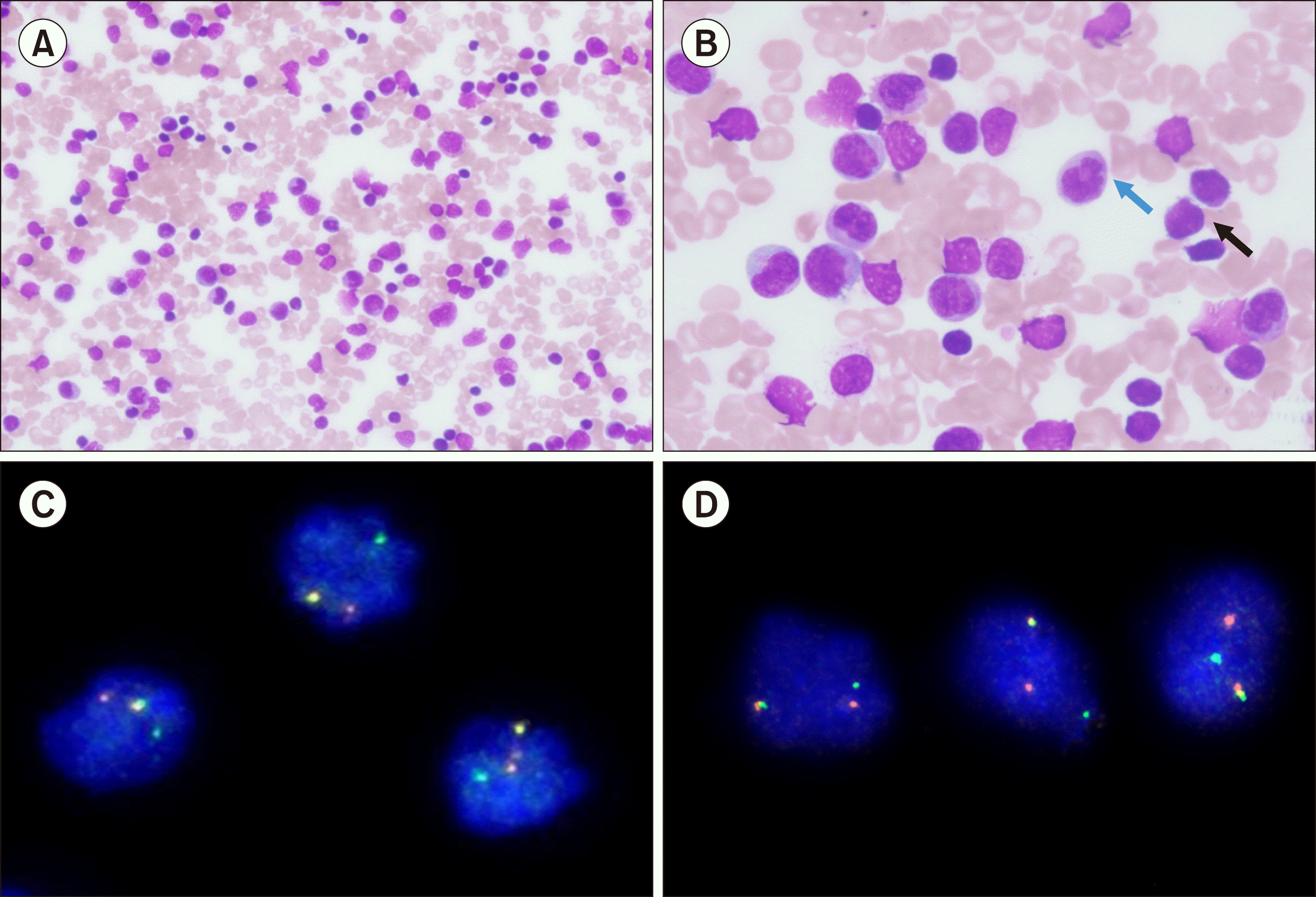
Fig. 2
Immunophenotyping by flow cytometry; 45% progenitor cells (blue color coded) show positivity for CD19, CD38, cytoplasmic CD79a, and HLA-DR. They are negative for CD10, CD20, CD34, CD22, myeloid, and T cell markers (not shown), confirming B-lineage leukemic blasts. Nearly 40% of the cells (magenta color-coded) were observed in the monocyte window of the CD45 versus the side scatter plot. These cells showed moderate CD45 expression and dim-to-moderate-sided scatter. These cells were positive for CD14, CD33, CD36, CD64, CD86, HLA-DR, CD7 (dim), and CD56 (subset) but negative for CD34, B cells, and other T cell markers (not shown).




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download