1. Abecassis IJ, Nerva JD, Ghodke BV, Sekhar LN, Levitt MR, Kim LJ, et al. The dual microcatheter technique for transvenous embolization of dural arteriovenous fistulae. J Neurointerv Surg. 2017; Jun. 9(6):578–82.

2. Borota L, Mahmoud E, Nyberg C, Lewén A, Enblad P, Ronne-Engström E. Dual lumen balloon catheter - An effective substitute for two single lumen catheters in treatment of vascular targets with challenging anatomy. J Clin Neurosci. 2018; May. 51:91–9.

3. Case D, Folzenlogen Z, Rochon P, Kumpe D, Roark C, Seinfeld J. Embolization of head and neck vascular malformations using serial arterial embolization followed by dominant arterial embolization with two microcatheter technique. J Vasc Interv Neurol. 2018; Nov. 10(2):47–51.
4. Chapot R, Stracke P, Velasco A, Nordmeyer H, Heddier M, Stauder M, et al. The pressure cooker technique for the treatment of brain AVMs. J Neuroradiol. 2014; Mar. 41(1):87–91.
5. Flores BC, See AP, Weiner GM, Jankowitz BT, Ducruet AF, Albuquerque FC. Use of the Apollo detachable-tip microcatheter for endovascular embolization of arteriovenous malformations and arteriovenous fistulas. J Neurosurg. 2018; Mar. 130(3):963–71.

6. Gentric JC, Raymond J, Batista A, Salazkin I, Gevry G, Darsaut TE. Dual-lumen balloon catheters may improve liquid embolization of vascular malformations: an experimental study in swine. AJNR Am J Neuroradiol. 2015; May. 36(5):977–81.

7. Heit JJ, Faisal AGS, Telischak NA, Choudhri O, Do HM. Headway duo microcatheter for cerebral arteriovenous malformation embolization with n-BCA. J Neurointerv Surg. 2016; Nov. 8(11):1181–5.

8. Jagadeesan BD, Grigoryan M, Hassan AE, Grande AW, Tummala RP. Endovascular balloon-assisted embolization of intracranial and cervical arteriovenous malformations using dual-lumen coaxial balloon microcatheters and Onyx: initial experience. Neurosurgery. 2013; Dec. 73(2 Suppl Operative):ons238–43. discussion ons243.
9. Mehta T, Hassan A, Masood K, Tekle W, Grande A, Tummala R, et al. The next step in balloon assisted endovascular neurosurgical procedures: a case series of initial experience with the Scepter Mini balloon microcatheter. Interv Neuroradiol. 2021; Apr. 27(2):298–306.
10. Orozco LD, Luzardo GD, Buciuc RF. Transarterial balloon assisted Onyx embolization of pericallosal arteriovenous malformations. J Neurointerv Surg. 2013; Jul. 5(4):e18.

11. Ozpar R, Nas OF, Hacikurt K, Taskapilioglu MO, Kocaeli H, Hakyemez B. Endovascular treatment of intracranial arteriovenous malformations using detachable-tip microcatheters and Onyx 18®. Diagn Interv Imaging. 2019; Jun. 100(6):353–61.
12. Paramasivam S, Niimi Y, Fifi J, Berenstein A. Onyx embolization using dual-lumen balloon catheter: initial experience and technical note. J Neuroradiol. 2013; Oct. 40(4):294–302.

13. Spiotta AM, James RF, Lowe SR, Vargas J, Turk AS, Chaudry MI, et al. Balloon-augmented Onyx embolization of cerebral arteriovenous malformations using a dual-lumen balloon: a multicenter experience. J Neurointerv Surg. 2015; Oct. 7(10):721–7.
14. Spiotta AM, Miranpuri AS, Vargas J, Magarick J, Turner RD, Turk AS, et al. Balloon augmented Onyx embolization utilizing a dual lumen balloon catheter: utility in the treatment of a variety of head and neck lesions. J Neurointerv Surg. 2014; Sep. 6(7):547–55.
15. Vollherbst DF, Chapot R, Wallocha M, Saatci I, Cekirge S, Rouchaud A, et al. First clinical multicenter experience with the new Scepter Mini microballoon catheter. J Neurointerv Surg. 2021; Mar. 13(3):261–6.

16. Vollherbst DF, Otto R, Do TD, von Deimling A, Kauczor HU, Bendszus M, et al. Extra-small dual-lumen micro-balloon catheters can improve endovascular embolization: an experimental in vivo and in vitro study. J Neurointerv Surg. 2018; Nov. 10(11):1092–6.

17. Weber W, Kis B, Siekmann R, Kuehne D. Endovascular treatment of intracranial arteriovenous malformations with onyx: technical aspects. AJNR Am J Neuroradiol. 2007; Feb. 28(2):371–7.
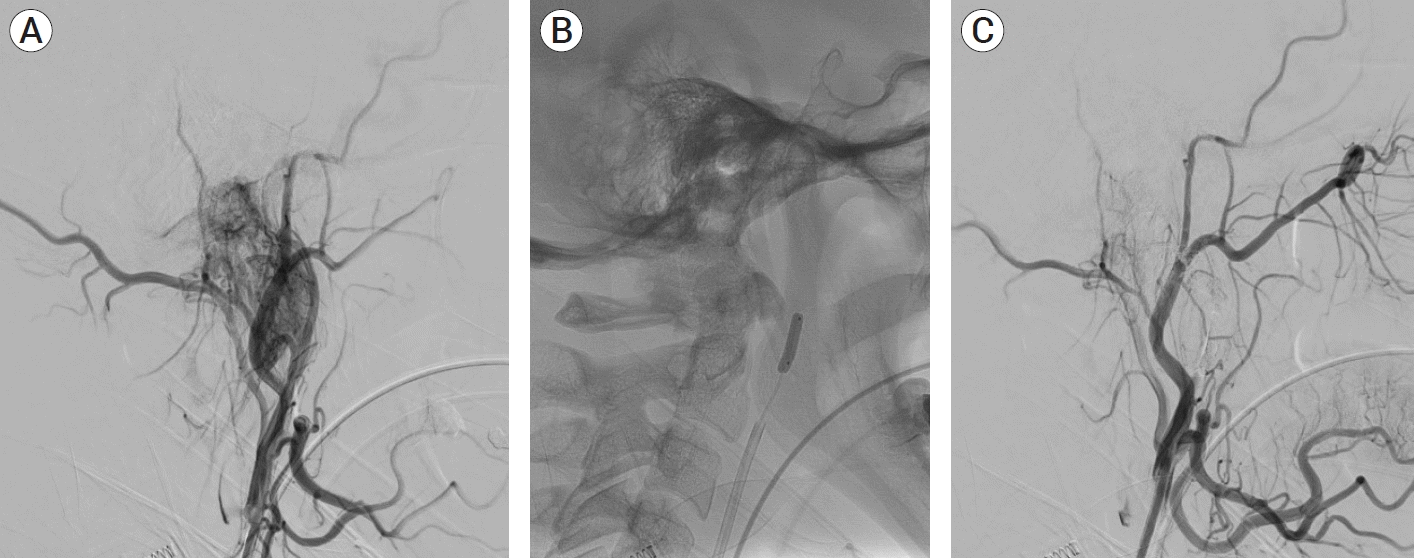
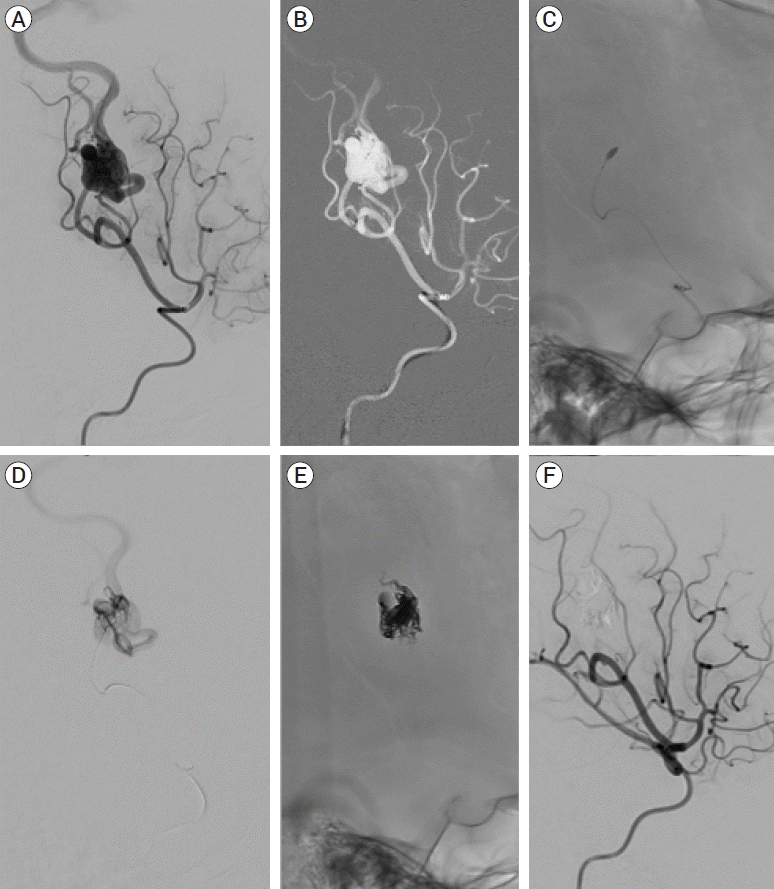




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download