Abstract
Background
This study aimed to evaluate the trend of β-lactam resistance in Escherichia coli and Klebsiella pneumoniae bacteremia, the prevalence of cefotaxime-susceptible extended-spectrum β-lactamase-producing (ESBL) E. coli and K. pneumoniae, and the clinical characteristics of these infections.
Methods
A retrospective study was conducted in a tertiary hospital in Korea between 2011 and 2020. All patients with E. coli or K. pneumoniae bacteremia were identified, along with the results for the antibiotic susceptibility and ESBL production test for each isolate.
Results
Of 15 706 E. coli and K. pneumoniae bacteremia episodes, only 10 (0.06%) were caused by cefotaxime-susceptible ESBL-producing isolates, and their prevalence remained low during the study period. The proportion of ESBL-producing isolates gradually increased from 23.1% to 29.5%, whereas the proportion of carbapenem-resistant isolates rapidly increased from 0.9% to 12.1%. Furthermore, the proportion of carbapenem-resistant K. pneumoniae increased significantly and surpassed that of carbapenem-susceptible ESBL-producing K. pneumoniae in 2020. In a matched case-control study, the baseline characteristics of patients with cefotaxime-susceptible ESBL E. coli and K. pneumoniae bacteremia were similar to those with cefotaxime-resistant infections. There was no significant difference in 30-day mortality between patients with cefotaxime-susceptible and cefotaxime-resistant ESBL E. coli and K. pneumoniae bacteremia.
Broad-spectrum cephalosporins, including oxymino-β-lactam antimicrobial agents interfere with cell wall synthesis and are the main antibiotics used for treating Enterobacterales infections [1]. However, drug-resistant Enterobacterales are continuously increasing, causing high morbidity and mortality [2]. Since the initial report of extended-spectrum β-lactamase (ESBL) production in Klebsiella pneumoniae in 1983 [3], ESBL production has been one of the main mechanisms of drug resistance in Enterobacterales [4].
In 2010, the Clinical and Laboratory Standards Institute (CLSI) revised its guidelines for Enterobacterales by lowering MIC (minimum inhibitory concentration) interpretive thresholds for cefotaxime (or ceftriaxone) from ≤8 μg/mL to ≤1 μg/mL to indicate susceptibility and recommending reporting susceptibility results for cephalosporin “as found”, removing the requirement for ESBL phenotypic testing for organisms with cefotaxime MICs ≥2 μg/mL [5]. These changes were based on pharmacokinetics/pharmacodynamic analyses of MICs and clinical outcomes in patients with ESBL-producing Enterobacterales bacteremia, where reduced clinical responses were associated with increased MICs rather than β-lactamase resistance [6,7]. However, the clinical outcomes of patients with cefotaxime-susceptible ESBL-producing Enterobacterales bacteremia were based on a limited number of studies [7-9], and the thresholds used in these studies were based on the previous guidelines [10]. Since the 2010 revision of the CLSI recommendations, several studies have advocated the need for monitoring ESBL-producing isolates with ESBL confirmation tests [11-13].
This study aimed to identify the prevalence of bacteremia caused by ESBL-producing Escherichia coli and K. pneumoniae (the family Enterobacteriaceae), cefotaxime-susceptible ESBL-producing E. coli and K. pneumoniae, and carbapenem-resistant E. coli and K. pneumoniae since the revision of the CLSI guidelines in 2010. Furthermore, we aimed to evaluate the clinical characteristics and outcomes of patients with cefotaxime-susceptible ESBL-producing E. coli and K. pneumoniae bacteremia.
This retrospective study was conducted at the Asan Medical Center, a 2700-bed tertiary center in Seoul, Korea, between January 2011 and December 2020. All patients with E. coli or K. pneumoniae bacteremia were identified. Antibiotic susceptibility results for cephalosporin and carbapenem and the status of ESBL production by each isolate were obtained. The total number of episodes of bacteremia caused by E. coli or K. pneumoniae bacteremia was analyzed to evaluate the prevalence of ESBL-producing E. coli and K. pneumoniae (ESBL Enterobacteriaceae) and carbapenem-resistant Enterobacteriaceae (CRE).
A matched case-control study within the retrospective cohort was performed, and only the first episode of bacteremia caused by an ESBL-producing isolate was included. All patients with cefotaxime-susceptible ESBL E. coli and K. pneumoniae bacteremia were identified and were matched with a 1:3 ratio to control patients with cefotaxime-resistant ESBL E. coli and K. pneumoniae bacteremia based on age, sex, ward, and case year to evaluate the clinical characteristics and outcomes of cefotaxime-susceptible ESBL E. coli and K. pneumoniae bacteremia. Patients who had polymicrobial bacteremia were excluded.
Clinical data were collected from electronic medical records and included the following information: demographics, preexisting medical conditions, source of bacteremia, source control measures, microbiological data, antibiotic therapy, and outcomes. This study was approved by the Institutional Review Board of Asan Medical Center (IRB no. 2021-1568).
Bacteremia characterized by a positive blood culture obtained from a patient who had been hospitalized for at least 48 h was classified as a nosocomial infection [14]. Community-onset bacteremia characterized by a positive blood culture obtained within 48 h of hospitalization was classified as healthcare-associated or community-acquired infection, according to the definition by Friedman et al. [15]. Prognoses of underlying disease were classified according to the McCabe and Jackson system as follows: rapidly fatal (when death was expected within several months), ultimately fatal (when death was expected within four years), and non-fatal (when life expectancy was over four years) [16]. The Charlson comorbidity index was used to score the severity of the underlying disease [17]. The severity of illness at the time of bacteremia was assessed by the Acute Physiology and Chronic Health Evaluation II (APACHE II) score [18], and the severity of bacteremia was classified as sepsis, severe sepsis, and septic shock, as proposed by the International Sepsis Definitions Conference [19]. The source of bacteremia was identified according to the definition of the Centers for Disease Control and Prevention [20]. Eradicable foci and removal of eradicable foci were defined as described in a previous study [21]. Appropriate empirical treatment was defined as the use of an antimicrobial agent in a susceptible pathogen within 24 h of the index blood culture. Appropriate definitive treatment was defined as the use of an in vitro active antimicrobial agent within 5 days of the acquisition of the first positive blood culture and treatment for more than 48 h after antimicrobial susceptibility data became available [9]. β-lactam/β-lactamase inhibitor combinations were considered appropriate if the agent had an in vitro activity on the susceptibility test. The main outcome of bacteremia was 30-day mortality after its onset.
Species and antimicrobial susceptibilities were determined using a MicroScan WalkAway 96 plus System and Neg Combo Panel Type 44 or 72 (Beckman Coulter, Brea, CA, USA), according to the standard criteria of the CLSI [22]. The clinical microbiology laboratory in our hospital reported the results of phenotypic detection of ESBL using the MicroScan ESBL detection test (included in the Neg Combo Panel), which included testing with cefotaxime and ceftazidime alone or together with a fixed concentration of clavulanate. CRE was defined as a member of the family Enterobacteriaceae (E. coli and K. pneumoniae in this study) demonstrating resistance to any carbapenem (ertapenem, meropenem, or imipenem), based on antimicrobial susceptibility testing [23]. Six out of the ten isolates of cefotaxime-susceptible ESBL Enterobacteriaceae were collected, and ESBL production was phenotypically determined by the combined disk test using cefotaxime and ceftazidime alone and in combination with clavulanic acid, according to the CLSI guidelines [22].
Trend analysis of prevalence rates of ESBL-producing isolates and CRE isolates was evaluated using Poisson regression models. The Student’s t-test or Mann–Whitney U test was used to compare continuous variables, and the Pearson chi-squared test or Fisher’s exact test was used to compare corresponding categorical variables, as appropriate. A two-tailed P-value<0.05 was considered statistically significant. Analyses were performed using SPSS software version 21.0 (SPSS Inc., Chicago, IL, USA).
This study was conducted according to the research protocol approved by the Asan Medical Center IRB in accordance with the Declaration of Helsinki and the Good Clinical Practice set by the Korea Food and Drug Administration. Identifying information in the electronic database was encrypted to protect personal privacy. Given the retrospective and observational nature of the study, written informed patient consent was waived by the IRB of the Asan Medical Center as no intervention was involved and no patient-identifying information was included.
A total of 10 205 episodes of E. coli bacteremia and 5501 episodes of K. pneumoniae bacteremia were identified during the study period. Of these, 32.5% (3183/10 205) of the E. coli isolates and 24.2% (1332/5501) of the K. pneumoniae isolates were ESBL-producing. A total of 10 (0.06% [10/15 706], 95% confidence interval [CI], 0.03%-0.12%) episodes of bacteremia with cefotaxime-susceptible ESBL E. coli and K. pneumoniae were identified from January 2011 to December 2020 (Fig. 1). The number of bacteremias caused by cefotaxime-susceptible ESBL E. coli and K. pneumoniae remained low, and there was no significant change in the prevalence over the study period (P=0.43 for trend). All six cefotaxime-susceptible ESBL isolates collected during the study period were confirmed as ESBL producers by the combined disk test.
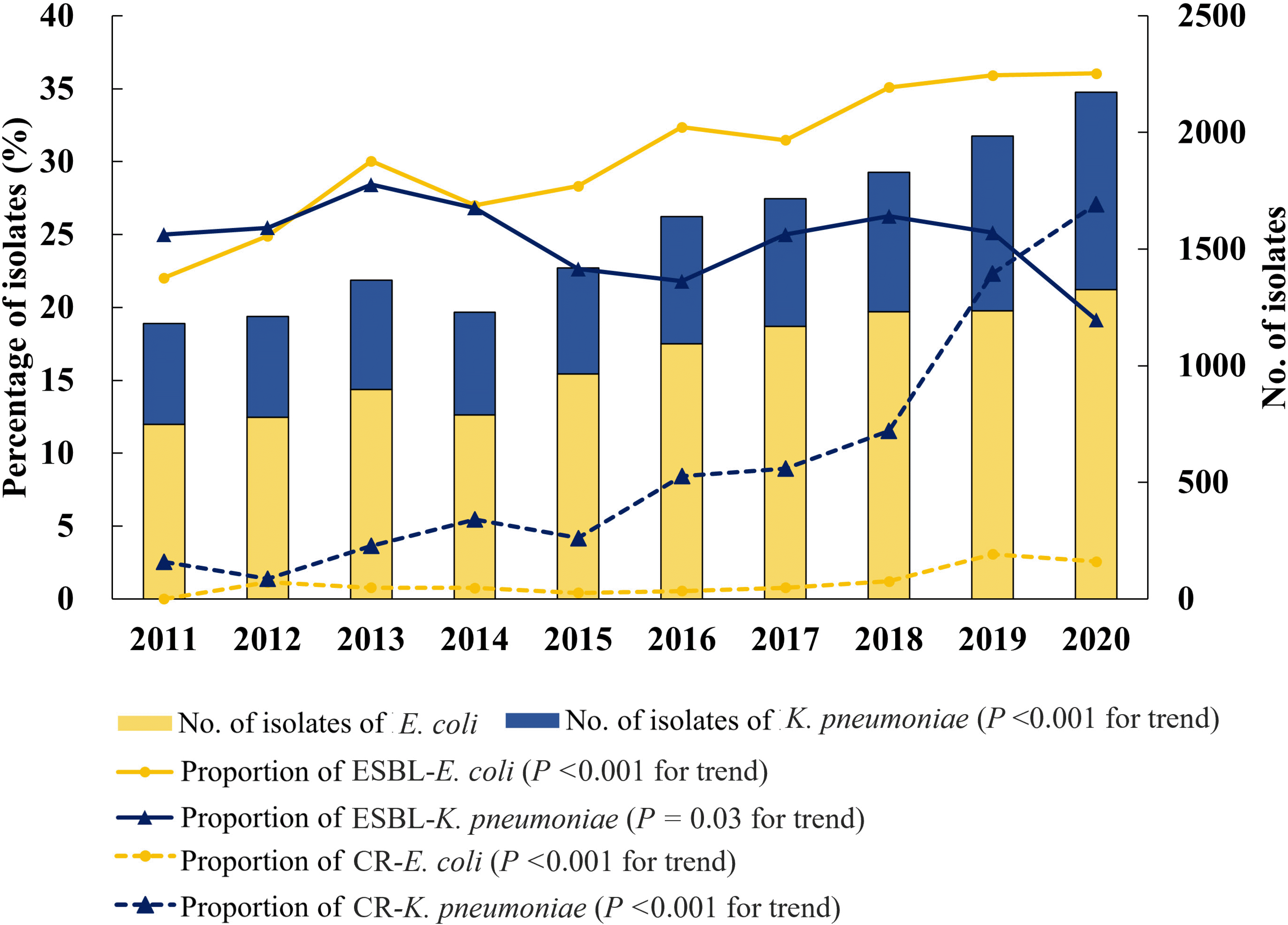
The total number of episodes of bacteremia caused by E. coli and K. pneumoniae increased from 2011 to 2020 (P<0.001 for trend), as shown in Fig. 1. The proportions of ESBL-producing isolates and CRE isolates also increased (both P<0.001 for trend). The proportion of ESBL-producing isolates gradually increased from 23.1% in 2011 to 29.5% in 2020 (incidence rate ratio [IRR], 1.03; 95% CI, 1.02-1.04), whereas the proportion of carbapenem-resistant isolates increased rapidly from 0.9% in 2011 to 12.1% in 2020 (IRR, 1.37; 95% CI, 1.33-1.42). Furthermore, the proportion of carbapenem-resistant K. pneumoniae increased rapidly and surpassed that of ESBL-producing K. pneumoniae in 2020 (Fig. 2).
The baseline and clinical characteristics of patients with cefotaxime-susceptible ESBL E. coli and K. pneumoniae bacteremia are shown in Table 1. Only one patient (10%) had community-acquired infection, and the majority had healthcare exposure (90%) and previous antibiotic use within 3 months (90%). The antibiotic susceptibility test reported susceptibility for cefotaxime in these 10 isolates; however, none of the patients received cephalosporin as definitive treatment, and half received antibiotics other than carbapenem (Table 2). None of the patients with cefotaxime-susceptible ESBL E. coli and K. pneumoniae bacteremia died within 30 days of the initial positive culture.
Patients with cefotaxime-susceptible ESBL E. coli and K. pneumoniae bacteremia were compared with those with cefotaxime-resistant ESBL E. coli and K. pneumoniae bacteremia. The two groups were largely comparable in terms of demographic and underlying variables (Table 1). They displayed similar patterns in terms of site of acquisition and previous antibiotic use (both P=0.44). The Charlson comorbidity index scores, severity of sepsis, and APACHE II scores were also comparable. Approximately 70% of the patients in both groups received appropriate empirical treatment, and most received appropriate definitive treatment. Patients with cefotaxime-susceptible ESBL E. coli and K. pneumoniae bacteremia tended to receive carbapenem less frequently for definitive treatment than those with cefotaxime-resistant ESBL E. coli and K. pneumoniae bacteremia (P=0.09). There was no significant difference in 30-day mortality in patients with cefotaxime-susceptible and cefotaxime-resistant ESBL E. coli and K. pneumoniae bacteremia (0% [0/10] vs. 23% [7/30], P=0.16).
The numbers of ESBL-producing E. coli and K. pneumoniae and CRE bacteremia increased; however, cefotaxime-susceptible ESBL E. coli and K. pneumoniae bacteremia remained rare over the study period. The CLSI recommends that susceptibility results for cephalosporin be reported “as found” without a mandatory requirement for ESBL testing. In this study, the microbiology laboratory in our hospital identified ESBL production via an automated antimicrobial susceptibility test (MicroScan Neg Combo Panel) in all the Enterobacteriaceae isolates. Previous studies have reported the specificity of the ESBL confirmation panel in MicroScan to be 73-97% [24, 25]. We performed ESBL confirmation tests on the six available isolates to exclude any false positives. Infante et al. [26] reported the incidence of very major errors (VME) for cefotaxime susceptibility testing using MicroScan to be 0.63%, and Chung et al. [27] reported 0% VME for cefotaxime in our hospital. Therefore, we considered the possibility of false positives in cefotaxime-susceptible isolates to be low.
The underlying diseases/conditions and severities of infection in patients with cefotaxime-susceptible ESBL E. coli and K. pneumoniae bacteremia were similar to those of patients with cefotaxime-resistant ESBL E. coli and K. pneumoniae, particularly in terms of healthcare and antibiotic exposure. These epidemiological characteristics were more similar to those of cefotaxime-resistant ESBL E. coli and K. pneumoniae compared with those of ESBL-non-producing E. coli and K. pneumoniae [28,29]. Half of the cefotaxime-susceptible patients received antibiotics other than carbapenem, and their mortality was numerically lower than that of the cefotaxime-resistant group. Our results are consistent with previous studies suggesting that cefotaxime MIC, rather than ESBL production, is associated with mortality [7-9,30]. However, we failed to demonstrate a statistically significant difference in mortality between the two groups due to the limited number of patients with cefotaxime-susceptible isolates. Multicenter studies with larger numbers of patients are required. Furthermore, studies comparing the clinical outcomes of antibiotics other than carbapenem in patients with cefotaxime-susceptible ESBL isolates are required.
We found that the prevalence of cefotaxime-susceptible ESBL isolates since the revision of the CLSI recommendations was 0.06% over 10 years. Ku et al. [30] reported a prevalence of 0.38% over the five years from 2006-2010. The low prevalence in both studies supports the CLSI guidelines, which recommend reporting cefotaxime susceptibility “as found,” without mandatory ESBL confirmation tests.
Since the first detection of Klebsiella pneumoniae carbapenemase-producing K. pneumoniae in 2010 in Korea [31], a rising trend of carbapenemase-producing Enterobacteriaceae has been reported on sentinel surveillance conducted by the Korea Disease Control and Prevention [32]. Noteworthy, the proportion of CRE surpassed that of ESBL among K. pneumoniae in our study.
Our study had several limitations. First, none of the patients received cephalosporin as a definitive treatment despite their susceptibility. Therefore, we were unable to evaluate whether clinical outcomes differed if patients with cefotaxime-susceptible ESBL E. coli and K. pneumoniae bacteremia received extended-spectrum cephalosporin based on the susceptibility test. Second, we could not perform ESBL confirmation tests on four of the cefotaxime-susceptible ESBL isolates since they were not collected and stored. Furthermore, we could not perform the broth test for cefotaxime; therefore, we cannot exclude the possibility of false negative results for cefotaxime susceptibility, although the VME for cefotaxime in our hospital was low. Third, we reviewed antibiotic use within 3 months based on electronic medical records; however, we cannot exclude antibiotic use prescribed outside the hospital, which was not recorded in our charts. Fourth, the study consisted of patients from a single center, indicating potential selection bias. Therefore, a larger study including more diverse hospital settings is required.
In conclusion, our study indicates that the prevalence of cefotaxime-susceptible ESBL E. coli and K. pneumoniae bacteremia and its mortality are low. These results support the current CLSI recommendation for elective ESBL testing. The rapidly increasing carbapenem resistance, particularly in K. pneumoniae, is a potential concern.
ACKNOWLEDGEMENTS
This study was supported by a grant (grant number: 2021IL0042) from the Asan Institute for Life Sciences, Asan Medical Center, Seoul, Korea.
We sincerely thank Eun Sil Kim, Kyoung A Jhang, and Sun Hwa Park for help with the data collection.
REFERENCES
1. Bennett JE, Dolin R, Blaser MJ. 2019. Mandell, Douglas, and Bennett's principles and practice of infectious diseases. 9th ed. Elsevier;Philadelphia: p. 270.
2. Kadri SS. 2020; Key takeaways from the U.S. CDC's 2019 antibiotic resistance threats report for frontline providers. Crit Care Med. 48:939–45. DOI: 10.1097/CCM.0000000000004371. PMID: 32282351. PMCID: PMC7176261.

3. Knothe H, Shah P, Krcmery V, Antal M, Mitsuhashi S. 1983; Transferable resistance to cefotaxime, cefoxitin, cefamandole and cefuroxime in clinical isolates of Klebsiella pneumoniae and Serratia marcescens. Infection. 11:315–7. DOI: 10.1007/BF01641355. PMID: 6321357.

4. Diekema DJ, Hsueh PR, Mendes RE, Pfaller MA, Rolston KV, Sader HS, et al. 2019; The microbiology of bloodstream infection: 20-year trends from the SENTRY antimicrobial surveillance program. Antimicrob Agents Chemother. 63:e00355–19. DOI: 10.1128/AAC.00355-19. PMID: 31010862. PMCID: PMC6591610.

5. Clinical. 2010. Performance standards for antimicrobial susceptibility testing. CLSI supplement M100. 20th ed. Clinical and Laboratory Standards Institute;Wayne: DOI: 10.1016/s0196-4399(01)88009-0.
6. Dudley MN, Ambrose PG, Bhavnani SM, Craig WA, Ferraro MJ. Dudley MN, Ambrose PG, Bhavnani SM, Craig WA, Ferraro MJ, Jones RN. Antimicrobial Susceptibility Testing Subcommittee of the Clinical and Laboratory Standards Institute. 2013; Background and rationale for revised clinical and laboratory standards institute interpretive criteria (Breakpoints) for Enterobacteriaceae and Pseudomonas aeruginosa: I. Cephalosporins and Aztreonam. Clin Infect Dis. 56:1301–9. DOI: 10.1093/cid/cit017. PMID: 23334813.
7. Andes D, Craig WA. 2005; Treatment of infections with ESBL-producing organisms: pharmacokinetic and pharmacodynamic considerations. Clin Microbiol Infect. 11 Suppl 6:10–7. DOI: 10.1111/j.1469-0691.2005.01265.x. PMID: 16209701.

8. Paterson DL, Ko WC, Von Gottberg A, Casellas JM, Mulazimoglu L, Klugman KP, et al. 2001; Outcome of cephalosporin treatment for serious infections due to apparently susceptible organisms producing extended-spectrum beta-lactamases: implications for the clinical microbiology laboratory. J Clin Microbiol. 39:2206–12. DOI: 10.1128/JCM.39.6.2206-2212.2001. PMID: 11376058. PMCID: PMC88112.

9. Kang CI, Kim SH, Park WB, Lee KD, Kim HB, Kim EC, et al. 2004; Bloodstream infections due to extended-spectrum beta-lactamase-producing Escherichia coli and Klebsiella pneumoniae: risk factors for mortality and treatment outcome, with special emphasis on antimicrobial therapy. Antimicrob Agents Chemother. 48:4574–81. DOI: 10.1128/AAC.48.12.4574-4581.2004. PMID: 15561828. PMCID: PMC529180.

10. National Committee for Clinical Laboratory Standards. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically: approved standard. 5th ed. National Committee for Clinical Laboratory Standards;Wayne: DOI: 10.1111/j.1469-0691.2005.01265.x.
11. Hombach M, Mouttet B, Bloemberg GV. 2013; Consequences of revised CLSI and EUCAST guidelines for antibiotic susceptibility patterns of ESBL- and AmpC β-lactamase-producing clinical Enterobacteriaceae isolates. J Antimicrob Chemother. 68:2092–8. DOI: 10.1093/jac/dkt136. PMID: 23633681.

12. Livermore DM, Andrews JM, Hawkey PM, Ho PL, Keness Y, Doi Y, et al. 2012; Are susceptibility tests enough, or should laboratories still seek ESBLs and carbapenemases directly? J Antimicrob Chemother. 67:1569–77. DOI: 10.1093/jac/dks088. PMID: 22461311.

13. Hsueh PR, Ko WC, Wu JJ, Lu JJ, Wang FD, Wu HY, et al. 2010; Consensus statement on the adherence to Clinical and Laboratory Standards Institute (CLSI) Antimicrobial Susceptibility Testing Guidelines (CLSI-2010 and CLSI-2010-update) for Enterobacteriaceae in clinical microbiology laboratories in Taiwan. J Microbiol Immunol Infect. 43:452–5. DOI: 10.1016/S1684-1182(10)60070-9. PMID: 21075714.

14. Garner JS, Jarvis WR, Emori TG, Horan TC, Hughes JM. 1988; CDC definitions for nosocomial infections, 1988. Am J Infect Control. 16:128–40. Erratum in: Am J Infect Control 1988;16:177. DOI: 10.1016/0196-6553(88)90053-3. PMID: 2841893.

15. Friedman ND, Kaye KS, Stout JE, McGarry SA, Trivette SL, Briggs JP, et al. 2002; Health care--associated bloodstream infections in adults: a reason to change the accepted definition of community-acquired infections. Ann Intern Med. 137:791–7. DOI: 10.7326/0003-4819-137-10-200211190-00007. PMID: 12435215.

16. McCabe WR, Jackson GG. 1962; Gram-negative bacteremia: II. Clinical, laboratory, and therapeutic observations. Arch Intern Med. 110:856–64. DOI: 10.1001/archinte.1962.03620240038007.
17. Charlson ME, Pompei P, Ales KL, MacKenzie CR. 1987; A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 40:373–83. DOI: 10.1016/0021-9681(87)90171-8. PMID: 3558716.

18. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. 1985; APACHE II: a severity of disease classification system. Crit Care Med. 13:818–29. DOI: 10.1097/00003246-198510000-00009. PMID: 3928249.
19. Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2003; ; SCCM/ESICM/ACCP/ATS/SIS. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 31:1250–6. DOI: 10.1097/01.CCM.0000050454.01978.3B. PMID: 12682500.
20. Horan TC, Andrus M, Dudeck MA. 2008; CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control. 36:309–32. Erratum in: Am J Infect Control 2008;36:655. DOI: 10.1016/j.ajic.2008.03.002. PMID: 18538699.

21. Seo H, Lee SC, Chung H, Ra SH, Sung H, Kim MN, et al. 2020; Clinical and microbiological analysis of risk factors for mortality in patients with carbapenem-resistant Enterobacteriaceae bacteremia. Int J Antimicrob Agents. 56:106126. DOI: 10.1016/j.ijantimicag.2020.106126. PMID: 32755654.

22. Clinical. 2021. Performance standards for antimicrobial susceptibility testing. CLSI supplement M100. 31st ed. Clinical and Laboratory Standards Institute;Wayne: DOI: 10.1016/s0196-4399(01)88009-0.
23. Tamma PD, Goodman KE, Harris AD, Tekle T, Roberts A, Taiwo A, et al. 2017; Comparing the outcomes of patients with carbapenemase-producing and non-carbapenemase-producing carbapenem-resistant Enterobacteriaceae bacteremia. Clin Infect Dis. 64:257–64. DOI: 10.1093/cid/ciw741. PMID: 28013264. PMCID: PMC5241781.
24. Wiegand I, Geiss HK, Mack D, Stürenburg E, Seifert H. 2007; Detection of extended-spectrum beta-lactamases among Enterobacteriaceae by use of semiautomated microbiology systems and manual detection procedures. J Clin Microbiol. 45:1167–74. DOI: 10.1128/JCM.01988-06. PMID: 17287329. PMCID: PMC1865808.

25. Linscott AJ, Brown WJ. 2005; Evaluation of four commercially available extended-spectrum beta-lactamase phenotypic confirmation tests. J Clin Microbiol. 43:1081–5. DOI: 10.1128/JCM.43.3.1081-1085.2005. PMID: 15750065. PMCID: PMC1081251.

26. Infante A, Martín C, Gázquez G, Buñuel F. Ortiz de la Tabla V. 2021; Rapid identification and antimicrobial susceptibility testing of Gram-negative rod on positive blood cultures using MicroScan panels. Eur J Clin Microbiol Infect Dis. 40:151–7. DOI: 10.1007/s10096-020-04014-3. PMID: 32860091.

27. Chung JW, Jeon HS, Sung H, Kim MN. 2009; Evaluation of MicroScan and Phoenix system for rapid identification and susceptibility testing using direct inoculation from positive BACTEC blood culture bottles. Korean J Lab Med. 29:25–34. DOI: 10.3343/kjlm.2009.29.1.25. PMID: 19262075.

28. Ceken S, Iskender G, Gedik H, Duygu F, Mert D, Kaya AH, et al. 2018; Risk factors for bloodstream infections due to extended-spectrum β-lactamase producing Enterobacteriaceae in cancer patients. J Infect Dev Ctries. 12:265–72. DOI: 10.3855/jidc.9720. PMID: 31851636.

29. van Hout D, Verschuuren TD, Bruijning-Verhagen PCJ, Bosch T, Schürch AC, Willems RJL, et al. 2020; Extended-spectrum beta-lactamase (ESBL)-producing and non-ESBL-producing Escherichia coli isolates causing bacteremia in the Netherlands (2014-2016) differ in clonal distribution, antimicrobial resistance gene and virulence gene content. PLoS One. 15:e0227604. DOI: 10.1371/journal.pone.0227604. PMID: 31935253. PMCID: PMC6959556.
30. Ku NS, Chung HS, Choi JY, Yong D, Lee K, Kim JM, et al. 2015; Clinical usefulness of the 2010 clinical and laboratory standards institute revised breakpoints for cephalosporin use in the treatment of bacteremia caused by Escherichia coli or Klebsiella spp. Biomed Res Int. 2015:831074. DOI: 10.1155/2015/831074. PMID: 25793209. PMCID: PMC4352463.
31. Hong SK, Yong D, Kim K, Hong SS, Hong SG, Khosbayar T, et al. 2013; First outbreak of KPC-2-producing Klebsiella pneumoniae sequence type 258 in a hospital in South Korea. J Clin Microbiol. 51:3877–9. DOI: 10.1128/JCM.01730-13. PMID: 24006005. PMCID: PMC3889788.

32. Ahn YS, Bahk HJ, Lee Y. 2021; Epidemiology of carbapenem-resistant Enterobacteriaceae in Korea between 2018 and 2019. Public Health Wkly Rep. 14:413–20.
Fig. 1
Ten-year trends of bacteremia caused by ESBL-producing and carbapenem-resistant Escherichia coli and Klebsiella pneumoniae. ESBL indicates carbapenem-susceptible ESBL-producing Escherichia coli and Klebsiella pneumoniae.
Abbreviations: CTX, cefotaxime; CRE, carbapenem-resistant Enterobacteriaceae; ESBL, extended-spectrum β-lactamase.
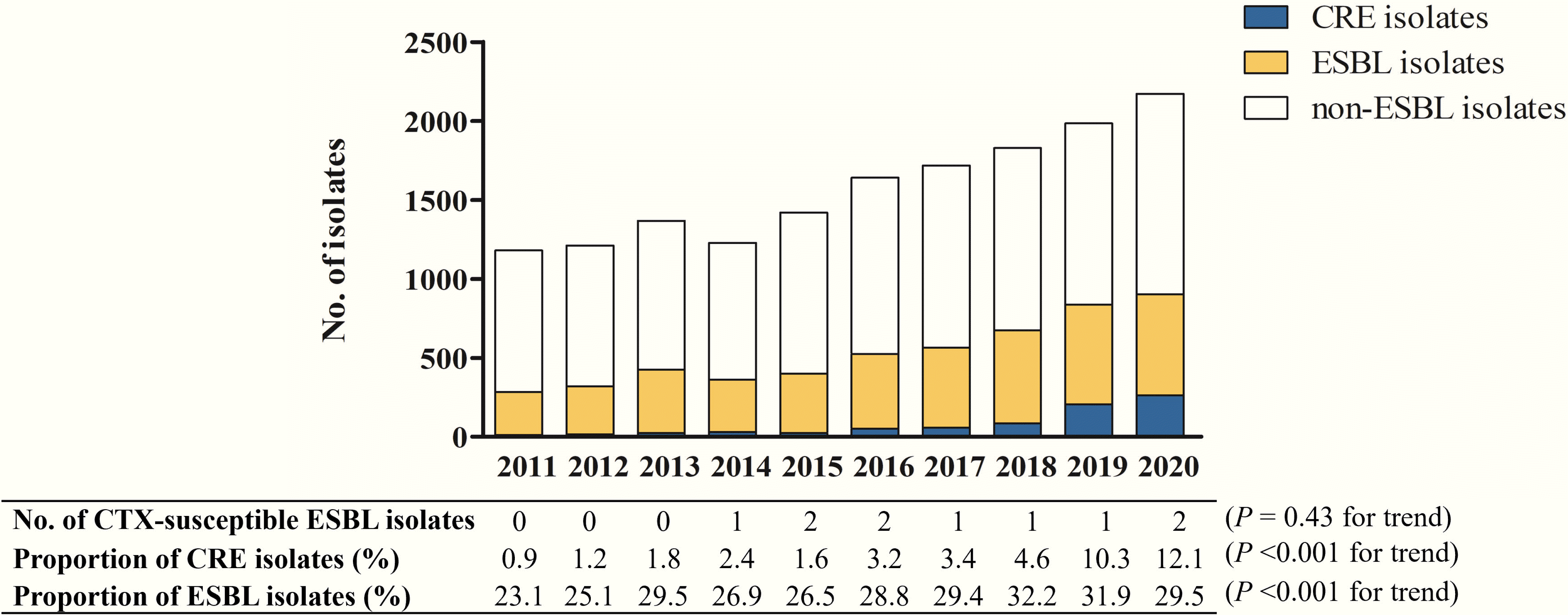
Fig. 2
Ten-year trends in proportions of ESBL-production and carbapenem-resistance in Escherichia coli and Klebsiella pneumoniae bacteremia.
Abbreviations: ESBL, extended-spectrum β-lactamase; CR, carbapenem-resistant.
Baseline and clinical characteristics of patients with ESBL-producing Escherichia coli and Klebsiella pneumoniae bacteremia according to cefotaxime susceptibility
Table 2
Clinical management and outcomes of patients with ESBL-producing Escherichia coli and Klebsiella pneumoniae bacteremia




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download