Abstract
Strongyloidiasis, a chronic helminth infection caused by the parasitic nematode Strongyloides stercoralis, has various clinical manifestations. Although rare, duodenal obstructions and venous thromboembolism are possible complications of strongyloidiasis. This paper presents the case of a 47-year-old Vietnamese male with a history of right lower limb edema, anorexia, nausea, vomiting, diarrhea, and abdominal discomfort lasting for four months. Venous Doppler ultrasound detected a thrombus in the right femoral vein, while an abdominal CT scan revealed a mass lesion suggestive of a lower bile duct tumor. Esophageogastroduodenoscopy showed a friable duodenal cap mucosa with multiple ulcers and edematous mucosa of the second part of the duodenum that caused a partial lumen obstruction. The final histological examination of the biopsy specimen revealed chronic duodenitis with larvae consistent with Strongyloides stercoralis. The patient was treated with Ivermectin for two weeks and anticoagulation therapy for three months. After treatment and a six-month follow-up, the patient's gastrointestinal symptoms and leg swelling resolved completely. This is the first documented case of a patient in Vietnam with strongyloidiasis who presented with venous thromboembolism and duodenal obstruction.
Chronic strongyloidiasis is an infrequently reported and often underrated infection with nonspecific gastrointestinal symptoms.1-3 The clinical presentation of a Strongyloides stercoralis infection can vary from mild to severe, with a severe and disseminated infection potentially leading to serious complications in immunocompromised patients.4 Despite their rarity, duodenal obstruction and venous thrombosis are possible complications of strongyloidiasis. Comorbid deep vein lower limb thrombosis and duodenal obstruction have not been reported in Vietnam. This paper describes the case of a 47-year-old Vietnamese male who was immunocompetent and presented with vomiting, abdominal pain, and lower limb deep venous thrombosis, all of which were confirmed through multiple mucosal biopsies from the duodenum. In addition, the literature on this rare infection is reviewed.
According to the Declaration of Helsinki by the World Medical Association, the study is under the authors' instructions and with ethical and legal principles (according to the Declaration of Helsinki by the World Medical Association). The patient has given his written informed consent to publish his case.
A 47-year-old male patient was admitted to the authors’ hospital with a four-month history of right lower limb edema, poor oral intake, nausea, vomiting, and abdominal discomfort. The patient denied any past medical history of significance, as well as the use of alcohol, tobacco, herbal medicine, or inhaled corticosteroids. He worked as a farmer in a rural area in southern Vietnam, and there was no significant family history.
A physical examination revealed the patient to be in poor clinical condition, malnourished, but afebrile. The vital signs showed a blood pressure of 100/40 mmHg, a pulse rate of 100 beats per minute, and a respiratory rate of 24 breaths per minute. There was no lymphadenopathy, and the lung and heart auscultation was normal. An abdominal examination showed no guarding, rebound tenderness, or epigastric distention. The spleen and liver were not palpable, and the right leg showed mild edema. Laboratory tests revealed leukocytosis with a white blood cell count of 13.62 (G/L) (73% neutrophils, 8.6% lymphocytes, and 6.4% eosinophils), serum protein of 4.6 g/dL, and albumin level of 2.6 g/dL. The renal and liver function tests, bilirubin (total, direct), international normalized ratio, partial thromboplastin time, and lipase were within normal limits.
In addition, serological tests for human immunodeficiency viruses (HIV), hepatitis B surface antigen, hepatitis C virus antibody, and Venereal Disease Research Laboratory tests were negative. Stool testing with concentration technique for ova and parasites was negative. On the other hand, the patient tested positive for Strongyloides (IgG-ELISA), while antibodies of Toxocara spp. and Ancaras were negative. IgE was elevated at 1,440 mg/dL. Laboratory analysis to identify hypercoagulable defects was negative for protein C, protein S, and antithrombin III deficiency. Lupus anticoagulant or anti- cardiolipin antibodies were not detected.
The patient underwent a venous Doppler ultrasound, which revealed a thrombus in the right femoral vein. The tumor markers and thrombophilia blood test were within the normal limits. Especially, CEA and CA 19.0 were 6.30 ng/mL (normal value <5 ng/mL) and 35.57 IU/mL (normal value <37 IU/mL), respectively. An abdominal CT scan and a computed tomography pulmonary angiography (CTPA) were performed to determine the presence of a primary tumor and pulmonary embolism. The CTPA revealed no abnormalities. The abdomen scan showed a 1.2 cm mass resembling a lower common bile duct tumor at the second part of the duodenum (Fig. 1). Esophagogastroduodenoscopy revealed a friable duodenal cap mucosa with multiple ulcers and edematous mucosa of the second part of the duodenum that partially obstructed the lumen (Fig. 2). The differential diagnosis of a duodenal ulcer was considered, and biopsies were obtained to exclude a malignancy or H. pylori infection. The histology examination of the duodenal mucosal biopsy showed eosinophilic infiltrates and multiple S. stercoralis larvae (Fig. 3). Endoscopic ultrasound suggested a mass lesion at the end of the bile duct, causing biliary dilation, but a diagnostic evaluation with ERCP later confirmed no tumor. The combination of biliary sludge and the swelling of the duodenal mucosa led to a misdiagnosis of a tumor at the end of the bile duct. The final diagnosis was Strongyloidiasis, with the complications of a gastric outlet obstruction caused by duodenitis and venous thromboembolism.
The patient was treated with a two-week course of Ivermectin and was anticoagulated with rivaroxaban for three months. The patient's gastrointestinal symptoms and leg swelling improved after therapy. During the six-month follow-up period, the patient underwent regular monitoring and additional laboratory tests, which revealed negative parasitological stool examinations, normal thrombotic profile, and complete ulcer healing, as confirmed by esophagogastroduodenoscopy. As a result, the hematology service recommended no further workup for thrombophilia.
Strongyloidiasis, a common intestinal infection caused by two species of the nematode Strongyloides, was first described in 1876 by French colonial troops suffering from diarrhea in Vietnam. S. Stercoralis is the most clinically significant pathogenic species in humans, while another species, Strongyloides fuelleborni, causes limited human infections. The risk factors for disease severity include long-term corticosteroid therapy, older age, and malignancies.4-8 The early detection and treatment of Strongyloidiasis can reduce the risk of hyperinfection, autoinfection, dissemination, and mortality.2,4 The life cycle of this parasite is complex. Filariform larvae penetrate intact skin through contact with infected soil and enter the venous microcirculation via lymphatics. They then penetrate the pulmonary alveoli, ascend the respiratory tree, and enter the pharynx. From there, they are swallowed into the stomach and small intestine. In the duodenum, the larvae mature into adult females. Female worms produce up to 40 eggs per day via parthenogenesis. The eggs are 50-58 μm×30-36 μm in size and contain a transparent, thin cuticle with either the morula or the tadpole stages of rhabditoid embryos inside. Once the eggs hatch, the rhabditiform larvae are released, which can either be passed in stools to continue their soil-based cycle or cause autoinfection.2,4 After penetrating the skin and respiratory system, the third-stage filariform larvae migrate along swallowed sputum and enter the gastrointestinal tract, particularly in the duodenum and upper jejunum. Patients may experience vague and nonspecific symptoms, including anorexia, nausea, vomiting, weight loss, abdominal pain, flatulence, and diarrhea.4-6,9 The term disseminated disease is used when the infective larvae migrate from the intestine to other organs not involved in the normal helminthic life cycle. Larva currens, a pathognomonic cutaneous manifestation of Strongyloides infection, can also occur.2,4
A negative stool result cannot exclude Strongyloidiasis because of the high false negative rate (up to 70% cases), probably due to the low parasite load and irregular larval output.1,3,10 Multiple studies have suggested that obtaining 3-7 serial stool samples can significantly improve the diagnostic sensitivity from 50-99%.1,3,10 On the other hand, the limitations of these tests include cross-reactions with other parasites and the prolonged persistence of antibodies even after treatment, making it difficult to distinguish between acute and chronic conditions based solely on the serum antibody levels.11 In this case, the patient tested positive for several parasites, which may be a cross-reaction or being infected by multiple endemic agents.
In this patient, esophagogastroduodenoscopy and biopsy were conducted to determine the cause of the patient's symptoms and detect a duodenal obstruction as the underlying issue. Several case reports have described the endoscopic findings of the duodenum in strongyloidiasis, including edema, erythema, erosion, swollen folds, fine granules, tiny ulcers, polyps, hemorrhage, megaduodenum, deformity, and stenosis. Normal mucosa has also been reported in some cases.12 Given the broad range of possible endoscopic findings, a biopsy should be performed if there is any evidence from the clinical presentation. Inflammatory cell infiltration in the lamina propria, consisting of lymphocytes, plasma cells, and eosinophils, is commonly observed in the histological findings, with the possibility of detecting larvae in biopsy samples in severe cases.4,13 Nevertheless, pathologists must differentiate the types of helminths based on the stages of development and morphological features to avoid misidentification as arthropods or artifacts.
A duodenal obstruction, a rare complication of strongyloidiasis, can occur due to severe mucosal edema or extrinsic compression and can be challenging to diagnose, often requiring exploratory laparotomies in some instances.1,4 Benign and malignant causes, including strictures, tumors, and masses outside the intestinal tract, can also lead to a duodenal obstruction.1,5,14 In this case, a mass lesion was detected on CT, and a malignant etiology could not be excluded. Therefore, EUS and ERCP were performed to confirm the diagnosis, which revealed a severe edematous mucosa of the duodenal segment causing obstructions and biliary sludge mimicking a cancerous mass.
On the other hand, eosinophilia has traditionally been used as a diagnostic tool for strongyloidiasis. Eosinophilia has limited predictive value for individuals presenting with a disseminated form of the disease. The absence of eosinophilia may be attributable to peripheral eosinophil suppression, corticosteroid therapy, or concurrent bacterial infection.6,10,15-18 Nevertheless, a normal eosinophilic state was observed in some previous cases involving compromised patients because it may vary in chronic situations.1,14,19
In the present case, the patient was admitted to hospital with a thrombosis in the right femoral vein. Although thrombotic events are rare in cases of strongyloidiasis, there are various potential etiological factors, including systemic and local predisposing factors. Abdominal inflammatory diseases and the systemic inflammation caused by S. stercoralis infection should be considered because the present case was non-obese with no history of familial thrombophilia and cigarette smoking, the involvement of local factors, including recent surgery and trauma to the venous system. Multiple cytokines, such as interleukin-1, -6, and -8, interferon, and tumor necrosis factor, mediate the inflammation in strongyloidiasis, which can contribute to thrombus formation by damaging endothelial cells, activating and aggregating platelets, increasing the tissue factor protein levels, and decreasing the fibrinolytic mechanisms.20 Individuals with elevated eosinophil in peripheral blood may have a higher likelihood of thrombotic events, but hypereosinophilia was not reported in the present case. This relationship was strengthened because the patient had recovered completely, and his thrombotic profile was normal after a six-month follow-up.
Regarding treatment, Ivermectin has been reported to be the most effective medication for treating severe strongyloidiasis in most cases. The drug is more tolerated than thiabendazole and has a higher rate of larvae clearance from stool than albendazole.2,4
A literature review was conducted using the MEDLINE database to identify articles published in English related to duodenal obstruction caused by Strongyloides stercolaris in adults. The inclusion criteria were limited to cases reported in full text and published in English. Only confirmed cases of duodenal obstruction were included in this report, as documented in Table 1. Most cases presented as male individuals in an immunocompromised state, and the diagnoses were established by examining duodenal biopsy specimens.
In conclusion, the coexistence of duodenal obstruction and venous thrombosis is a rare but significant differential diagnosis that should be considered in endemic countries. Strongyloidiasis presents a wide range of clinical manifestations, low parasitic burden, and atypical symptoms, making its diagnosis challenging. Peripheral eosinophilia alone cannot be relied upon for diagnosis. An endoscopic examination and biopsy should be performed to confirm the infection and exclude other possible diagnoses. This case illustrates the diagnosis of an unusual manifestation of strongyloidiasis in low-resource countries, emphasizing the importance of a multimodal approach for diagnosis. Furthermore, the patient in this case study had an uncommon coexistence of gastric outlet obstruction and venous thromboembolism, which was not previously reported in the scientific literature from Vietnam.
Acknowledgements
We would like to thank the Cho Ray hospital Gastroenterology Department staff for their support.
REFERENCES
1. Juchems MS, Niess JH, Leder G, et al. 2008; Strongyloides stercoralis: a rare cause of obstructive duodenal stenosis. Digestion. 77:141–144. DOI: 10.1159/000128597. PMID: 18446028.

2. Malézieux-Picard A, Saint-Paul MC, Dellamonica J, et al. 2017; Severe intestinal obstruction due to Strongyloides stercoralis in a pregnant woman. Med Mal Infect. 47:429–431. DOI: 10.1016/j.medmal.2017.05.007. PMID: 28651833.

3. Yazdanpanah F, Saba H, Rahmani R, Schreiber ZJ, Hindy P. 2020; Strongyloides hyperinfection presenting as a gastric outlet obstruction. Cureus. 12:e6603. DOI: 10.7759/cureus.6603. PMID: 32064185. PMCID: PMC7008763.

4. Cruz RJ Jr, Vincenzi R, Ketzer BM. 2010; Duodenal obstruction - an unusual presentation of Strongyloides stercoralis enteritis: a case report. World J Emerg Surg. 5:23. DOI: 10.1186/1749-7922-5-23. PMID: 20698992. PMCID: PMC2925357.

5. Cohen J, Spry CJ. 1979; Strongyloides stercoralis infection and small intestinal lymphoma. Parasite Immunol. 1:167–178. DOI: 10.1111/j.1365-3024.1979.tb00704.x. PMID: 317839.

6. Zeitler K, Jariwala R, Restrepo-Jaramillo R, et al. 2018; Successful use of subcutaneous ivermectin for the treatment of Strongyloides stercoralis hyperinfection in the setting of small bowel obstruction and paralytic ileus in the immunocompromised population. BMJ Case Rep. 2018:bcr2017223138. DOI: 10.1136/bcr-2017-223138. PMID: 29866667. PMCID: PMC5990086.
7. Patra AA, Nath P, Pati GK, et al. 2019; Strongyloides infection presenting as proximal small intestinal obstruction. ACG Case Rep J. 6:e00124. DOI: 10.14309/crj.0000000000000124. PMID: 31616778. PMCID: PMC6722340.

8. Rothe K, Katchanov J, Schneider J, et al. 2020; Strongyloides stercoralis hyperinfection syndrome presenting as mechanical ileus after short-course oral steroids for chronic obstructive pulmonary disease (COPD) exacerbation. Parasitol Int. 76:102087. DOI: 10.1016/j.parint.2020.102087. PMID: 32087332.

9. Aslam A, Barlas U, Yassan LJ, Lodhi M. 2022; An unusual case of gastric outlet obstruction and melena. Clin J Gastroenterol. 15:374–380. DOI: 10.1007/s12328-021-01584-3. PMID: 35064555.

10. Beltrán Rosel A, Sanjoaquín Conde I, Pérez Muñoz A, Alcalá PM, Marta CB, Irigoyen von Sierakowski A. 2020; Strongyloides stercoralis: a rare and severe presentation in a pregnant woman. New Microbiol. 43:44–46. PMID: 31814031.
11. Kakati B, Dang S, Heif M, Caradine K, McKnight W, Aduli F. 2011; Strongyloides duodenitis: case report and review of literature. J Natl Med Assoc. 103:60–63. DOI: 10.1016/s0027-9684(15)30246-7. PMID: 21329250.

12. Kishimoto K, Hokama A, Hirata T, et al. 2008; Endoscopic and histopathological study on the duodenum of Strongyloides stercoralis hyperinfection. World J Gastroenterol. 14:1768–1773. DOI: 10.3748/wjg.14.1768. PMID: 18350608. PMCID: PMC2695917.

13. Franco AR, Mendo R, Figueiredo P, Albuquerque AC. An unusual cause of duodenal obstruction: Watch your feet! GE Port J Gastroenterol. 2022; Oct. 21. doi: https://doi.org/10.1159/000526040. DOI: 10.1159/000526040.

14. Zyngier FR, Lemme A, Pereira EG, Leal BB, Liberal MH. 1983; Surgical perpetuation of serious infection with Strongyloides stercoralis. Trans R Soc Trop Med Hyg. 77:425. DOI: 10.1016/0035-9203(83)90182-7. PMID: 6414126.

15. Baaten GG, Sonder GJ, van Gool T, Kint JA, van den Hoek A. 2011; Travel-related schistosomiasis, strongyloidiasis, filariasis, and toxocariasis: the risk of infection and the diagnostic relevance of blood eosinophilia. BMC Infect Dis. 11:84. DOI: 10.1186/1471-2334-11-84. PMID: 21466667. PMCID: PMC3079652.

16. Hindy P, Parvin R, Hanna K, Gress F. 2011; Strongyloidiasis presenting as duodenal obstruction in a patient infected with human T-cell lymphotropic virus type 1. Gastrointest Endosc. 74:439–441. DOI: 10.1016/j.gie.2010.11.035. PMID: 21704987.

17. Li W, Chattree A. 2019; Small-bowel obstruction due to Strongyloides stercoralis infection. Clin Gastroenterol Hepatol. 17:A41–A42. DOI: 10.1016/j.cgh.2018.03.017. PMID: 29559358.

18. Martyn E, Gration B, Somasundaram C, Chiodini PL. 2019; Strongyloides, HTLV-1 and small bowel obstruction. BMJ Case Rep. 12:e232461. DOI: 10.1136/bcr-2019-232461. PMID: 31822534. PMCID: PMC6904184.
19. Harish K, Sunilkumar R, Varghese T, Feroze M. 2005; Strongyloidiasis presenting as duodenal obstruction. Trop Gastroenterol. 26:201–202. DOI: 10.1016/j.gie.2010.11.035. PMID: 16737052.
20. Waters M, Krajden S, Kim C, et al. 2019; Case report: Two cases of strongyloidiasis presenting with thrombotic events. Am J Trop Med Hyg. 101:418–421. DOI: 10.4269/ajtmh.19-0347. PMID: 31218995. PMCID: PMC6685577.

Fig. 1
CT findings. Abdominal CT, coronal plane (A), and transversal plane (B) showed the showing a soft tissue density filling defect in the distal common bile duct representing cancerous mass (the yellow arrows).
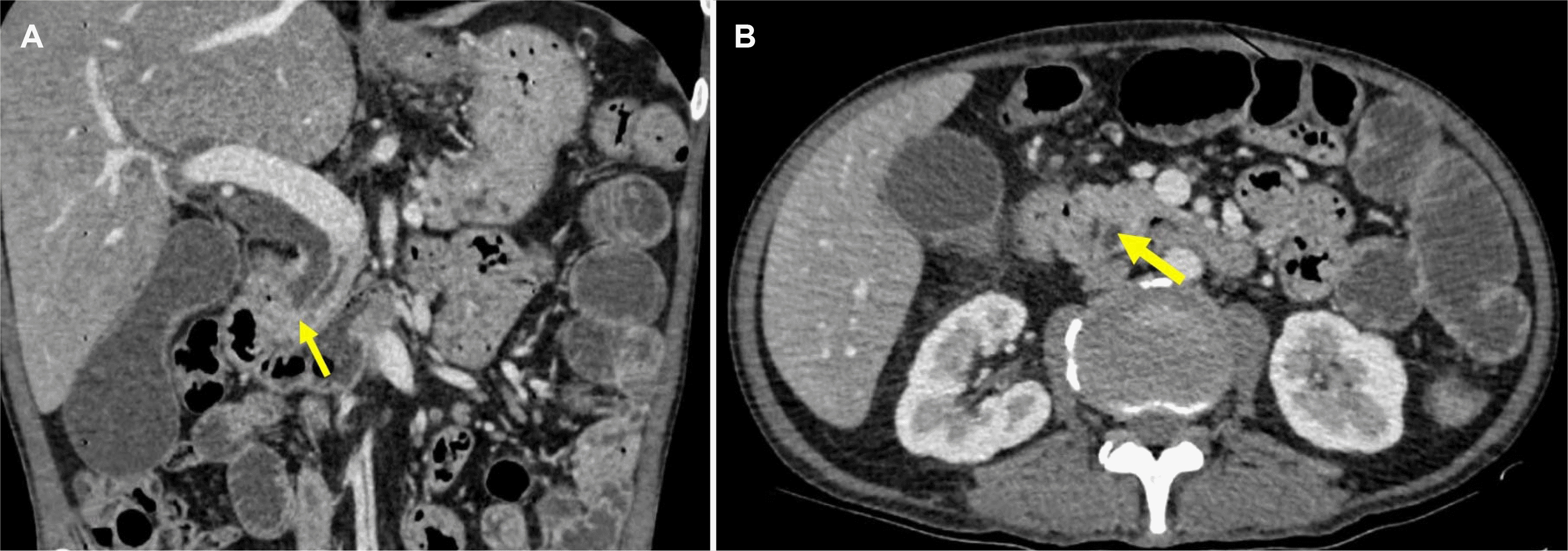
Fig. 2
Images from EGD. Ulceration and erosions (the white arrow) at the duodenal cap (A). Edema cause semi-obstruction (the white arrow) at the superior duodenal flexure and the second part of the duodenum (B). EGD, esophagogastroduodenoscopy.
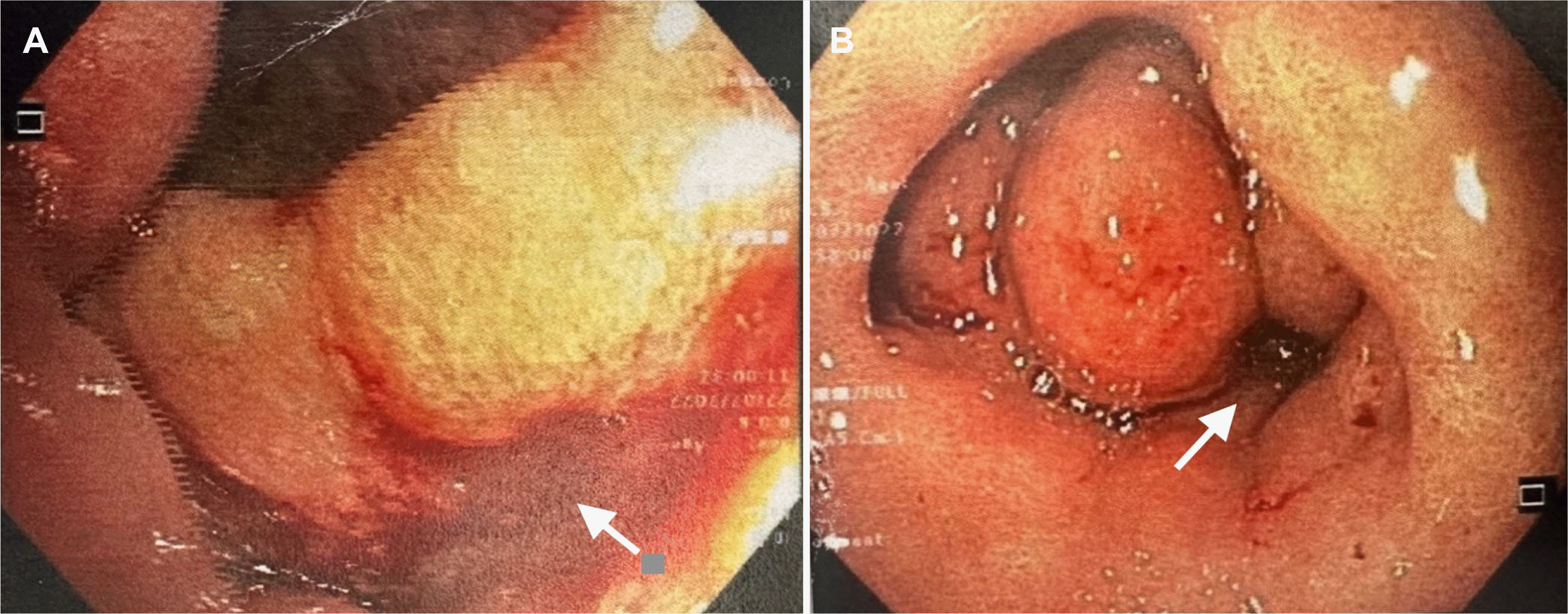
Fig. 3
Histological aspects of strongyloidiasis in the duodenum. The section of parasite larvae in the duodenum biopsy was observed (H&E, ×400). H&E stained sections of the duodenal biopsy. (A) The image shows numerous Strongyloides stercoralis larvae (green arrows) in the tissue. There is marked eosinophilic infiltration. (H&E, ×100). (B) The image shows larvae and lots of S. stercoralis eggs (blue arrow) inside the nematode in the tissue (H&E, ×400). (C) The image shows S. stercoralis larvae in the tissue (H&E, ×400).
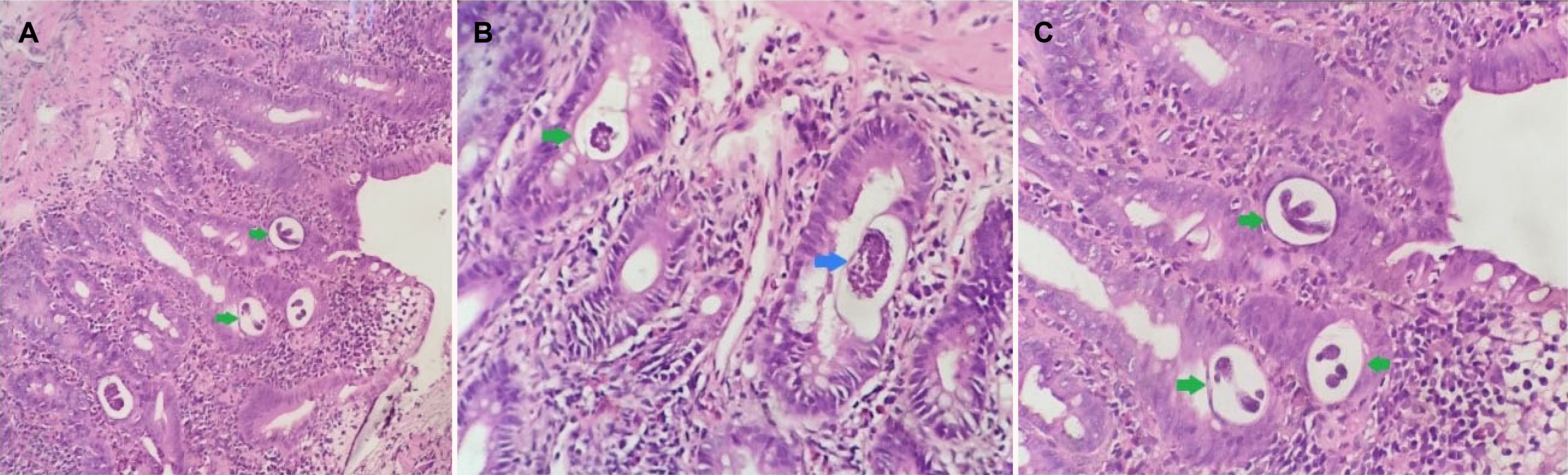
Table 1
Literature Review of Strongyloidiasis Cases with Duodenal Obstruction
| Author | Age-Sex | Immunosuppressive state | Thrombosis | Eosinophilia | Diagnostic method | Surgical treatment | Drug treatment | Outcome | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Aslam et al.9 | 57-F | HIV Breast cancer (Taxol-based chemotherapy) | None | None | DB | None | Ivermectin | Dead |
| 2 | Beltrán Rosel et al.10 | 23 F | HIV Pregancy | None | None | DB, Stoool examination | None | Ivermectin | Alive |
| 3 | Cohen and Spry5 | 40-M | Lymphoma | None | + | DA, DB | None | Thiabendazole | Dead |
| 4 | Cruz et al.4 | 42-F | None | None | None | Surgical specimen | Duodenal resection | Ivermectin Albendazole | Dead |
| 5 | Franco et al.13 | 28-F | None | None | + | DB, Stool examination | None | Ivermectin | Alive |
| 6 | Harish et al.19 | 45-M | None | None | + | Dudodenal aspirate, DB | None | Ivermectin | Alive |
| 7 | Hindy et al.16 | 50-F | HIV | None | NR | Duodenal biopsy | None | Albendazole -> ivermectin | Alive |
| 8 | Juchem et al.1 | 63-M | None | None | NR | DB and surgical specimen | Duodenal resection | Ivermectin | Alive |
| 10 | Li and Chattree17 | 52-M | Ulcerative colitis with immunosuppressive agents | None | NR |
DB+ Stool analysis Strogyloides serology |
None | NR | NR |
| 11 | Martyn et al.18 | 81-M | HIV Diabetes mellitus Prostate cancer | None | None | DB + Strogyloides serology + Stool examination | None | Ivermectin | Alive |
| 12 | Patra et al.7 | 46-M | Lichen planus with corticosteroid treatment | None | NR | DB Stool analysis | None | Ivermectin | Alive |
| 13 | Malézieux-Pica rd et al.2 | 32-F | HIV Pregnancy | None | + | DB+ GA | None | Ivermectin | Alive |
| 14 | Rothe et al.8 | 56-F | HIV Diabetes mellitus COPD | None | None | DB+ Bronchoalveolar lavage | None | Ivermectin | Dead |
| 15 | Yazdanpanah et al.3 | 67-F | Diabetes mellitus | None | None | DB | None | Ivermectin | Alive |
| 16 | Zeitler et al6 | 33 F | HIV | None | NR | DB, BAL cytology, Stool examination | None | Ivermectin | Alive |
| 17 | Zyngier et al.14 | 30-M | None | None | None | GA, sputum | None | Thiabendazole | Alive |
| 18 | Our case | 47M | None | + | None | DB | None | Ivermectin | Alive |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download