1. Banerjee C, Chimowitz MI. Stroke caused by atherosclerosis of the major intracranial arteries. Circ Res. 2017; 120:502–513.

2. Wong LK. Global burden of intracranial atherosclerosis. Int J Stroke. 2006; 1:158–159.

3. Sacco RL, Kargman DE, Zamanillo MC. Race-ethnic differences in stroke risk factors among hospitalized patients with cerebral infarction: the Northern Manhattan Stroke Study. Neurology. 1995; 45:659–663.

4. Tsivgoulis G, Vadikolias K, Heliopoulos I, Katsibari C, Voumvourakis K, Tsakaldimi S, et al. Prevalence of symptomatic intracranial atherosclerosis in Caucasians: a prospective, multicenter, transcranial Doppler study. J Neuroimaging. 2014; 24:11–17.

5. Yaghi S, Rostanski SK, Boehme AK, Martin-Schild S, Samai A, Silver B, et al. Imaging parameters and recurrent cerebrovascular events in patients with minor stroke or transient ischemic attack. JAMA Neurol. 2016; 73:572–578.

6. Derdeyn CP, Chimowitz MI, Lynn MJ, Fiorella D, Turan TN, Janis LS, et al. Aggressive medical treatment with or without stenting in high-risk patients with intracranial artery stenosis (SAMMPRIS): the final results of a randomised trial. Lancet. 2014; 383:333–341.

7. Chimowitz MI, Lynn MJ, Derdeyn CP, Turan TN, Fiorella D, Lane BF, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011; 365:993–1003.
8. Zaidat OO, Fitzsimmons BF, Woodward BK, Wang Z, Killer-Oberpfalzer M, Wakhloo A, et al. Effect of a balloon-expandable intracranial stent vs medical therapy on risk of stroke in patients with symptomatic intracranial stenosis: the VISSIT randomized clinical trial. JAMA. 2015; 313:1240–1248.

9. Sangha RS, Naidech AM, Corado C, Ansari SA, Prabhakaran S. Challenges in the medical management of symptomatic intracranial stenosis in an urban setting. Stroke. 2017; 48:2158–2163.

10. Holmstedt CA, Turan TN, Chimowitz MI. Atherosclerotic intracranial arterial stenosis: risk factors, diagnosis, and treatment. Lancet Neurol. 2013; 12:1106–1114.

11. Flusty B, de Havenon A, Prabhakaran S, Liebeskind DS, Yaghi S. Intracranial atherosclerosis treatment: past, present, and future. Stroke. 2020; 51:e49–e53.
12. Yaghi S, Prabhakaran S, Khatri P, Liebeskind DS. Intracranial atherosclerotic disease. Stroke. 2019; 50:1286–1293.

13. Narwal P, Cutting S, Prabhakaran S, Yaghi S. Diagnosis and management of active intracranial atherosclerotic disease: a case study. Stroke. 2018; 49:e221–e223.
14. Dakay K, Yaghi S. Symptomatic intracranial atherosclerosis with impaired distal perfusion: a case study. Stroke. 2018; 49:e10–e13.
15. de Havenon A, Khatri P, Prabhakaran S, Yeatts SD, Peterson C, Sacchetti D, et al. Hypoperfusion distal to anterior circulation intracranial atherosclerosis is associated with recurrent stroke. J Neuroimaging. 2020; 30:468–470.

16. Amin-Hanjani S, Pandey DK, Rose-Finnell L, Du X, Richardson D, Thulborn KR, et al. Effect of hemodynamics on stroke risk in symptomatic atherosclerotic vertebrobasilar occlusive disease. JAMA Neurol. 2016; 73:178–185.

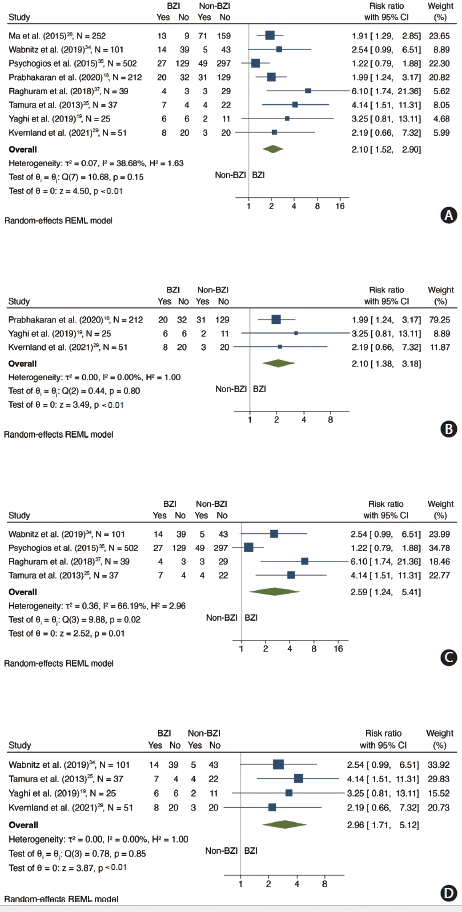
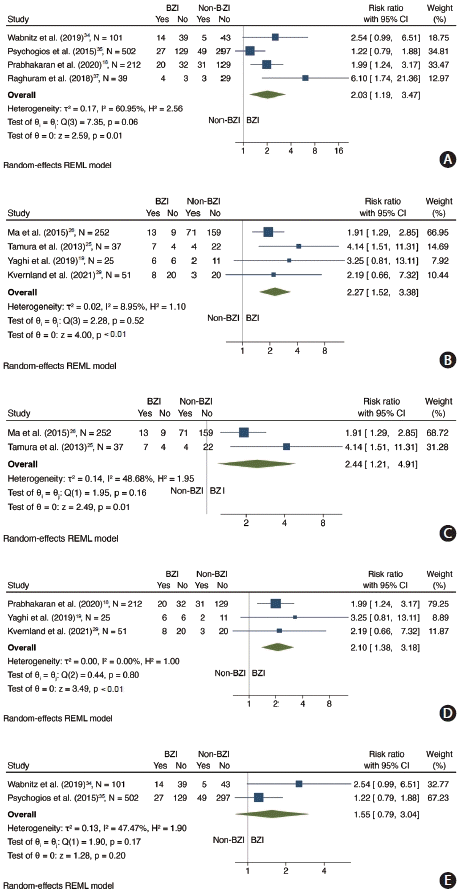
17. Prabhakaran S, Liebeskind DS, Cotsonis G, Nizam A, Feldmann E, Sangha RS, et al. Predictors of early infarct recurrence in patients with symptomatic intracranial atherosclerotic disease. Stroke. 2021; 52:1961–1966.

18. Prabhakaran S, Sangha R, Ansari S, Romano J, Sylaja P, Liebeskind DS. Abstract wmp45: Borderzone infarct pattern predicts recurrent stroke in patients with intracranial stenosis. Stroke. 2020; 51(Suppl 1):AWMP45.

19. Yaghi S, Grory BM, Prabhakaran S, Yeatts SD, Cutting S, Jayaraman M, et al. Infarct pattern, perfusion mismatch thresholds, and recurrent cerebrovascular events in symptomatic intracranial stenosis. J Neuroimaging. 2019; 29:640–644.

20. Momjian-Mayor I, Baron JC. The pathophysiology of watershed infarction in internal carotid artery disease: review of cerebral perfusion studies. Stroke. 2005; 36:567–577.

21. Yamauchi H, Nishii R, Higashi T, Kagawa S, Fukuyama H. Hemodynamic compromise as a cause of internal border-zone infarction and cortical neuronal damage in atherosclerotic middle cerebral artery disease. Stroke. 2009; 40:3730–3735.

22. Kim SJ, Morales JM, Yaghi S, Honda T, Scalzo F, Hinman JD, et al. Intracranial atherosclerotic disease mechanistic subtypes drive hypoperfusion patterns. J Neuroimaging. 2021; 31:686–690.

23. Liu D, Sun W, Scalzo F, Xiong Y, Zhang X, Qiu Z, et al. Early magnetic resonance imaging predicts early neurological deterioration in acute middle cerebral artery minor stroke. J Stroke Cerebrovasc Dis. 2016; 25:469–474.

24. Kim JT, Kim HJ, Yoo SH, Park MS, Kwon SU, Cho KH, et al. MRI findings may predict early neurologic deterioration in acute minor stroke or transient ischemic attack due to intracranial atherosclerosis. Eur Neurol. 2010; 64:95–100.

25. Tamura A, Yamamoto Y, Nagakane Y, Takezawa H, Koizumi T, Makita N, et al. The relationship between neurological worsening and lesion patterns in patients with acute middle cerebral artery stenosis. Cerebrovasc Dis. 2013; 35:268–275.

26. Ma Y, Liu L, Pu Y, Zou X, Pan Y, Soo Y, et al. Predictors of neurological deterioration during hospitalization: results from the Chinese intracranial atherosclerosis (CICAS) study. Neurol Res. 2015; 37:385–390.

27. Easton JD, Saver JL, Albers GW, Alberts MJ, Chaturvedi S, Feldmann E, et al. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke. 2009; 40:2276–2293.

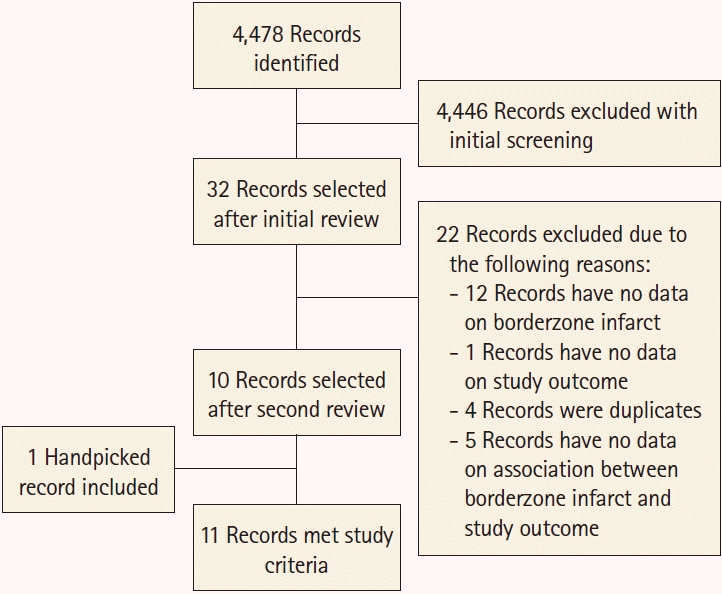
28. Wallace BC, Small K, Brodley CE, Lau J, Trikalinos TA. Deploying an interactive machine learning system in an evidencebased practice center: abstrackr. Proceedings of the 2nd ACM SIGHIT International Health Informatics Symposium. 2012. Jan. 28-30. Miami, FL, USA: Association for Computing Machinery;2012. p. 819–824.
29. Kvernland A, Prahbakaran S, Khatri P, Havenon AHD, Yeatts SD, Scher E, et al. Border-zone infarcts predict early recurrence in patients with large artery atherosclerotic subtype despite medical treatment. Stroke. 2021; 52:AP605.
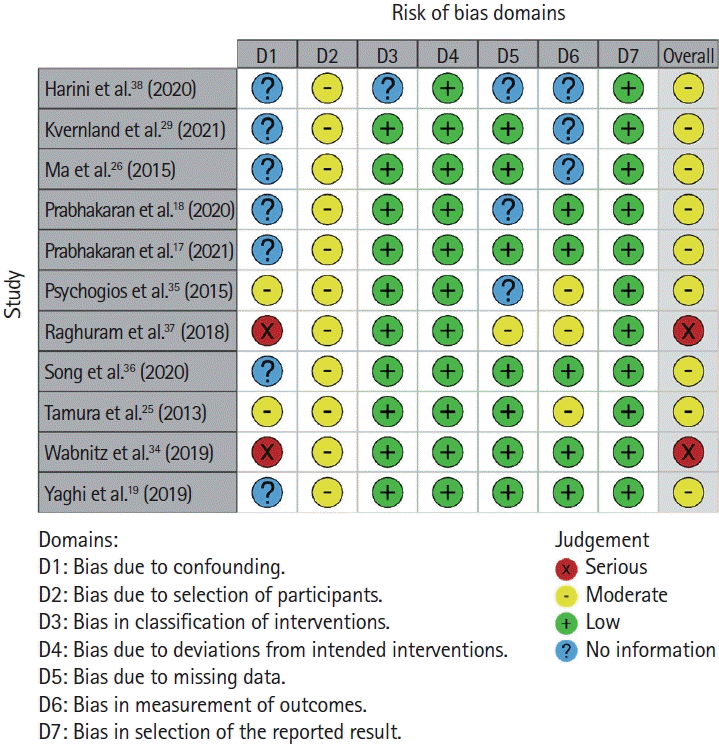
30. Sterne JA, Hernán MA, Reeves BC, Savovic´ J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016; 355:i4919.

31. Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane handbook for systematic reviews of interventions. Cochrane Database Syst Rev. 2019; 10:ED000142.

32. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997; 315:629–634.

33. Deeks JJ, Higgins JPT, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. In: Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, Welch VA, editors. Cochrane Handbook for Systematic Reviews of Interventions [Internet]. Version 6.3 [updated February 2022; accessed date]. London: Cochrane, 2022. Available from:
https://training.cochrane.org/handbook/archive/v6.2/chapter-10.
34. Wabnitz AM, Derdeyn CP, Fiorella DJ, Lynn MJ, Cotsonis GA, Liebeskind DS, et al. Hemodynamic markers in the anterior circulation as predictors of recurrent stroke in patients with intracranial stenosis. Stroke. 2019; 50:143–147.

35. Psychogios K, Stathopoulos P, Takis K, Vemmou A, Manios E, Spegos K, et al. The pathophysiological mechanism is an independent predictor of long-term outcome in stroke patients with large vessel atherosclerosis. J Stroke Cerebrovasc Dis. 2015; 24:2580–2587.
36. Song X, Zhao X, Liebeskind DS, Wang L, Xu W, Xu Y, et al. Incremental value of plaque enhancement in predicting stroke recurrence in symptomatic intracranial atherosclerosis. Neuroradiology. 2020; 62:1123–1131.

37. Raghuram K, Durgam A, Kohlnhofer J, Singh A. Relationship between stroke recurrence, infarct pattern, and vascular distribution in patients with symptomatic intracranial stenosis. J Neurointerv Surg. 2018; 10:1161–1163.

38. Harini P, Senthilvelan S, Saraf U, Kesavadas C, Sylaja PN, Sarma P. Infarct pattern as a predictor of outcome in symptomatic intracranial atherosclerotic disease. Int J Stroke. 2020; 15(Suppl 1):488.
39. Caplan LR, Hennerici M. Impaired clearance of emboli (washout) is an important link between hypoperfusion, embolism, and ischemic stroke. Arch Neurol. 1998; 55:1475–1482.

40. Gao P, Wang T, Wang D, Liebeskind DS, Shi H, Li T, et al. Effect of stenting plus medical therapy vs medical therapy alone on risk of stroke and death in patients with symptomatic intracranial stenosis: the CASSISS randomized clinical trial. JAMA. 2022; 328:534–542.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download