Abstract
Candida auris (C. auris) is a pathogen that causes severe infections, particularly in healthcare settings, and is resistant to common antifungal agents, leading to a global public health threat. Infections of the cavernous sinus are rare but potentially fatal. We report a rare case of cavernous sinus infection caused by drug-resistant C. auris. A 60-year-old woman with chronic otitis media underwent a mastoidectomy and tympanoplasty. The patient developed a surgical site infection with methicillin-resistant Staphylococcus aureus after the surgery, which improved with antibiotic treatment. However, she developed purulent otorrhea, ear pain, and a limitation of eye movement. Cultures of ear discharge repeatedly identified C. auris, and brain magnetic resonance imaging (MRI) findings were consistent with the presence of cavernous sinus infection and pachymeningitis. Therefore, amphotericin B and micafungin combination therapy was initiated for the C. auris infection. The patient showed no clinical improvement despite combination therapy with amphotericin B and micafungin; therefore, flucytosine was added to the treatment regimen. Symptoms improved after the triple-agent combination therapy, and a follow-up MRI showed improvement in the cavernous sinus infection and pachymeningitis.
Recently, strains of Candida, a fungus resistant to antifungal agents, have increased. Candida auris (C. auris) infection is a serious global health threat [1]. Healthcare facilities in several countries have reported severe C. auris infections among inpatients [2]. C. auris is often resistant to common antifungal agents, causing difficulty in treatment [1,2].
Cavernous sinus infection is a rare, potentially life-threatening condition that requires prompt treatment and is associated with a high mortality rate despite antimicrobial therapy [3]. Herein, we report the case of a 60-year-old woman diagnosed with a cavernous sinus infection and pachymeningitis caused by C. auris. We successfully administered triple antifungal combination therapy, and the patient fully recovered from the severe infection. To the best of our knowledge, there have been no previous reports of cavernous sinus infections caused by C. auris in South Korea. Therefore, we report this case and discuss the use of combination antifungal therapy to treat C. auris.
A 60-year-old woman was admitted to the Department of Otolaryngology on November 2, 2020, with bilateral chronic otitis media (COM). She was on medications for hypertension and diabetes mellitus. She underwent a left mastoidectomy and tympanoplasty. She developed earache and purulent otorrhea 2 days after the operation and was diagnosed with surgical site infection (SSI) caused by methicillin-resistant Staphylococcus aureus (MRSA), which was treated with vancomycin (1 g intravenously twice daily). The clinical symptoms improved after 5 days of vancomycin treatment, and she was discharged on oral trimethoprim/sulfamethoxazole treatment (960 mg twice daily). The patient was prescribed oral trimethoprim/sulfamethoxazole until December 11, 2020, and the infection resolved.
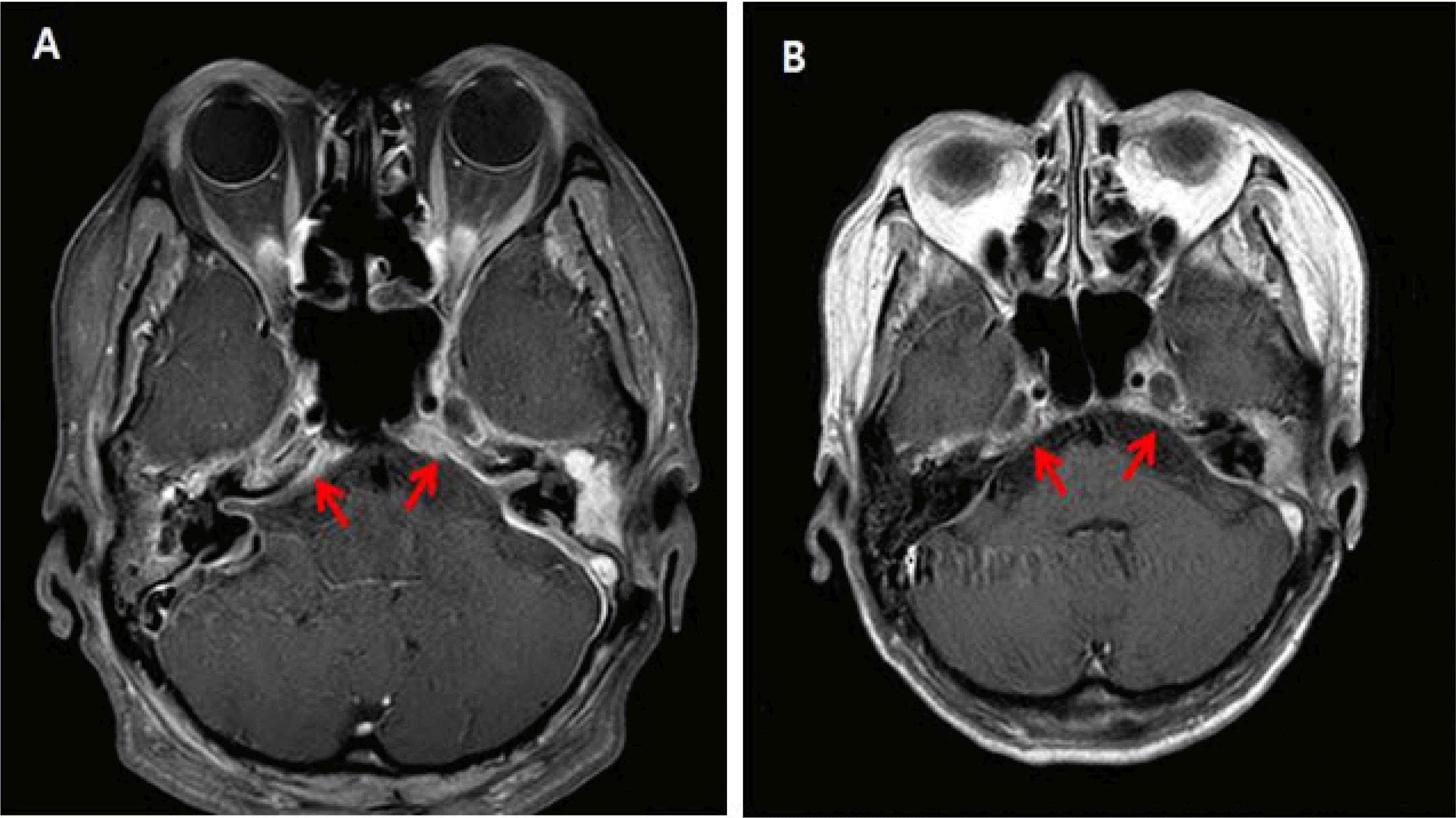
In February 2021, 3 months after the operation, the patient presented to the emergency department with dizziness and a headache. Magnetic resonance imaging (MRI) revealed soft tissue infection and pachymeningitis around the otomastoiditis lesion. We assumed that the patient had recurrent SSI and reinitiated vancomycin therapy. MRSA was identified in an ear pus culture from a sample collected in the emergency department. The patient’s clinical condition improved after antibiotic treatment. C-reactive protein (CRP) levels decreased from 60.2 to 46.7 mg/L after 14 days of vancomycin treatment. A follow-up brain MRI performed on March 4, 2021, after 27 days of vancomycin treatment, revealed improvement. However, the patient’s clinical condition worsened after 3 weeks of treatment. The patient developed limited extraocular muscle movement (EOM) and ptosis of the right eye, and her headache and ear discharge worsened. Furthermore, the CRP level increased to 74.5 mg/L. Brain MRI and orbital computed tomography (CT) were performed. A brain MRI revealed aggravated diffuse pachymeningitis and an additional cavernous sinus infection (Fig. 1A). The repeated ear pus cultures identified C. auris. Repeated blood cultures yielded negative results. The patient’s symptoms and the clinical findings continued to worsen despite the antibiotic treatment. The cavernous sinus infection and worsening of symptoms were believed to be caused by C. auris entering the cavernous sinus through the inner ear since C. auris was repeatedly identified in the patient’s ear pus.
The antibiotics were discontinued, and intravenous injections of 100 mg micafungin and 50 mg amphotericin B were administered once daily starting from March 23, 2021. The otorrhea and headache were not relieved after 1 week of antifungal therapy, and the CRP level increased to 87.1 mg/L. Due to the lack of clinical improvement, 1,250 mg of flucytosine (procured from the Korea Orphan and Essential Drug Center) was added to the treatment regimen on March 30, 2021. We monitored the CRP levels after adding flucytosine and found that the CRP level decreased to 39 mg/L after 10 days. The patient developed acute renal injury 4 weeks after the initiation of amphotericin B. Therefore, amphotericin B was discontinued on April 29, 2021. After the discontinuation of amphotericin B, we administered a combination therapy of micafungin and flucytosine. Subsequently, the patient’s CRP level, otorrhea, and pain decreased. The follow-up MRI after the two-drug combination therapy for 4 weeks showed improvement in the pachymeningitis and cavernous sinus infection. Subsequently, her EOM also improved, and the patient was treated with micafungin monotherapy starting June 7, 2021. A follow-up brain MRI on June 30, 2021, showed complete resolution of the cavernous sinus infection (Fig. 1B), and blood biochemistry showed a CRP level of <0.5 mg/L. Therefore, the micafungin treatment was discontinued.
Matrix-assisted laser desorption ionization time-of-flight mass spectrometry (Bruker Daltonics, Bremen, Germany) of the ear pus revealed C. auris. DNA was extracted from the four isolates of C. auris for DNA sequencing. Internal transcribed spacer 1 was used as the forward primer to conduct 23S ribosomal RNA sequencing, which yielded a 23S ribosomal RNA DNA sequence of 400 base pairs. ribosomal RNA. Sequence analysis using the GenBank Basic Local Alignment Search Tool (BLAST) showed 100% matching of the identified sequence with C. auris (GenBank accession no.: CP043535.1) in three samples and 99.49% matching in the fourth sample. Antifungal susceptibility was tested using the AST-YS08 card (bioMérieux, Inc., Durham, NC, USA) of the VITEK 2 system. The identified C. auris strain was susceptible to amphotericin B and echinocandins and resistant to fluconazole and voriconazole (Table 1).
C. auris was first discovered in the ear pus of a Japanese patient in 2009. This multidrug-resistant fungus is considered a global health threat because it causes invasive nosocomial infections, which are associated with high mortality [2]. In addition, it is the main Candida species causing nosocomial outbreaks [2]. Its mortality rate in medical institutions is 30-60% [4], and it is resistant to existing antifungal agents. Among C. auris infections, 88-90% and 10-35% of cases are resistant to fluconazole and amphotericin B, respectively, which are the first-line antifungal agents used to treat Candida infections in South Korea [4]. Echinocandins are the treatment of choice for C. auris [4,5]. However, FSK1 mutations that confer resistance to echinocandins are observed in approximately 4% of the cases [4].
Cavernous sinus infection is a rare but severe infection, with a mortality rate of 30-50% [3]. Patients who receive adequate treatment and survive may develop long-term cranial nerve dysfunction [6]. Infection of the cavernous sinus spreads mainly from facial cellulitis, sinusitis, and dental infections [7], and the main causative pathogens are Staphylococcus, Streptococcus, and Pneumococcus [8]. Fungal infections of the cavernous sinus are rare. Infection of the cavernous sinus by Candida species has been previously reported [9]. To our knowledge, this is the first case report of cavernous sinus infection caused by C. auris in South Korea [8,10,11]. Since C. auris mostly colonizes the ear canals, most cases in South Korea are identified in ear discharge cultures [10]. Invasive infections are rare [10,11]. The C. auris identified in South Korea is resistant to fluconazole but sensitive to amphotericin B and ecchinocandin [10].
The patient had COM. The SSI caused by MRSA improved after antibiotic treatment. However, symptoms subsequently worsened, and the patient developed limited EOM. C. auris colonization in the external ear canal might have developed as a result of repeated antibiotic therapy. In addition, the tympanic membrane remained ruptured postoperatively, and secondary infections by other pathogens could not be ruled out. C. auris was repeatedly cultured; therefore, we assumed that C. auris was the cause of the cavernous sinus infection and pachymeningitis. However, the choice of antifungal agents was limited because of the antifungal resistance of C. auris and the poor penetration of antifungal agents into the central nervous system (CNS) [5]. Echinocandins, the primary choice of treatment for C. auris, have limited penetration into the central nervous system [5]. According to literature reports, micafungin and amphotericin B have synergistic effects if administered together [5]. The combined administration of flucytosine and amphotericin B, voriconazole, or micafungin caused no antagonism [12]. Additionally, flucytosine is most effective in combination with other antifungal agents [1,12]. The treatment guidelines recommend liposomal amphotericin B with or without flucytosine [13].
We provided a combination therapy with synergistic antifungal agents, based on a review of the evidence. As a result, we initially chose micafungin and added amphotericin B since echinocandins have limited penetration into the CNS. However, amphotericin B, like micafungin, cannot penetrate well into the CNS and has low concentrations in the cerebrospinal fluid when administered [14]. We believe that this explains the lack of improvement in the patient’s clinical course with two antifungal drug combination therapy. We initiated a triple-therapy regimen of flucytosine, micafungin, and amphotericin B due to the lack of clear clinical improvement. The patient developed an acute kidney injury after 4 weeks of treatment, and amphotericin B was discontinued. After the patient’s laboratory and clinical findings improved, micafungin monotherapy was administered as maintenance therapy.
Only a limited number of case reports and clinical studies are available on combination antifungal therapy for C. auris [15]. Therefore, further studies on the synergistic effects of different antifungal agents for the effective treatment of C. auris infections should be conducted.
Fungal infections of the cavernous sinus are life-threatening condition that requiring prompt treatment [9]. This is a rare case of a severe infection of the cavernous sinus caused by drug-resistant C. auris. We treated the patient with a combination antifungal therapy. The mortality rate of C. auris infections remains high despite appropriate antifungal therapy [4]. Several in vitro studies have shown the synergistic effects of antifungal combination treatment, with no antagonism [5,12]. Antifungal combination therapy should be considered for treating severe infections, such as severe sepsis or septic shock, or when the sites of infection have difficult penetrability to drugs, such as meningitis or endocarditis.
REFERENCES
1. O'Brien B, Chaturvedi S, Chaturvedi V. 2020; In vitro evaluation of antifungal drug combinations against multidrug-resistant Candida auris isolates from New York outbreak. Antimicrob Agents Chemother. 64:e02195–19. DOI: 10.1128/AAC.02195-19. PMID: 31932367. PMCID: PMC7179280.
2. Lockhart SR, Etienne KA, Vallabhaneni S, Farooqi J, Chowdhary A, Govender NP, et al. 2017; Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin Infect Dis. 64:134–40. Erratum in: Clin Infect Dis 2018;67:987. DOI: 10.1093/cid/ciw691. PMID: 27988485. PMCID: PMC5215215.
3. Yarington CT Jr. 1977; Cavernous sinus thrombosis revisited. Proc R Soc Med. 70:456–9. DOI: 10.1177/003591577707000703. PMID: 331338. PMCID: PMC1543142.
4. Cortegiani A, Misseri G, Fasciana T, Giammanco A, Giarratano A, Chowdhary A. 2018; Epidemiology, clinical characteristics, resistance, and treatment of infections by Candida auris. J Intensive Care. 6:69. DOI: 10.1186/s40560-018-0342-4. PMID: 30397481. PMCID: PMC6206635.
5. Vitale RG. 2021; Role of antifungal combinations in difficult to treat Candida infections. J Fungi (Basel). 7:731. DOI: 10.3390/jof7090731. PMID: 34575770. PMCID: PMC8468556.

6. Ebright JR, Pace MT, Niazi AF. 2001; Septic thrombosis of the cavernous sinuses. Arch Intern Med. 161:2671–6. DOI: 10.1001/archinte.161.22.2671. PMID: 11732931.

7. Pavlovich P, Looi A, Rootman J. 2006; Septic thrombosis of the cavernous sinus: two different mechanisms. Orbit. 25:39–43. DOI: 10.1080/01676830500506077. PMID: 16527775.

8. Desa V, Green R. 2012; Cavernous sinus thrombosis: current therapy. J Oral Maxillofac Surg. 70:2085–91. DOI: 10.1016/j.joms.2011.09.048. PMID: 22326173.

9. Ng BHK, Kho GS, Sim SK, Liew DNS, Tang IP. 2019; Cavernous sinus fungal infection: a rare case. Br J Neurosurg. 33:283–4. Erratum in: Br J Neurosurg 2017;31:631. DOI: 10.1080/02688697.2017.1335857. PMID: 28597698.

10. Kwon YJ, Shin JH. 2022; Detection and control of Candida auris in healthcare settings. Korean J healthc assoc Infect Control Prev. 27:4–17. DOI: 10.14192/kjicp.2022.27.1.4.

11. Jung J, Kim MJ, Kim JY, Lee JY, Kwak SH, Hong MJ, et al. 2020; Candida auris colonization or infection of the ear: a single-center study in South Korea from 2016 to 2018. Med Mycol. 58:124–7. DOI: 10.1093/mmy/myz020. PMID: 30874806.
12. Bidaud AL, Botterel F, Chowdhary A, Dannaoui E. 2019; In vitro antifungal combination of flucytosine with amphotericin B, voriconazole, or micafungin against Candida auris shows no antagonism. Antimicrob Agents Chemother. 63:e01393–19. DOI: 10.1128/AAC.01393-19. PMID: 31591129. PMCID: PMC6879228.
13. Pappas PG, Kauffman CA, Andes DR, Clancy CJ, Marr KA, Ostrosky-Zeichner L, et al. 2016; Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin Infect Dis. 62:e1–50. DOI: 10.1093/cid/civ933. PMID: 26679628. PMCID: PMC4725385.

14. Felton T, Troke PF, Hope WW. 2014; Tissue penetration of antifungal agents. Clin Microbiol Rev. 27:68–88. DOI: 10.1128/CMR.00046-13. PMID: 24396137. PMCID: PMC3910906.

15. Reque J, Arlandis R, Panizo N, Pascual MJ, Perez-Alba A. 2022; Candida auris invasive infection after kidney transplantation. Case Rep Nephrol. 2022:6007607. DOI: 10.1155/2022/6007607. PMID: 35127186. PMCID: PMC8816593.
Fig. 1
T1-weighted magnetic resonance imaging of the brain with gadolinium enhancement. (A) Image taken in March 2021 showing aggravated diffuse pachymeningeal thickening, bilateral temporal convexities, cerebellar convexities, tentorium, retrocaval area, cavernous sinuses, and a C-shaped spinal canal, consistent with aggravated meningitis. (B) Image taken in June 2021 showing a reduction in the diffuse pachymeningeal thickening, bilateral temporal convexities, cerebellar convexities, tentorium, retrocaval area, cavernous sinuses, and C-shaped spinal canal, consistent with improved meningitis.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download