1. Forgacs D, Silva-Moraes V, Sautto GA, Hanley HB, Gattiker JL, Jefferson AM, et al. The effect of waning on antibody levels and memory B cell recall following SARS-CoV-2 infection or vaccination. Vaccines (Basel). 2022; 10(5):696. PMID:
35632452.
3. Patrizio A, Ferrari SM, Antonelli A, Fallahi P. A case of Graves’ disease and type 1 diabetes mellitus following SARS-CoV-2 vaccination. J Autoimmun. 2021; 125:102738. PMID:
34653776.
4. Rela M, Jothimani D, Vij M, Rajakumar A, Rammohan A. Auto-immune hepatitis following COVID vaccination. J Autoimmun. 2021; 123:102688. PMID:
34225251.
5. Vuille-Lessard É, Montani M, Bosch J, Semmo N. Autoimmune hepatitis triggered by SARS-CoV-2 vaccination. J Autoimmun. 2021; 123:102710. PMID:
34332438.
6. Garrido I, Lopes S, Simões MS, Liberal R, Lopes J, Carneiro F, et al. Autoimmune hepatitis after COVID-19 vaccine - more than a coincidence. J Autoimmun. 2021; 125:102741. PMID:
34717185.
7. Sagy I, Zeller L, Raviv Y, Porges T, Bieber A, Abu-Shakra M. New-onset systemic lupus erythematosus following BNT162b2 mRNA COVID-19 vaccine: a case series and literature review. Rheumatol Int. 2022; 42(12):2261–2266. PMID:
36098769.
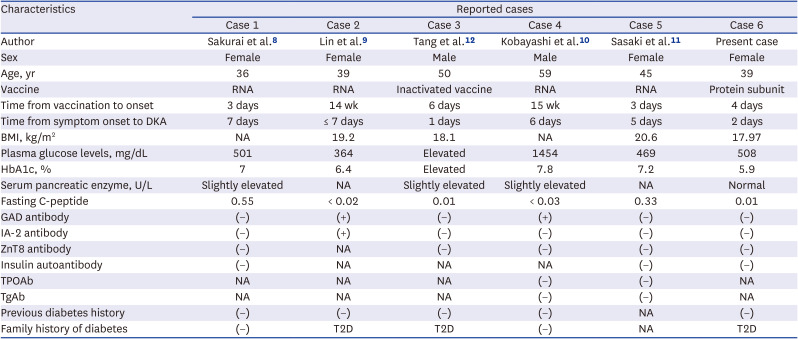
8. Sakurai K, Narita D, Saito N, Ueno T, Sato R, Niitsuma S, et al. Type 1 diabetes mellitus following COVID-19 RNA-based vaccine. J Diabetes Investig. 2022; 13(7):1290–1292.
9. Lin R, Lin YW, Chen MH. Fulminant type 1 diabetes mellitus after SARS-CoV-2 vaccination: a case report. Vaccines (Basel). 2022; 10(11):1905. PMID:
36423001.
10. Kobayashi T, Yakou F, Saburi M, Hirose A, Akaoka H, Hirota Y, et al. New-onset atypical fulminant type 1 diabetes after COVID-19 vaccination: a case report. Clin Case Rep. 2022; 10(10):e6473. PMID:
36267825.
11. Sasaki K, Morioka T, Okada N, Natsuki Y, Kakutani Y, Ochi A, et al. New-onset fulminant type 1 diabetes after severe acute respiratory syndrome coronavirus 2 vaccination: a case report. J Diabetes Investig. 2022; 13(7):1286–1289.
12. Tang X, He B, Liu Z, Zhou Z, Li X. Fulminant type 1 diabetes after COVID-19 vaccination. Diabetes Metab. 2022; 48(2):101324. PMID:
35091092.
13. Chee YJ, Ng SJ, Yeoh E. Diabetic ketoacidosis precipitated by Covid-19 in a patient with newly diagnosed diabetes mellitus. Diabetes Res Clin Pract. 2020; 164:108166. PMID:
32339533.
14. Fadini GP, Morieri ML, Boscari F, Fioretto P, Maran A, Busetto L, et al. Newly-diagnosed diabetes and admission hyperglycemia predict COVID-19 severity by aggravating respiratory deterioration. Diabetes Res Clin Pract. 2020; 168:108374. PMID:
32805345.
15. Liao HC, Wu WL, Chiang CY, Huang MS, Shen KY, Huang YL, et al. Low-dose SARS-CoV-2 S-trimer with an emulsion adjuvant induced th1-biased protective immunity. Int J Mol Sci. 2022; 23(9):4902. PMID:
35563292.
16. Silveira MM, Moreira GM, Mendonça M. DNA vaccines against COVID-19: perspectives and challenges. Life Sci. 2021; 267:118919. PMID:
33352173.
17. Ibrahim S, Monaco GS, Sims EK. Not so sweet and simple: impacts of SARS-CoV-2 on the β cell. Islets. 2021; 13(3-4):66–79. PMID:
33970787.
18. Drucker DJ. Diabetes, obesity, metabolism, and SARS-CoV-2 infection: the end of the beginning. Cell Metab. 2021; 33(3):479–498. PMID:
33529600.
19. Yang S, Li Y, Dai L, Wang J, He P, Li C, et al. Safety and immunogenicity of a recombinant tandem-repeat dimeric RBD-based protein subunit vaccine (ZF2001) against COVID-19 in adults: two randomised, double-blind, placebo-controlled, phase 1 and 2 trials. Lancet Infect Dis. 2021; 21(8):1107–1119. PMID:
33773111.
20. Tran TN, May BP, Ung TT, Nguyen MK, Nguyen TT, Dinh VL, et al. Preclinical immune response and safety evaluation of the protein subunit vaccine Nanocovax for COVID-19. Front Immunol. 2021; 12:766112. PMID:
34938290.
21. Lui DT, Lee KK, Lee CH, Lee AC, Hung IF, Tan KC. Development of Graves’ disease after SARS-CoV-2 mRNA vaccination: a case report and literature review. Front Public Health. 2021; 9:778964. PMID:
34888290.
22. Oikawa Y, Shimada A. Possible involvement of autoimmunity in fulminant type 1 diabetes. Diabetol Int. 2020; 11(4):329–335. PMID:
33088639.
23. Luo S, Ma X, Li X, Xie Z, Zhou Z. Fulminant type 1 diabetes: a comprehensive review of an autoimmune condition. Diabetes Metab Res Rev. 2020; 36(6):e3317. PMID:
32223049.
24. Imagawa A, Hanafusa T, Miyagawa J, Matsuzawa Y. Osaka IDDM Study Group. A novel subtype of type 1 diabetes mellitus characterized by a rapid onset and an absence of diabetes-related antibodies. N Engl J Med. 2000; 342(5):301–307. PMID:
10655528.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download