This article has been
cited by other articles in ScienceCentral.
Abstract
A ruptured brain arteriovenous malformation (bAVM) presenting with a hematoma may have unseen parts of the shunts in diagnostic angiography in the acute phase, which may lead to innate incomplete evaluation for the whole angioarchitecture of the bAVM. Even though it is generally accepted that the nidus of a ruptured bAVM may be underestimated in angiography during the acute phase due to hematoma compression, documentation of the underestimated parts has not been described in the literature. The authors report 2 cases of ruptured bAVMs in which the obscured segments were cast with liquid embolic material, which suggests a potential presence of obscured segments in bAVMs.
Go to :

Keywords: Intracranial arteriovenous malformation, Endovascular procedure, Therapeutic embolization
INTRODUCTION
Brain arteriovenous malformations (bAVMs) are treated by surgical resection, embolization, and/or radiosurgery. There are remaining controversies regarding the timing of treatment in ruptured bAVMs. Some authors have reported notable success with curative transvenous embolization in the acute hemorrhagic phase [
1]. However, during the acute phase of hemorrhage, targeted embolization has been reported to be safe and effective [
2-
5], and delayed curative treatment is still preferred by many institutions [
6].
During the acute hemorrhagic phase, caution is warranted when interpreting angiography, because the mass effect of hematoma or edema may compress the nidus of the ruptured bAVM resulting in underestimation of the size. Furthermore, “early recurrence” after complete obliteration or resection may not be an actual recurrence, but only a reappearance of the once obscured parts of the treated bAVM. However, as far as the authors have searched, no literature has reported imaging evidence of the unseen parts in ruptured bAVMs.
We present 2 cases of a ruptured bAVM with an obscured segment that could be visualized during Onyx casting, and we discuss the potential of suboptimal angiographic evaluation during the acute hemorrhagic phase.
Go to :

CASE REPORT
Case 1
A pediatric patient presented with intracranial hemorrhage in the right temporal lobe (
Fig. 1A). Though magnetic resonance angiography (MRA) failed to visualize any frank bAVM, a small nodular arterial signal was observed on the lateral surface of the hematoma (
Fig. 1B). Digital subtraction angiography (DSA) performed on the same day revealed a small bAVM fed by the posterior temporal branch of the right posterior cerebral artery with a perinidal aneurysm corresponding to the nodular structure observed on MRA images. This bAVM was drained to the lateral sinus and the deep vein system. We proceeded directly to emergency embolization to occlude the perinidal aneurysm. A casting of embolic material (Onyx 18; Medtronic) was started at the distal-most accessible part of the proper feeder. After obliteration of the visible nidus and aneurysmal dilatation, the Onyx continued to flow into a nidus-like structure at the medial posterior aspect of the previously cast nidus (
Fig. 1G,
H). This structure was not anticipated by pre-embolization angiography or superselective microangiography (
Fig. 1C–
F). Using a microcatheter with a detachable tip (Apollo; Medtronic), 0.7 mL of Onyx was injected, and final angiography revealed no evidence of an angiographically visible shunt. The patient did not experience any periprocedural complication or re-bleeding, and early follow-up DSA at 3 months did not show evidence of a remnant or recurred shunt. Two-year follow-up DSA was also angiographically negative for recurrence.
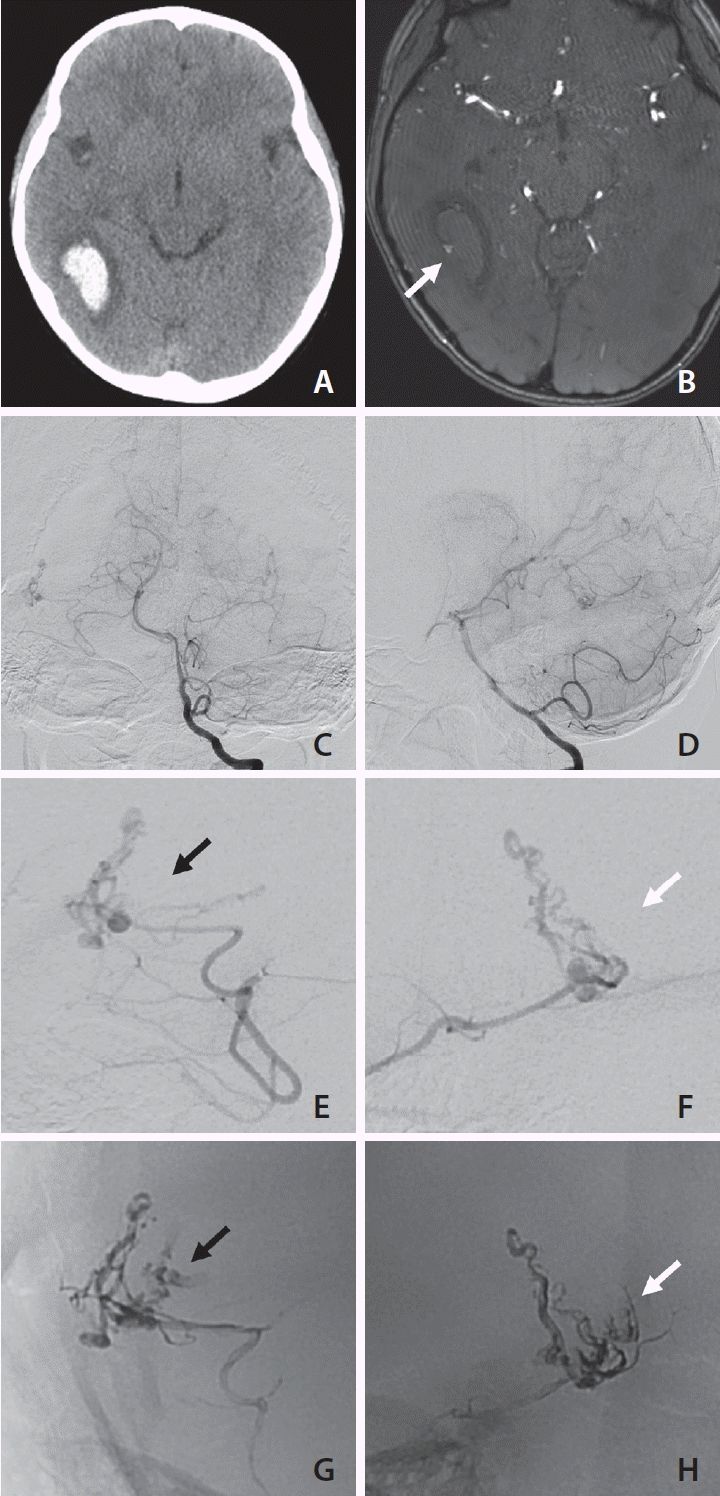 | Fig. 1.A pediatric patient with a ruptured brain arteriovenous malformation (bAVM) presented with a right temporal lobe hematoma (A). Magnetic resonance angiography (MRA) source image (B) shows a nodular flow-related signal suspected to be an aneurysm and the source of bleeding (white arrow). Digital subtraction angiography image obtained on the day of presentation (C, D) demonstrates a small bAVM fed by the right posterior cerebral artery posterior temporal branch and a small perinidal aneurysm corresponding to the MRA finding. As compared with magnified images of anteroposterior (E) and lateral (F) microangiography views during emergency embolization, images of the final Onyx cast (G and H, obtained using the same anteroposterior and lateral views as E and F) show some nidus-like structures not observed by pre-embolization angiography (black arrows on E and G; white arrows on F and H). 
|
Case 2
A pediatric patient presented with acute onset severe headache and aphasia. Initial imaging work up displayed left frontal lobe hematoma (
Fig. 2A) caused by rupture of a bAVM. MR imaging and angiographic findings indicated the nidus deviated to inferolateral side of the hematoma (
Fig. 2B). Emergency diagnostic angiography performed on the day of presentation revealed a small bAVM supplied by superior division branch of the left middle cerebral artery (
Fig. 2C,
D). The bAVM drained to a cortical vein then to the superior sagittal sinus. In addition, a large venous ectasia was observed just distal to the nidal structure. The venous portion of the nidus or distorted nidal structure exhibited slightly congested flow with contrast stagnation. Although the nidus had some ‘en-passage’ feeders from the main feeder artery, the attending neurosurgeon and interventionist agreed to proceed to emergency embolization to relieve intranidal pressure and avoid the potential risk of acute re-bleeding. The final Onyx cast appears to be larger compared to the pre-embolization angiographic finding and includes some nidal structures that were not apparent on pre-embo images (
Fig. 2E,
F). A total of 1.7 mL of Onyx was cast and after the procedure, and immediate post-embolization angiography produced no evidence of a remnant shunt. Follow-up DSA performed 7 months after the procedure at another hospital revealed a small nidus at the inferior portion of the Onyx cast that drained into the same draining vein observed by pre-embolization angiography (not shown). This reappeared small nidus was managed by radiosurgery at an outside hospital.
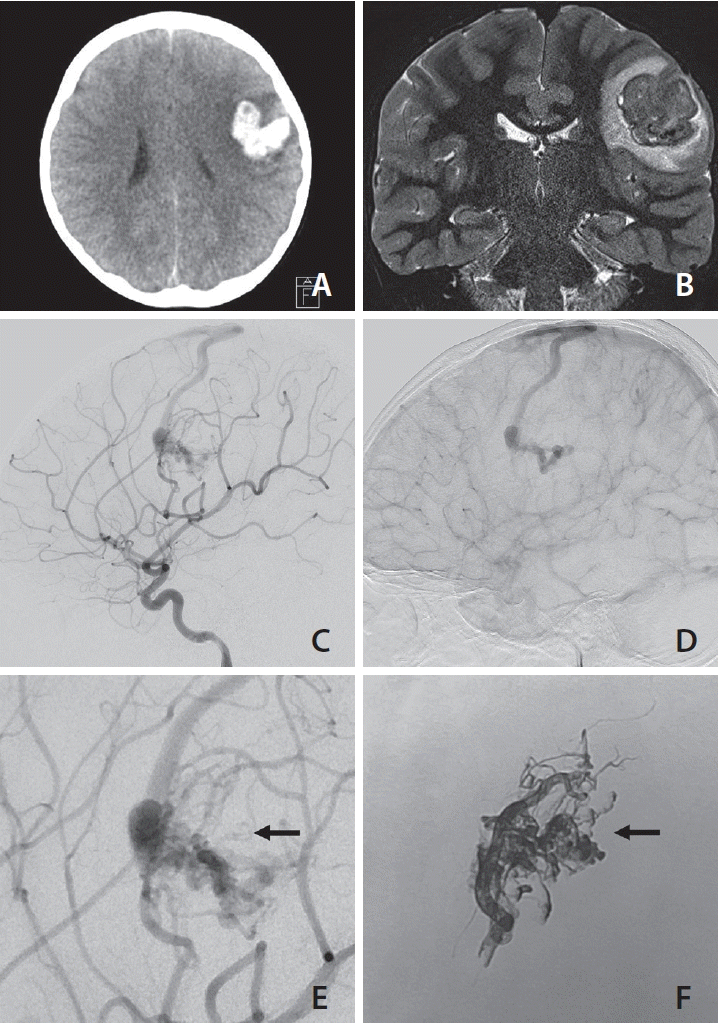 | Fig. 2.A pediatric ruptured brain arteriovenous malformation (bAVM) with left frontal lobe hematoma (A). T2-weighted magnetic resonance image coronal view (B) shows tangled flow voids, considered to be a vascular lesion causing hematoma. Lateral view digital subtraction angiography images of early (C) and late (D) phases reveal a small bAVM associated with large venous ectasia and slightly congested venous outflow of the shunt. Comparison of a magnified pre-embolization angiography lateral image (E) and an image of the final Onyx cast (F) at the same projection demonstrates that a remarkable portion of the Onyx cast was filled into the nidus which was not visualized by pre-procedural angiography (arrows on E and F). 
|
Go to :

DISCUSSION
We report 2 cases of visualization of an obscured segment during Onyx embolization of pediatric bAVMs presenting with acute hemorrhage. Embolization of a ruptured bAVM in the acute phase may be advocated when the weak point can be secured by partial embolization with acceptable risk, or shunt flow can be reduced with reasonable risk. The targeted embolization of acute ruptured bAVMs has been reported to produce remarkable results [
2-
5], and even curative embolization during the acute hemorrhagic phase through transvenous access was described [
1].
Changes in the angioarchitectures of ruptured bAVMs were well-described by Jin et al. [
6] and reportedly were observed in roughly 40% of cases. As hematomas resolve, obscured segments may revive or regress spontaneously with thrombosis. Initial DSA may fail to demonstrate the whole bAVM angioarchitecture; because of this, some parts of the shunts may be compressed by hematomas.
It is not new and is generally agreed that some parts of shunts may be compressed by hematomas; therefore, initial DSA may fail to demonstrate the whole bAVM angioarchitecture. We refer to these collapsed parts of bAVMs as ‘obscured segments’ to distinguish them from ‘hidden compartments’ [
7]. These physiologically or hemodynamically unfilled parts located at the periphery of the nidus may be visualized during embolization because of flow re-direction and altered shunt hemodynamics after partial obliteration. We deliberately used the term ‘obscured segment’ to describe our observation of Onyx casting into a compressed and/or collapsed portion of a bAVM primarily caused by the mass effect of a hematoma. The term ‘compartment’ has been used to indicate a hemodynamic structural unit of a bAVM in the field of microsurgery [
8]. Parts compressed by a hematoma are not necessarily ‘compartments’ but can be any component of a bAVM; and thus, we refer to them as ‘segments’.
Even with meticulous microcatheter angiographic workup, an obscured segment may not be visualized during the acute phase of hemorrhage. Furthermore, it is difficult to presume the original location of an obscured segment on delayed angiography because anatomical orientations and local geometries can be altered significantly after hematoma resorption. And the bleeding point of a ruptured bAVM may be destroyed by the rupture event and not revived even after hematoma resorption.
Flow of liquid embolic material to an obscured segment during emergency embolization was not intended but proved to be an effective means of revealing unseen segments. Onyx casted obscured segments represent the original bAVM architecture, at least in part, because the time interval between rupture and embolization is short enough to be considered concurrent.
We believe the presence of an obscured segment is not a reason to hesitate or delay the embolization of a ruptured bAVM during the acute phase. Instead, we would like to point out the underappreciation of the hematoma effect causing incomplete angiographic evaluation during the acute rupture phase or immediate post-treatment stage. The reappearance of a shunt may be misinterpreted as recurrence when follow-up DSA is not executed at the proper time. Brain AVM recurrences have been reported even after angiographically documented complete surgical resection, and notably, pediatric patients and ruptured bAVMs have higher risks of recurrence [
9-
13]. Even though immediate post-operative angiography is often taken as a standard for treatment outcome assessments, it should be interpreted with caution, especially when bAVMs have bled. The optimal timing of the first follow-up DSA 30 after the first-ever documented negative angiography has not been systematically established. However, immediate post-operative angiography may be inappropriate for documenting the curative treatment of bAVMs, because edema, vasospasm, or thrombosis near resection sites can mask small remaining shunt components. We suspect some cases reported as recurrence after complete bAVM obliteration or excision were reappearances of obscured segments. Hence, the timings of follow-up studies should be carefully determined. An appropriate time interval should be chosen for total hemorrhage resorption and the resolution of post-operative changes, but also to capture early reappearance for timely management. Cross-sectional imaging may play an important role in determining when to perform follow-up DSA.
Our report has limitations as it is based on observations with 2 cases that were not systematically selected to represent all ruptured bAVM patients treated at our institution. A comprehensive review of cases is required to evaluate the frequency of the phenomenon and its clinical implications. A lack of microsurgical or histopathologic correlation is another weak point of this report. Lastly, we need future studies to systematically evaluate better imaging tools to visualize those obscured segments in ruptured bAVMs. Nevertheless, although this case report lacks scientific veracity, Onyx filling into the obscured segments of ruptured bAVMs may interest neurointerventionists who are up to treating bAVMs endovascularly.
In conclusion, ruptured bAVMs with hematoma may have obscured segments, and we report 2 cases with visualization of those unseen parts through Onyx casting. When interpreting acute phase angiography findings as negative for remnant shunts, caution should be exercised.
Go to :







 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download