INTRODUCTION
Antimicrobial resistance (AMR) is a leading cause of death and has recently been recognized as a potential threat to public health worldwide [
1]. Approximately 1.27 million deaths were attributed to bacterial AMR in 2019, and
Acinetobacter is one of the six leading pathogens responsible for 73.4% of total deaths [
2].
Acinetobacter primarily causes bacteremia, hospital-acquired pneumonia, and central line-related infections in healthcare settings [
3,
4]. The World Health Organization and Centers for Disease Control and Prevention have categorized AMR in
Acinetobacter as an urgent threat and a critical priority owing to its rapidly increasing incidence through multiple drug resistance mechanisms, causing high mortality [
3-
6].
Considering its difficult-to-treat resistance (DTR), which is resistance to all first-line agents,
Acinetobacter infection has limited therapeutic options including, high-dose ampicillin/sulbactam, meropenem prolonged infusion, polymyxins (colistin), minocycline, tigecycline, or combinations of these regimens [
7-
10]. However, these agents have minimal effectiveness, and no clear therapy standard exists [
8,
10]. Novel agents with microbiological activity against
Acinetobacter have been developed but remain unavailable in several countries, including South Korea.
Understanding the recent AMR patterns of Acinetobacter is crucial to selecting optimal antibiotics for treatment. This study aimed to suggest insight into the clinical significance of Acinetobacter infection, particularly bacteremia with pneumonia, by providing data regarding its recent resistance trends and analyzing its mortality risk factors.
MATERIALS AND METHODS
1. Study design and population
This retrospective study was conducted at two tertiary referral hospitals in the Republic of Korea from January 2016 to December 2019. Patients ≥18 years of age with at least one positive blood culture for Acinetobacter species were enrolled in our study. Case patients were defined as individuals whose blood and sputum cultures grew the same Acinetobacter species to limit the cases to those in which the cause of bacteremia was pneumonia. Cases with other causes of bacteremia were excluded: 1) intra-abdominal infections, 2) catheter-related bloodstream infections, 3) skin and soft tissue infections, and 4) urinary tract infections. If patients presented with more than one episode of Acinetobacter species bacteremia, then only the first bacteremia episode of each patient was included in the analysis.
We analyzed the prevalence of AMR phenotypes and the results of drug resistance via each antimicrobial agent for the clinical isolates of the Acinetobacter species. In addition, we compared the baseline characteristics and clinical outcomes between the non-survivor and survivor groups and evaluated the risk factors for the 28-day mortality of Acinetobacter species bacteremia.
2. Data collection
Demographic, clinical, and microbiological data were reviewed retrospectively from medical records. The following data were collected: age, sex, body mass index, duration of stay before and after bacteremia, duration of antibiotic use before bacteremia, underlying diseases, corticosteroid use within 30 days, chemotherapy within 30 days, hospitalization within 90 days, prior invasive procedures, i.e., central catheter, intubation, and tracheostomy during hospitalization, the presence of shock on bacteremia onset, antimicrobial susceptibility test results, antimicrobial therapy within two weeks after the onset of bacteremia, in-hospital mortality, duration of febrile status, and duration of bacteremia. The Charlson Comorbidity Index (CCI) was used to assess the severity of underlying diseases, and the Pitt Bacteremia Score (PBS) was used to assess the severity of acute illness at the time of positive blood cultures.
All isolates were identified using a matrix-assisted laser desorption ionization-time of flight mass spectrometry system. Susceptibility tests were performed using automated antimicrobial susceptibility testing systems, and the minimum inhibitory concentrations (MICs) were reported. The susceptibility of isolates based on the reported MIC was determined using the Clinical and Laboratory Standards Institute guidelines [
11].
3. Definitions
The survivor group was defined as patients alive on the day of discharge. Comorbid conditions were defined using the
International Classification of Disease, 11th Revision coding system [
12]. Steroid use was defined as corticosteroids (≥20 mg of prednisolone taken daily for more than three weeks) used within four weeks of bacteremia onset. Shock was defined as hypotension (either a systolic blood pressure of <90 or 30 mmHg less than baseline values or requiring the use of a vasopressor to maintain blood pressure) with evidence of end-organ dysfunction. A PBS of four or higher indicated the most severe infection. Thrombocytopenia was defined as a platelet count of less than 150×10
3/µL, and hypoalbuminemia was defined as an albumin count of less than 3g/dL at the time of bacteremia.
Bacteremia onset was defined as the day of blood culture collection. The afebrile date was defined as the first out of two consecutive days when body temperature was <38℃. The culture-negative date was defined as the first day when “no growth” was consecutively confirmed twice. The duration of febrile status was measured from the bacteremia onset to the afebrile date, and the duration of bacteremia was measured from the bacteremia onset to the culture-negative date. Only patients who underwent a culture test at least once within a week were included in the evaluation of the duration of bacteremia. We set the period of analysis for antibiotic treatment at two weeks to focus only on the treatment of the Acinetobacter infection. Only antibiotics administered for more than half of the total antibiotic treatment period were considered the main antibiotic regimens.
We encountered the following resistance phenotypes in our study: multidrug-resistant (MDR) was defined as non-susceptible to at least one agent in three or more antimicrobial categories; extensively drug-resistant (XDR) was defined as non-susceptible to at least one agent in all but two or fewer antimicrobial categories; pandrug-resistant (PDR) was defined as non-susceptible to all agents in all antimicrobial categories; DTR was defined as intermediate or resistant to all reported agents in carbapenem, β-lactam, and fluoroquinolone categories (including piperacillin-tazobactam and ampicillin-sulbactam when results were available); carbapenem-resistant (CR) was defined as intermediate or resistant to ≥1 carbapenem (imipenem, meropenem, doripenem, and ertapenem); extended-spectrum cephalosporin-resistant (ECR) was defined as resistance to ≥1 extended-spectrum cephalosporin (ceftazidime, cefepime, ceftriaxone, and cefotaxime); and fluoroquinolone-resistant (FQR) was defined as resistance to ≥1 fluoroquinolone (ciprofloxacin, levofloxacin, and moxifloxacin) [
7,
13,
14].
4. Statistical analysis
Categorical variables were expressed as numbers (percentages) and continuous variables as means±standard deviations (SDs). Data without normal distributions were expressed as medians and interquartile ranges (IQRs). Categorical variables were compared using the χ2 or Fisher’s exact tests and continuous variables using the Student’s t or Mann–Whitney U tests, as appropriate. The significance tests were two-tailed, and statistical significance was set at P<0.05. Risk factors for the 28-day mortality of patients with Acinetobacter species bacteremia were analyzed by logistic regression analysis. Variables that were significant in the univariate analysis and other variables of clinical importance were included in a multiple logistic regression model. Results from the multivariate analysis are expressed as an odds ratio (OR) with a 95% confidence interval (CI). All statistical analyses were performed using the Statistical Package for the Social Sciences (SPSS) for Windows software package version 26 (SPSS Inc., Chicago, IL, USA).
5. Ethics
This study was reviewed and approved by the Institutional Review Board of Severance Hospital, Yonsei University College of Medicine in Seoul, Korea (Reg. No. 4-2021-1817). Informed consent was waived due to the retrospective, observational nature of the study.
RESULTS
1. Study population
We retrospectively evaluated 231 patients with
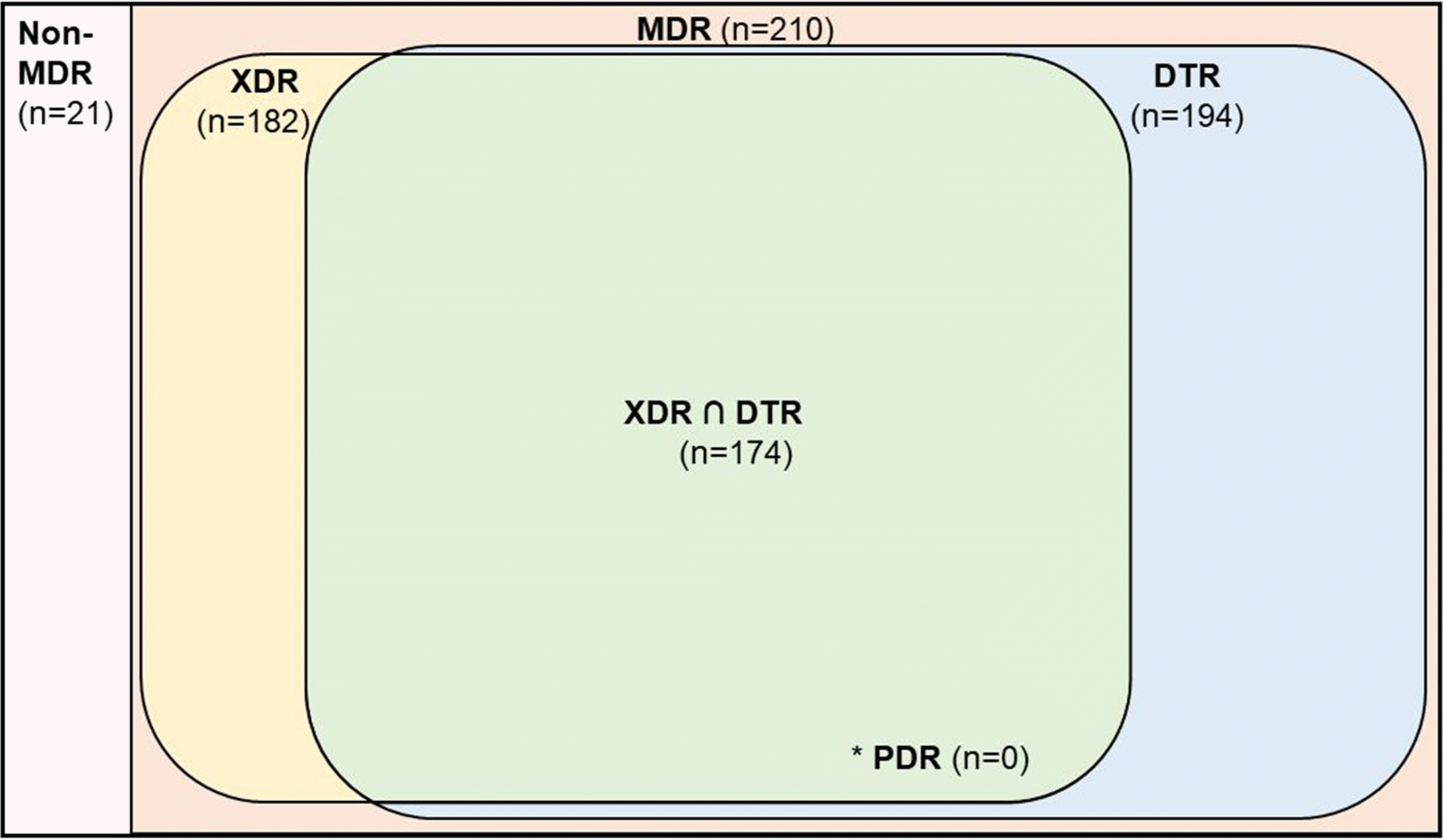
Acinetobacter bacteremia caused by pneumonia. These patients were classified into the MDR (n=210), XDR (n=182), DTR (n=194), and CR groups (n=206) according to the resistance category (
Fig. 1). These groups were not mutually exclusive (
Fig. 2). In addition, we divided the 231 patients into survivor (n=83) and non-survivor (n=148) groups to analyze the risk factors for in-hospital mortality.
2. Clinical characteristics of patients with Acinetobacter bacteremia
The baseline characteristics of the study population are shown in
Table 1. The mean age for all the patients was 64.7±14.9 years, and 145 patients (62.8%) were men. The median duration of hospitalization before the diagnosis of bacteremia was 22 days (IQR, 10-47), and the duration of antibiotic use before the diagnosis of bacteremia was 21 days (IQR, 10-46). The mean CCI score was 3.3±3.5. The mean PBS was 4.1±2.6, and 61.5% (n=142) presented a score of ≥4.
The overall mortality rate until discharge was 64.1% (n=148), and the 14- and 28-day mortality rates were 40.3% (n=93) and 45.9% (n=106), respectively. The non-survivor group was older than that of the survivor group (66.2±13.6 and 61.9±16.7 years, respectively, P=0.045) and had higher rates of hematologic malignancy (10.8% and 2.4%, respectively, P=0.02) and corticosteroid use (31.1% and 10.8%, respectively, P=0.001). The non-survivor group had a higher CCI score than the survivor group; however, this was not significant (3.6±3.4 and 2.8±3.6, respectively, P=0.12). The non-survivor group exhibited higher rates of thrombocytopenia (72.3% and 37.3%, respectively, P<0.001), hypoalbuminemia (85.1% and 60.2%, respectively, P<0.001), and higher C-reactive protein (CRP) levels (131.6±90.5 and 96.7±83.6, respectively, P<0.001) compared with that of the survivor group. Regarding severity, the non-survivor group had a higher rates of PBSs of ≥4 (68.9% and 48.2%, respectively, P=0.002) and septic shock on bacteremia onset (68.2% and 34.9%, respectively, P<0.001) compared with that of the survivor group. Antibiotic resistance was more prevalent in the non-survivor group than the survivor group: MDR (95.3% and 83.1%, respectively, P=0.002), XDR (83.8% and 31.9%, respectively, P=0.01), DTR (87.8% and 77.1%, respectively, P=0.03), CR (93.2% and 81.9%, respectively, P=0.008), ECR (93.9% and 84.3%, respectively, P=0.02), and FQR (97.3% and 81.9%, respectively, P<0.001). The median duration from bacteremia onset to death was 5.5 days (IQR, 1-35.5) in the non-survivor group.
3. Strain types for the Acinetobacter species
The most common strain identified was Acinetobacter baumannii (n=207, 89.6%), and the other strains were Acinetobacter nosocomialis (n=5), Acinetobacter baumannii complex (n=4), Acinetobacter bereziniae (n=3), Acinetobacter ursingii (n=2), Acinetobacter junii (n=1), Acinetobacter pittii (n=1), Acinetobacter soli (n=1), and unspecified Acinetobacter species (n=7). All isolates of Acinetobacter nosocomialis, Acinetobacter pittii, and Acinetobacter soli were not MDR pathogens.
4. AMR phenotypes
Of the total clinical isolates, 84.0% (n=196) exhibited DTR, compared with 90.9% (n=210) for MDR, 78.8% (n=182) for XDR, 89.2% (n=206) for CR, 90.5% (n=209) for ECR, and 91.8% (n=212) for FQR (
Fig. 3A). Among the isolates in this study, there were no PDR strains, and all DTR strains were CR, ECR, or FQR.
The detection rates for DTR, MDR, and CR ranged between 85.5% and 91.3% from 2016 to 2019, and the XDR detection rate increased over time from 72.5% to 85.7% (
Fig. 3B). The drug susceptibility results for each antibiotic are summarized in
Fig. 3C.
Acinetobacter species were resistant to carbapenem, aminoglycosides, cephalosporins, and sulfa drugs. Their resistance rates were above 85%, with the following results: cefepime (88.7%), ceftazidime (86.6%), ciprofloxacin (90.9%), trimethoprime/sulfamethoxazole (80.1%), imipenem (89.2%), meropenem (88.3%), and piperacillin/tazobactam (89.2%). Only colistin, tigecycline, and minocycline had high sensitivities to the
Acinetobacter species (96.5%, 77.9%, and 61.9%, respectively).
5. Antibiotic regimens
An analysis of antibiotic regimens for
Acinetobacter bacteremia was performed (
Table 1). Patients treated with monotherapy accounted for 33.8% (n=78) of all patients, including meropenem (n=53, 22.9%) and colistin (n=12, 5.2%). Dual combination therapy was the most common treatment (n=114, 49.4%). Eighty-four patients (36.4%) received a combination regimen containing both carbapenem and colistin, 22 (9.5%) received colistin-based treatment (not including carbapenem), and 21 (9.1%) were treated with a carbapenem-based regimen (not including colistin).
6. Risk factors for mortality
Significant variables in the univariate analysis (hematologic malignancy, corticosteroid use, PBS ≥4, DTR, thrombocytopenia, hypoalbuminemia, and CRP) and other variables of clinical significance (age, antibiotic use before BSI for more than 30 days, and recent hospitalization) were included in a multiple logistic regression model to identify independent risk factors for mortality. Only significant correlations between the variables that reflected the severity of bacteremia were presented; therefore, only PBSs of ≥4 were retained. Multivariate analysis revealed that age (OR, 1.025; 95% CI 1.002-1.049;
P=0.03), hematologic malignancy (OR, 4.691; 95% CI, 1.226-17.946;
P=0.02), PBS ≥4 (OR, 2.401; 95% CI, 1.213-4.751;
P=0.01), DTR (OR, 2.912; 95% CI, 1.129-7.515;
P=0.03), and thrombocytopenia (OR, 4.769; 95% CI, 2.384-9.539;
P<0.001) were independent risk factors for 28-day mortality (
Table 2).
7. Kaplan–Meier survival curves 28 days after bacteremia onset
Kaplan–Meier survival analysis showed that the 28-day mortality rate for patients infected with DTR
Acinetobacter strains was significantly higher than for patients infected with non-DTR strains (
P=0.006,
Fig. 4A). MDR and CR showed a similar pattern (
P=0.002, and
P=0.001, respectively,
Fig. 4B). We also performed a Kaplan–Meier survival analysis for the subgroup of DTR strains to compare the effects of the treatment regimens. In this analysis, the colistin-containing regimen had a significantly lower 28-day mortality rate than the regimen without colistin (
P=0.001,
Fig. 4D).
DISCUSSION
In this study, we identified that the prevalence of Acinetobacter species with resistance phenotypes, such as DTR, MDR, and CR, remained constant at a level of ≥80% from 2016-2019 (84%, 90.9%, and 89.2%, respectively). In addition, the clinical isolates showed high resistance to all antibiotics except for colistin, tigecycline, and minocycline. Furthermore, we observed high in-hospital mortality (64.1%) and 28-day mortality (45.9%) in patients with Acinetobacter bacteremia. DTR, age, hematological malignancy, high PBS, and thrombocytopenia were found to be independent risk factors for 28-day mortality.
We included only patients with pneumonia-related bacteremia among the bloodstream infections caused by
Acinetobacter species. In previous studies,
Acinetobacter infections were classified and analyzed according to infection sources (i.e., primary bacteremia, pneumonia, urinary tract infection, or skin and soft tissue infection) [
15-
19]. However, primary bacteremia is rarely observed in clinical practice, and bacteremia generally occurs secondary to other primary infections. Among the infection sources, pneumonia is the most frequent manifestation of
Acinetobacter nosocomial infection, and pneumonia-related bacteremia has higher mortality compared with that of non-pneumonia-related bacteremia [
3,
4,
20,
21]. Therefore, bacteremia caused by pneumonia is of clinical significance.
AMR in
Acinetobacter species is the result of a combination of various mechanisms, including the production of acquired β-lactamases (i.e., OXA-23-like enzymes, which are plasmid-borne carbapenemases) and several intrinsic mechanisms (i.e., intrinsic β-lactamase production, efflux pump upregulation, porin changes, and target binding site mutations) [
8,
22]. According to previous studies conducted during the early 2000s, the prevalence of CR
A. baumannii bacteremia in Korea was approximately 42.3-55.8% [
15,
17,
23]. However, this study reported that the prevalences of CR- and MDR-
Acinetobacter species bacteremia in 2016 were 89.9% and 91.3%, respectively, and the prevalences remained almost constant until 2019. This information may suggest that the AMR of
Acinetobacter can spread within a short period, and once resistance has spread, it is difficult to reduce it.
Resistance phenotypes, such as MDR, XDR, and CR, have been widely used for effective surveillance of AMR [
13,
14]. However, despite their epidemiologic usefulness, conventional resistance categories have limitations in that all classes of antibiotics are evaluated equally regardless of efficacy and toxicity and that testing is required for broad classes of antibiotics [
12]. In 2018, Kadri et al. [
7] proposed a new definition, termed DTR, for AMR in gram-negative bacterial infections, focusing on the effect of resistance on treatment decisions and clinical outcomes. All first-line antibiotics are unavailable for DTR infections; therefore, clinicians must use less effective and more toxic antibiotics. This leads to treatment failure and adverse events associated with high mortality [
7,
24]. Our study confirmed that DTR is an independent risk factor for 28-day mortality in
Acinetobacter species bacteremia, which was consistent with previous studies [
7,
24].
Currently, no definitive treatment regimen for DTR
Acinetobacter infection exists since few antibiotics remain available and clinical data proving their effectiveness are insufficient [
8-
10,
25]. According to the Infectious Diseases Society of America Guidance, combination therapy with at least two active agents is preferred for treating moderate-to-severe CR
A. baumannii infections [
10]. High-dose ampicillin-sulbactam, polymyxins, tigecycline, and extended-infusion meropenem can be considered components of combination therapy. Colistin and carbapenem are the most frequently used antibiotics as combination therapy in clinical practice [
26]. However, colistin in combination with meropenem did not show survival benefits over colistin monotherapy in CR
A. baumnnaii infections in the two large randomized clinical trials (AIDA and OVERCOME) [
26,
27].
In this study, carbapenem was the most frequently used antibiotic (68.4%), followed by colistin (51.1%). Regarding the number of antibiotics used, dual combination therapy was the most common (49.4%), followed by monotherapy (33.8%). This study is unique in that antibiotic regimens including ampicillin-sulbactam or tigecycline were scarcely used compared to other studies [
18,
19,
28]. We also performed a Kaplan–Meier analysis of the survival probabilities in the subgroup of DTR strains according to treatment regimens containing each of the two most commonly used antibiotics. As a result, we identified that colistin-containing regimens have survival benefits for patients with DTR strains. Therefore, treatment regimens that include colistin can be considered to increase survival in severe infections caused by DTR
Acinetobacter infections in the absence of standard treatment.
Colistin is generally used as a last-resort drug for highly resistant
Acinetobacter strains because of its remaining in vitro susceptibility [
10,
29]. The colistin susceptibility rate was also high in this study. However, given the recommendations by a joint European Committee on Antimicrobial Susceptibility Testing (EUCAST)/Clinical and Laboratory Standards Institute (CLSI) subcommittee, the colistin susceptibility results in this study are not entirely reliable since we used automated antimicrobial susceptibility testing systems [
30]. In addition, some experts are concerned that the increased use of colistin will lead to a rise in colistin-resistant strains worldwide [
29,
31]. Accordingly, the development of novel antibiotics is increasing to overcome the limitations of existing antibiotics. Cefiderocol, the only novel Food and Drug Administration-approved beta-lactam agent against CR
Acinetobacter species, has been used in some countries [
9,
10]. However, it is recommended as a rescue therapy owing to the lack of consistent data on a favorable outcome [
10,
32]. An additional issue is that the introduction of novel antibiotics is delayed in resource-limited countries, and most novel antibiotics are unavailable in a developed country like South Korea.
Age, underlying medical conditions, i.e., malignancy, mechanical ventilation, or previous surgery, and severity of diseases are predictors of death in patients with CR- or MDR-
Acinetobacter species bacteremia [
15,
17-
19]. We also confirmed that age, hematologic malignancies, and PBSs of ≥4 were associated with higher mortality rates. Other studies have reported inadequate antibiotic treatment and ineradicable or non-eradicable foci as other independent risk factors for mortality [
17,
19]. A previous study reported that the mortality rate was higher in patients with pneumonia as the cause of bacteremia, consistent with our results [
15].
Our study had several limitations. First, the retrospective study design might have resulted in selection bias during data collection despite the large study population. Second, polymicrobial infection was not considered an exclusion criterion in our study and may have served as a confounding variable. Third, when Acinetobacter species were isolated from both sputum and blood, we considered the strains to be true pathogens of pneumonia. Each chest image of the patients should have been individually evaluated to establish a pneumonia diagnosis; however, this was not performed due to a lack of capability. Fourth, we did not identify specific durations and doses of antibiotics in our study, which might have affected clinical outcomes. Fifth, we could not suggest the optimal antibiotic regimen because the use of antibiotics such as high-dose ampicillin-sulbactam or tigecycline was scarce. Finally, we could not analyze the pattern of resistance trends for Acinetobacter species that might have been affected by the COVID-19 pandemic since our study concluded in 2019.
In conclusion, we found that the prevalence of DTR Acinetobacter strains was approximately 80% and did not decrease over time. DTR was an independent risk factor for death and caused substantial therapeutic challenges in clinical settings. Regarding DTR Acinetobacter infections in resource-limited circumstances, colistin may contribute to improved clinical outcomes in patients with mortality risk factors, such as older age, comorbidity diseases, high PBS, and thrombocytopenia. Moreover, strengthening the surveillance of AMR and encouraging the development of novel antibiotics is necessary.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download