INTRODUCTION
Gastric carcinoma (GC) is traditionally classified into intestinal- and diffuse-type adenocarcinomas based on Lauren’s criteria.
1 GC can also be classified into differentiated and undifferentiated types according to the degree of differentiation.
2 In 2007, a new histological type with differentiation towards the gastric fundic glands was proposed, called gastric adenocarcinoma of the fundic gland type (GA-FG).
3 GA-FG was recently added to the fifth edition of the World Health Organization’s (WHO 2019) classification of digestive system tumors.
4 This type of GC occurs primarily in the deep portion of the normal fundic gland without atrophy or metaplasia in
Helicobacter pylori (
H. pylori)-negative patients.
5 The disease is usually found in the upper third of the stomach and frequently invades the submucosa, even if the lesions are small.
6 On the other hand, GA-FG is regarded as a low-grade malignancy with a favorable prognosis because of its low tumor cell proliferation index and the limited incidence of recurrence and metastasis.
3 Despite this, reports on the outcomes of patients who underwent endoscopic resection for GA-FG are scarce. This paper reports the authors’ experience with an endoscopic resection for GA-FG in five patients.
Go to :

CASE REPORT
We retrospectively searched our database for all patients who had undergone an endoscopic resection at the Pusan National University Hospital between January 2012 and June 2022. Five patients diagnosed with GA-FG after endoscopic resection were identified (
Table 1): two male and three female patients aged 35 to 73 years (median, 63 years). Four lesions were located in the upper third of the stomach and one in the lower third. A histopathological examination of the specimens obtained via endoscopic forceps biopsy revealed mild atypia in two patients, oxyntic gland neoplasm in two, and low-grade dysplasia in one. Atrophic gastritis was limited to the gastric antrum in two patients and was not detected in the three remaining patients.
H. pylori infections were not detected in any of the patients according to rapid urease test and histology. Macroscopically, three lesions showed a IIa morphology, and two resembled a subepithelial tumor (SET). Three lesions had a similar color to that of the surrounding normal mucosa, whereas two lesions were discolored. Dilated surface blood vessels were observed in three lesions. Magnifying endoscopy with narrow-band imaging (ME-NBI) was performed for three lesions. Two lesions had regular microsurface (MS) and microvascular (MV) patterns; one lesion had an irregular MS and a regular MV pattern. A demarcation line was observed in two lesions. Endoscopic ultrasonography was performed on three lesions before the endoscopic resection. All three lesions were hypoechoic; one lesion was limited to the second (deep mucosal) layer, and the other two lesions extended to the superficial portion of the third (submucosal) layer. An endoscopic mucosal resection was performed with a ligation device in one patient, and an endoscopic submucosal dissection (ESD) was performed in the other four patients.
Figs. 1 and
2 show two representative cases (cases 2 and 4).
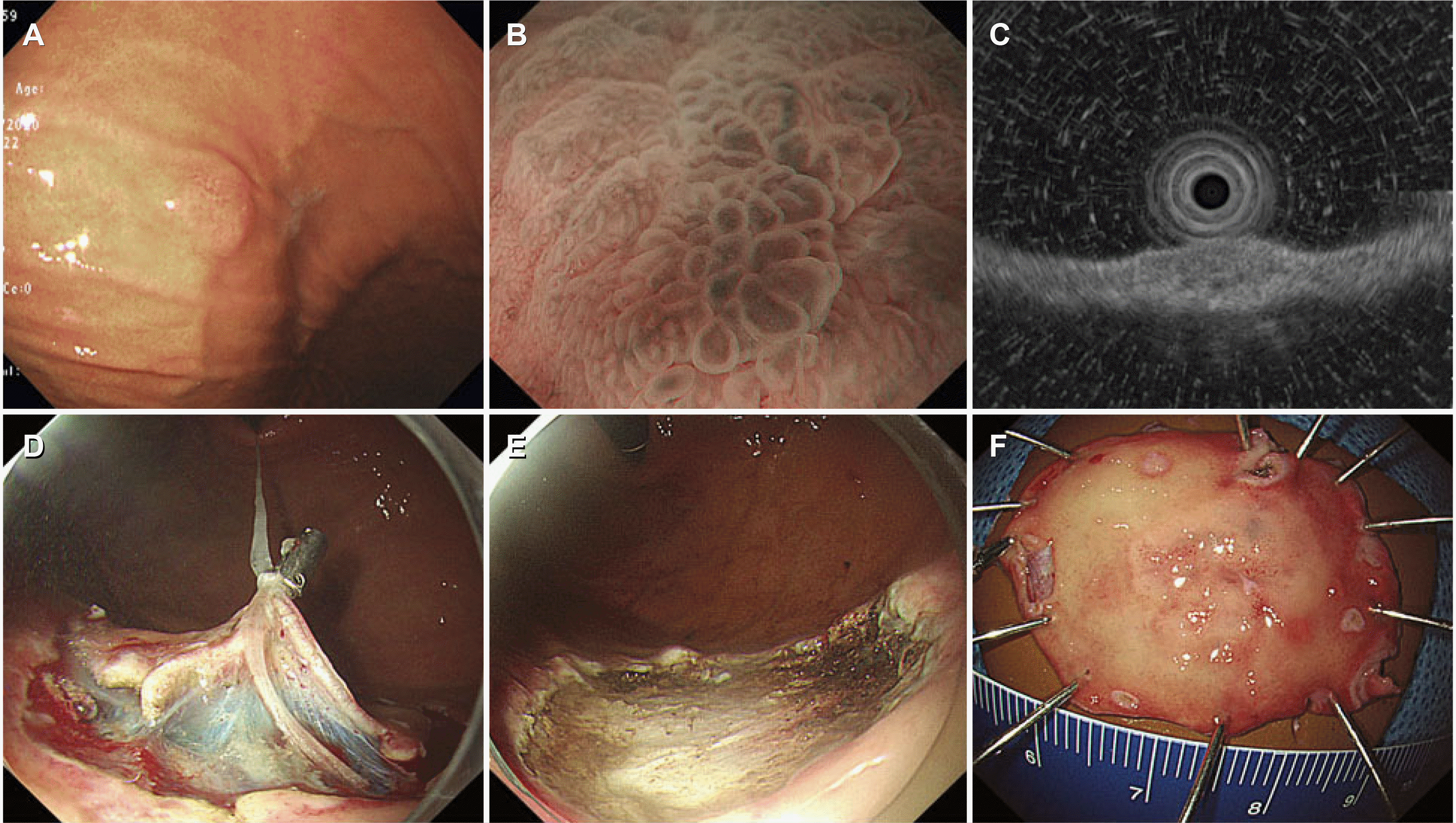 | Fig. 1A representative case of gastric adenocarcinoma of the fundic gland type (Case 2). (A) Conventional endoscopy shows a subepithelial tumor-like lesion on the greater curvature of the gastric upper body. (B) Magnifying endoscopy with narrow-band imaging shows irregular microsurface and microvascular patterns with a demarcation line. (C) On endoscopic ultrasound, the tumor extends up to the upper portion of the submucosal layer. (D, E) Traction-assisted endoscopic submucosal dissection is performed. (F) A resected specimen. 
|
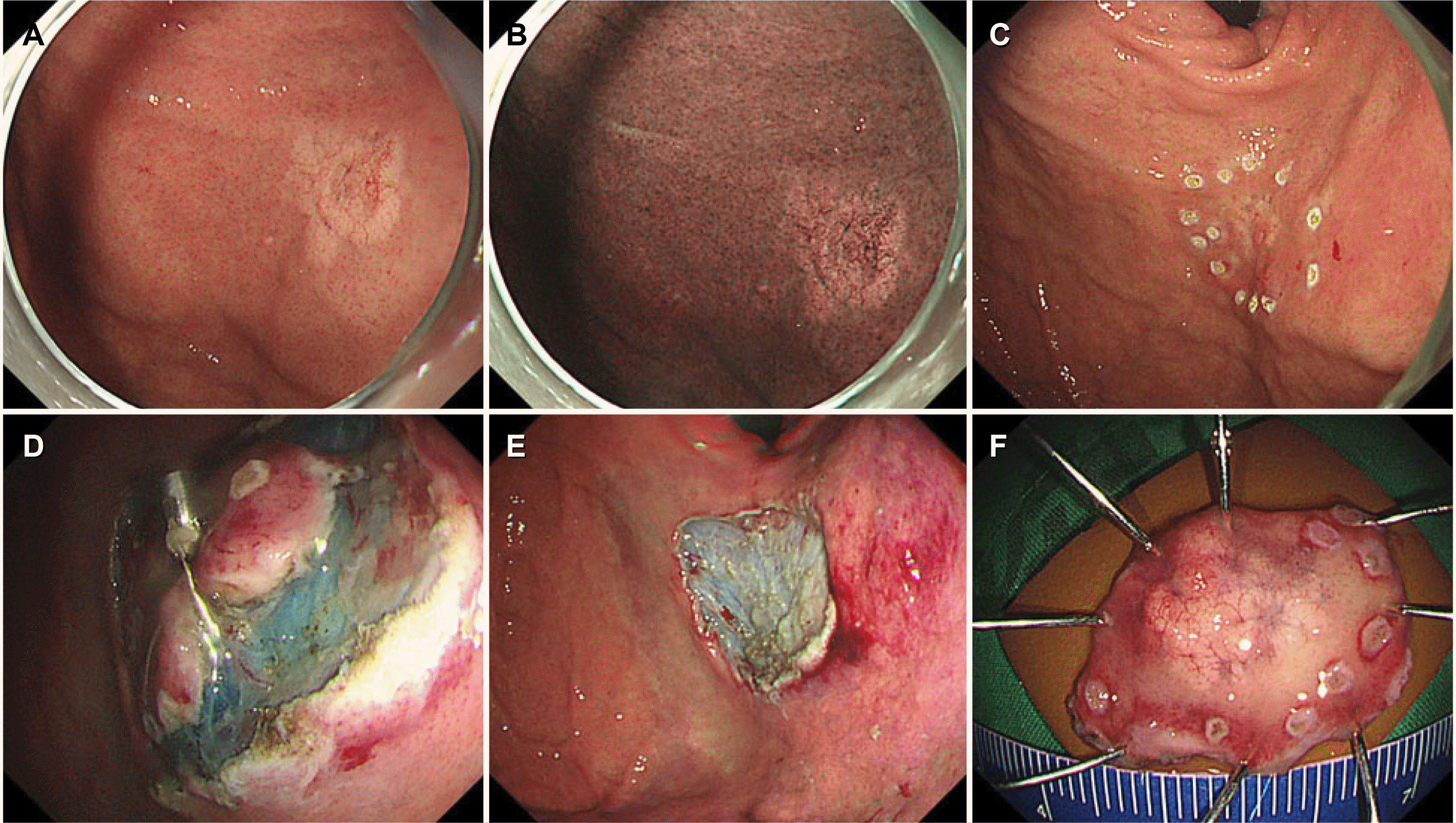 | Fig. 2A representative case of gastric adenocarcinoma of the fundic gland type (Case 4). (A, B) Conventional endoscopy and narrow-band imaging show a discolored, slightly elevated lesion on the anterior wall of the gastric fundus. Dilated blood vessels are observed on the surface of the lesion. (C-E) Traction-assisted endoscopic submucosal dissection is performed. (F) A resected specimen. 
|
Table 1
Clinicopathologic and Endoscopic Features of the Five Patients with Gastric Adenocarcinoma of the Fundic Gland Type
|
Variable |
Case 1 |
Case 2 |
Case 3 |
Case 4 |
Case 5 |
|
Sex |
Male |
Female |
Female |
Male |
Female |
|
Age (yr) |
34 |
65 |
41 |
63 |
73 |
|
Location |
Fundus |
Upper body |
Fundus |
Fundus |
Lower body |
|
Helicobacter pylori infection |
Absent |
Absent |
Absent |
Absent |
Absent |
|
Macroscopic shape |
IIa |
SET-like |
SET-like |
IIa |
IIa |
|
Color |
Discolored |
Normal |
Normal |
Discolored |
Normal |
|
Dilated blood vessels |
Absent |
Present |
Present |
Present |
Absent |
|
Background atrophy |
Absent |
Absent |
Absent |
Absent |
Absent |
|
Endoscopic biopsy |
Low-grade dysplasia |
Atypia |
Oxyntic gland neoplasm |
Atypia |
Oxyntic gland neoplasm |
|
Treatment method |
ESD |
ESD |
EMR |
ESD |
ESD |
|
Size (mm) |
51 |
10 |
6 |
10 |
4 |
|
Invasion depth |
Lamina propria |
Submucosa (550 μm) |
Submucosa (150 μm) |
Submucosa (300 μm) |
Lamina propria |
|
Lymphovascular invasion |
Absent |
Absent |
Absent |
Absent |
Absent |
|
MUC5AC stain |
Focally positive |
Negative |
Negative |
Negative |
Negative |
|
MUC6 stain |
Positive |
Positive |
Positive |
Positive |
Positive |
|
Follow-up period (mon) |
52 |
23 |
13 |
11 |
7 |

Histopathologically, most of the lesions were located in the deep layer of the lamina propria. The tumor cells were composed of well-differentiated columnar cells mimicking the fundic gland cells (mixed chief cells or parietal cells) (
Fig. 3). The median tumor size was 10 mm (4-51 mm). Three lesions invaded the submucosa (150 μm, 300 μm, and 550 μm from the muscularis mucosae, respectively). No lymphatic or venous invasion was observed. The horizontal and vertical margins were negative in four lesions; it was indeterminate in one lesion because of the poor orientation of the specimen. Immunohistochemical staining showed that the tumor cells were positive for MUC6 in all five cases and focally positive for MUC5AC in one case, indicating tumor differentiation into the fundic gland and foveolar epithelium, respectively. Additional ESD was performed in the patient with indeterminate margins and minimal submucosal invasion (150 μm). The final histopathological examination revealed no remnant tumor. Additional surgery was recommended for the patient with deep submucosal invasion (550 μm), but the patient refused to undergo gastrectomy. During the median follow-up period of 13 months (7-52 months), no recurrence was observed in any of the patients.
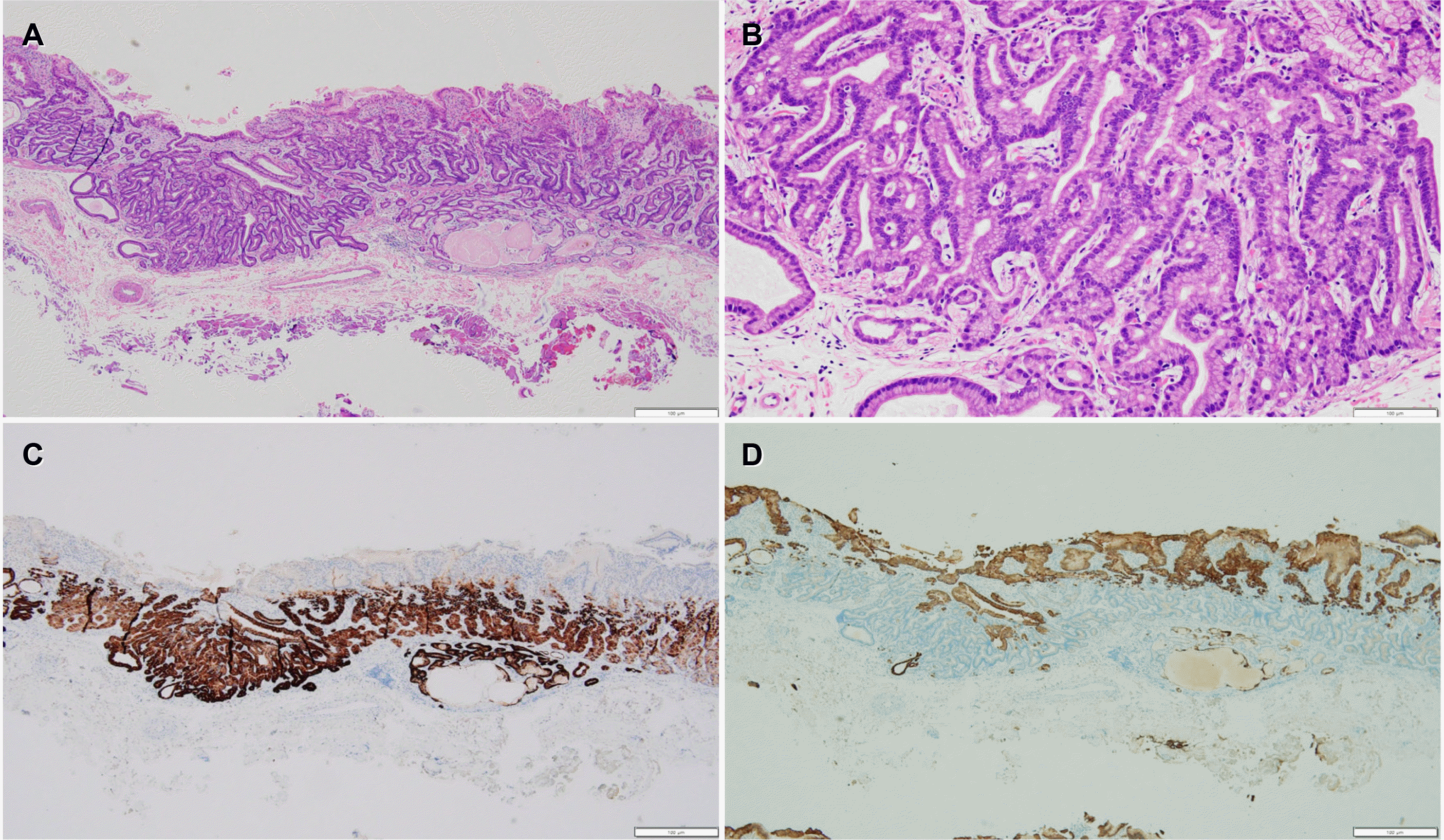 | Fig. 3Histopathological findings (Case 2). (A) Tumor arises from the deep layer of the lamina propria and invades the submucosal layer. Most of the surface is covered with non-atypical foveolar epithelium (H&E stain, ×40). (B) Tumor is composed of well-differentiated columnar cells mimicking the fundic gland cells with mild nuclear atypia (H&E stain, ×200). (C, D) Tumor is diffusely positive for MUC6 stain (C) but negative for MUC5AC stain (D) (immunohistochemical stain, ×40). 
|
This case series was reviewed and approved by the Institutional Review Board of the Pusan National University Hospital (2302-008-123).
Go to :

DISCUSSION
GA-FG is a rare variant of GC composed of cells resembling fundic gland cells.
3 According to a histopathologic review of more than 6,000 Korean GC specimens resected by endoscopy or surgery, only three were diagnosed with GA-FG.
7 A Japanese study of early GC treated with ESD reported a GA-FG incidence of 0.98%.
8 Another Japanese study on population-based registries for the cancer incidence detected a male-to-female ratio of approximately 1.4; the average age was 67.7 years (42-82 years).
9 None of the patients with GA-FG showed evidence of a
H. pylori infection.
10 In the present series, the male-to-female ratio was 2:3, and the average age was 63 years. Although two of the patients were suspected of having had a prior
H. pylori infection based on the endoscopic findings, such as the presence of atrophy in the gastric antrum,
11 none of them had a current infection.
GA-FG has characteristic endoscopic features that are not observed in conventional GC.
10 GA-FG is primarily located in the upper third of the stomach (>85% of cases), with 80% of tumors <10 mm in diameter (mean, 7.5 mm) at the time of the diagnosis. Macroscopically, GA-FG has an elevated shape, particularly SET-like, with poorly demarcated borders. One-quarter of the cases had a flat or depressed shape.
12 A higher frequency of SET-like shape can be explained by the histopathological findings that GA-FG lesions originate from the deep layer of the gastric mucosa and spread vertically into the submucosa and laterally into the surrounding tissue with minimal destruction.
12 Discoloration, dilated blood vessels with branching architecture, and non-atrophic background mucosa are also characteristic endoscopic features of GC-FG.
12 Although GA-FG was reported to frequently accompany fundic gland polyps, a fundic gland polyp is not a precursor to GA-FG.
13 Endoscopically, GA-FG is easily differentiated from conventional fundic gland polyps. In the present case series, all patients had an elevated lesion and a background mucosa without atrophy. Two patients had SET-like lesions, and two had discolored lesions. There have been few reports of ME-NBI for GA-FG.
8,10,14 Several patients have absent MS and irregular MV patterns, while others have regular MS and MV patterns. The demarcation line is not always detected in GA-FG because of the non-expoure of some tumors to the surface (those located in the deep mucosal layer). Similarly, two lesions had regular MS and MV patterns, and the demarcation line was absent in one lesion.
GA-FG is a well-differentiated tubular adenocarcinoma composed of mildly atypical columnar cells that mimic the fundic glands.
12 Considering the mild atypia of tumor cells, GA-FG appears to be a low-grade malignancy with regard to its biological behavior. GA-FG rarely exhibits submucosal and lymphovascular invasion.
8,12 Immunohistochemically, GA-FG is categorized as a purely gastric phenotype. The tumor cells in GA-FG are characteristic transitional type cells (from mucous neck cells to chief cells), designated immature chief cells. Therefore, these immature chief cells express MUC6, a biomarker for mucous neck cells.
15,16 In contrast, MUC5AC, a biomarker of foveolar cells, is rarely detected in GA-FG. Immunohistochemical analysis of pepsinogen-I, the most specific marker for differentiation into chief cells, is indispensable for diagnosing GA-FG.
3 In the present case series, all five patients were diagnosed with an atypia, oxyntic gland neoplasm, or low-grade dysplasia on the endoscopic forceps biopsy; three had a submucosal invasion. Lymphovascular invasion was not detected in any patient, and there was no tumor recurrence during the follow-up period. These results are consistent with those of previous studies in that GA-FG is generally considered to have low-grade malignant potential, regardless of its ability for submucosal invasion.
3,5,6
A
H. pylori infection is not involved in the development of GA-FG. This type of GC is believed to have been derived from non-atrophic mucosa.
12 H. pylori infection was not detected in any of the patients. Recently, GA-FG has been reported in non-atrophic gastric mucosa after successful
H. pylori eradication.
6 This suggests that GA-FG may develop in patients undergoing
H. pylori eradication. Therefore, the recognition and knowledge of GA-FG will reduce the incidence of overlooking GC during endoscopy without
H. pylori infection, including in post-eradication status. Although the oncogenic mechanism of GA-FG has not been elucidated in detail, it is associated with changes in the Wnt/β-catenin signaling pathway and mutation of GNAS and KRAS.
15,17
Considering its small size and low-grade malignant behavior, an endoscopic resection is frequently performed for GA-FG.
8,10,18-20 An endoscopic resection for GC with a submucosal invasion of ≥500 μm is beyond the curative resection criteria.
21 GA-FG rarely exhibits lymphovascular invasion despite the deep submucosal invasion. Although the number of reported cases is small, no patient has developed recurrence or metastasis, except for local residual recurrence.
10 In the present case series, there was no recurrence in the three patients with a submucosal invasion. Considering the rarity of this type of GC, further large-scale, multicenter studies on the long-term outcomes of endoscopic resection for GA-FG, particularly with submucosal invasion, are needed.
In conclusion, considering that GA-FG arises from the normal gastric mucosa in the fundic gland region without atrophy or intestinal metaplasia, the incidence of GA-FG might increase as the prevalence of H. pylori infections decreases. Therefore, it is vital to know the endoscopic features of GA-FG and perform immunohistochemical staining for an accurate diagnosis. Although the reported data are limited, an endoscopic resection is a feasible treatment modality for GA-FG because of its small size and low risk of recurrence or metastasis.
Go to :








 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download