TO THE EDITOR: Early T-cell precursor acute lymphoblastic leukemia (ETP-ALL) arises from early thymic progenitor cells that migrate from the bone marrow to the thymus and have the potential to differentiate into myeloid/dendritic or T cells. Gene expression profiling has revealed that ETP cells share similarities with hematopoietic stem cells and myeloid progenitor cells. ETP leukemic cells do not express CD1a, CD8, and CD5 (negative to dim). Instead, these cells express ≥1 stem cell/myeloid markers. However, in addition to other ETP-ALL diagnostic criteria, near-ETP-ALL usually shows brighter CD5 [1]. In the literature, the data on ETP-ALL varies markedly, ranging from outcomes poorer than those of other T-ALL to comparable outcomes, with complete remission (CR) rates ranging from 70% to more than 90% [2-5]. Combination chemotherapy is the mainstay treatment. ETP-ALL represents a high-risk subtype of ALL. These outcomes highlight the need for alternative therapeutic approaches that are prognosis-based, and ideally, aiming for minimal residual disease (MRD)-negative remission, which may help in preventing relapse.
Here, we present the results of a retrospective study on ETP/near-ETP-ALL in the Department of Hemato-Oncology of a dedicated cancer hospital in North India.
We identified consecutive patients who were newly diagnosed with ETP/near-ETP-ALL between January 2016 and December 2021 at our center. The diagnosis of ETP/near-ETP-ALL was based on ≥20% blasts in the pre-treatment peripheral blood and/or bone marrow specimens, which on multi-parameter flow-cytometry showed the ETP/near-ETP-ALL immunophenotype. This includes: 1) absent (<5% positive cells) CD1a and CD8 expression, 2) absent or dim (<75% positive cells) CD5 expression, and 3) expression (>25% positive cells) of 1 or more myeloid (e.g., CD11b, CD13, CD33, or CD117) or stem cell (e.g., CD34 or HLA-DR) markers. Leukemia that expressed brighter or more uniform CD5, but otherwise met the criteria for ETP-ALL was defined as near-ETP-ALL. Patients with mixed-phenotype acute leukemia, such as T-myeloid leukemia, were excluded.
Inclusion criterion: Newly diagnosed cases of ETP/near-ETP-ALL.
Exclusion criteria: 1. Relapsed or refractory ALL. 2. Mixed phenotype acute leukemia. 3. Secondary ALL.
Chemotherapy was given as per the Children’s Oncology Group (COG) regimen in patients aged ≤40 years, or the UKALL-14 regimen for those >40 years. CR was defined as ≤5% bone marrow blasts, no extramedullary leukemia, neutrophil count of ≤1.0×109/L, and platelet count of ≤100×109/L. Response assessments were performed after induction and after further phases of chemotherapy until the best response was achieved. MRD was assessed using multiparameter flow cytometry, with a sensitivity of 0.01% in remission BM samples. Allogenic stem cell transplantation (allo-SCT) was performed for all patients who achieved CR. All baseline features and outcomes were recorded in an e-case record form and analyzed statistically. Ethical approval was obtained from the institutional review board.
The Kaplan-Meier method was used to assess overall survival (OS) and progression-free survival (PFS). The OS was calculated as the time from the date of diagnosis to the date of the last follow-up or death from any cause. PFS was calculated from the date of diagnosis to the date of the last follow-up or disease progression. Descriptive and survival analyses were performed using R 4.0.3 version (R core Team, 2020. R: Language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL: https://www.R-project.org/).
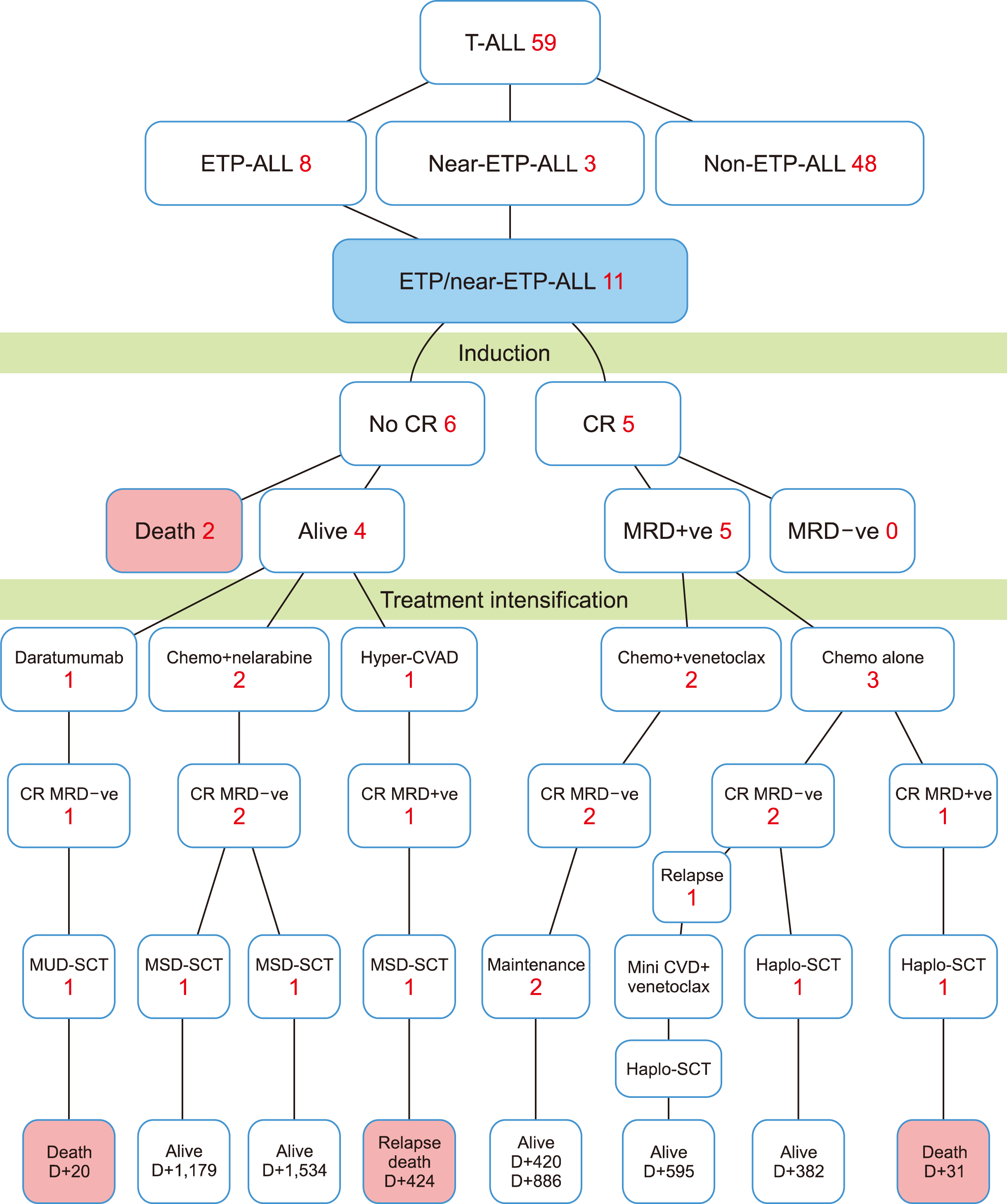
Of the 59 patients with newly diagnosed T-cell ALL, who received chemotherapy, during the study period, eight ETP-ALL (73%) and three near-ETP-ALL (27%) (total N=11, 19%) were identified. The median age was 35 years (24–61 yr), and there were nine men (82%) and two women (18%) (Table 1). One patient (9%) had a mediastinal mass. Conversely, CNS or other extramedullary involvement was not detected in any patients at baseline. The median white blood cell (WBC) count was 3.4×109/L (0.42–183.39). Five patients (45%) had normal cytogenetics, one (9%) demonstrated hyperdiploidy (48 XY, trisomy 4 and 15), and five (45%) did not have cytogenetics data (45%). Next-generation sequencing (NGS) was not performed. Upfront chemotherapy was administered according to the COG and UK-ALL-14 regimens, in nine (82%) and two (18%) patients, respectively (Table 1).
Baseline patient parameters, treatment, and results.
Five patients (45%) achieved CR following induction, although none achieved an MRD-negative status (Table 1). There was no induction mortality, but two patients (18%) died following the induction phase due to primary refractory disease.
Nine patients proceeded to the next phase of therapy including four patients with primary refractory disease. The regimens during the consolidation phase were: conventional chemotherapy alone (N=3), chemotherapy along with venetoclax (N=2; one patient received venetoclax 400 mg for 28 days while the other patient received venetoclax 400 mg for 4 days), chemotherapy (COG0232 protocol-based consolidation containing cyclophosphamide and cytarabine blocks for 8 wk) along with nelarabine (N=2), intensification with daratumumab monotherapy (N=1; 8 doses of daratumumab at 1,000 mg/dose), and Hyper-CVAD regimen (N=1). After consolidation/intensification, all patients achieved CR, with seven of nine (78%) patients achieving MRD-negative remission.
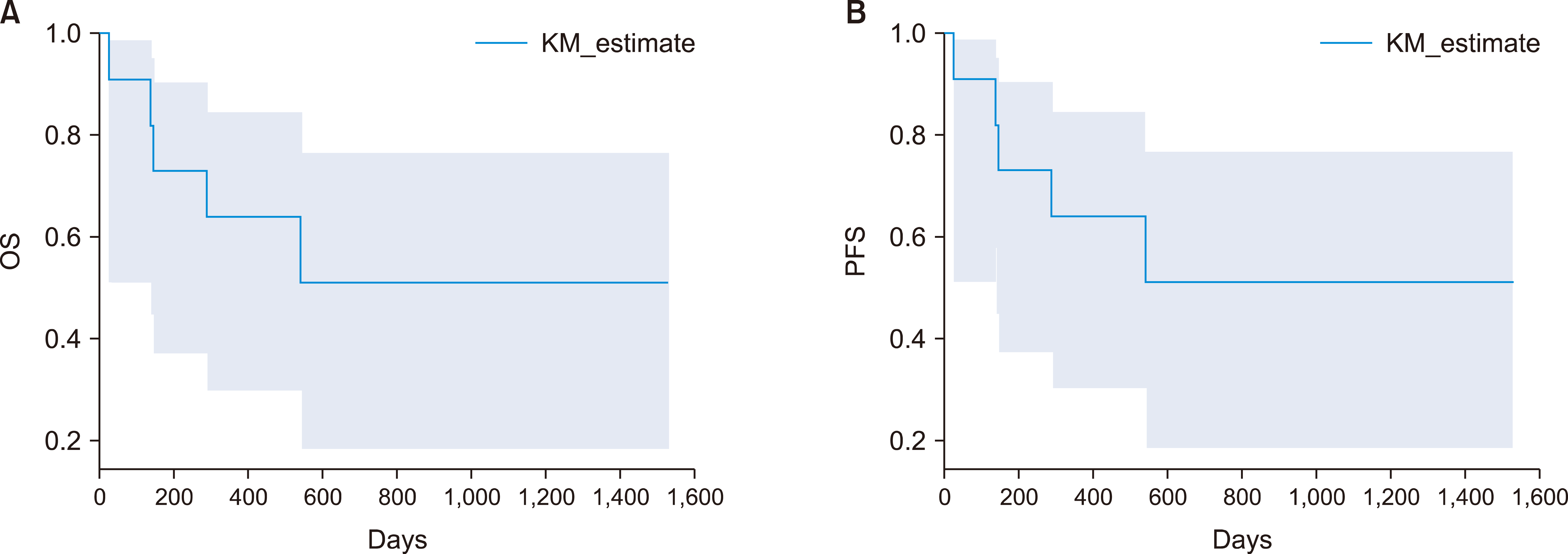
Seven patients underwent allo-SCT (MSD=3, MUD=1, Haplo=3). Three patients died (non-relapsed mortality after SCT, N=2; relapse-related death after SCT, N=1). Patients with post-transplant relapse (N=1) had significant pre-transplant MRD. Six of 11 patients (54.5%) survived, including four post-allo-SCT and two on chemotherapy. Five patients (45.5%) survived their first complete remission at a median follow-up of 13 months (Fig. 1). The estimate of the three-year OS and PFS was 50.9%, respectively. The median OS and PFS were not reached (Fig. 2).
The incidence of ETP-ALL among all T-ALL ranges from 17% (Jain et al. [2]) to 47% (Zhang et al. [3]). In our study, most of the patients presented with lower initial WBC count (<100×109/L). This finding concurred with Jain et al. [2]. Moreover, Jain et al. reported a higher incidence of clonal cytogenetic abnormalities [2].
In our study, the CR rate after four weeks of induction was 45%, with no MRD negativity. Previously reported CR rates were 68% by Jain et al. [2] and 64% by Zhang et al. [3]. The lack of adequate cytogenetics and baseline molecular data, as well as the small sample size, may have contributed to these lower rates. None of the patients in our study were MRD-negative following induction. In previously published data from our center, we cited how daratumumab-based early treatment intensification led to an MRD-negative status, which served as interim management before allo-SCT [4]. Bond et al. [5] and Jain et al. [2] reported post-induction MRD rates of 71% and 70%, respectively, in patients with ETP-ALL. However, MRD did not influence the outcomes of patients with ETP-ALL in either study.
All patients in our study achieved CR after consolidation chemotherapy, with or without intensification, and 78% attained MRD-negative remission.
According to a study by the COG, despite a higher MRD-positive status following induction in patients with ETP-ALL, their long-term outcomes matched those of non-ETP patients, and the 5-year event-free survival (EFS) and OS were 87% and 93%, respectively [6]. These were attributed to treatment intensification of the MRD-positive patients [6].
In our study, 63% of the patients underwent allo-SCT in CR1/CR2. In contrast, the allo-SCT rate was 15% in Jain et al. [2] 24% in Zhang et al. [3] and 48.9% in Bond et al. [5]. In these studies, response-based treatment intensification was performed using allo-SCT.
The relapse rate in our study was 18%, whereas Jain et al. reported 10% [2]. In our study, the estimated 3-year OS and PFS were 50.9%, respectively, with the median OS and median PFS not reached. Conversely, outcomes reported by Jain et al. [2] included a median OS of 20 months, while Bond et al. [5] determined a 5-yr-OS of 59.6%, and Zhang et al. [3] demonstrated a 2-yr-OS of 40.7% (Fig. 2).
After treatment intensification in nine patients, seven (78%) achieved MRD-negative CR in this study. As all of our patients who were refractory or MRD-positive post-induction received treatment intensification until they achieved MRD-negative CR status, a comparative analysis could not be performed with a control arm. In the future, a study with a larger sample size to verify our results is warranted.
In conclusion, ETP/near-ETP-ALL represents a high-risk subtype of ALL with a high rate of induction failure. The end-of-induction MRD assessment and early response-based treatment intensification with the addition of novel agents to obtain a deeper response can improve long-term treatment outcomes. Additionally, the use of novel agents for upfront treatment during induction may open new possibilities. The role of allo-SCT in ETP-ALL is still not clearly defined and requires further larger/multicenter studies.
Go to : 
Notes
Authors’ Disclosures of Potential Conflicts of Interest
No potential conflicts of interest relevant to this article were reported.
Go to : 
REFERENCES
1. Borowitz MJ, Chan JKC, Downing JR, Le Beau MM, Arber DA. 2017; Precursor lymphoid neoplasms. In: Swerdlow SH, Campo E, Harris NL, et al, eds. WHO classification of tumours of haematopoietic and lymphoid tissues. 4th ed. Lyon, France:. IARC. 199–213. DOI: 10.53347/rid-9250.
2. Jain N, Lamb AV, O'Brien S, et al. 2016; Early T-cell precursor acute lymphoblastic leukemia/lymphoma (ETP-ALL/LBL) in adolescents and adults: a high-risk subtype. Blood. 127:1863–9. DOI: 10.1182/blood-2015-08-661702. PMID: 26747249. PMCID: PMC4915808.

3. Zhang Y, Qian JJ, Zhou YL, et al. 2020; Comparison of early T-cell precursor and non-ETP subtypes among 122 Chinese adults with acute lymphoblastic leukemia. Front Oncol. 10:1423. DOI: 10.3389/fonc.2020.01423. PMID: 32974153. PMCID: PMC7473208. PMID: 04fa1d45900b4e4a84fe67e26e49d3d1.

4. Mirgh S, Ahmed R, Agrawal N, et al. 2019; Will daratumumab be the next game changer in early thymic precursor-acute lymphoblastic leukaemia? Br J Haematol. 187:e33–5. DOI: 10.1111/bjh.16154. PMID: 31452197.

5. Bond J, Graux C, Lhermitte L, et al. 2017; Early response-based therapy stratification improves survival in adult early thymic precursor acute lymphoblastic leukemia: a group for research on adult acute lymphoblastic leukemia study. J Clin Oncol. 35:2683–91. DOI: 10.1200/JCO.2016.71.8585. PMID: 28605290.

6. Wood BL, Winter SS, Dunsmore KP, et al. 2014; T-lymphoblastic leukemia (T-ALL) shows excellent outcome, lack of significance of the early thymic precursor (ETP) immunophenotype, and validation of the prognostic value of end-induction minimal residual disease (MRD) in Children's Oncology Group (COG) Study AALL0434. Blood (ASH Annual Meeting Abstracts). 124(Suppl):1. DOI: 10.1182/blood.V124.21.1.1.

Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download