INTRODUCTION
Subglottic cysts (SGCs) are rare, benign lesions that cause upper airway narrowing in infants and young children. First reported by Wigger and Tang in 1968, these lesions occur in the subglottic space, the narrowest part of the upper airway [
1]. SGCs are formed due to mucosal damage by endotracheal intubation, and the subsequent healing of this damaged mucosa results in the blockage of mucosal glands, leading to the formation of subepithelial or submucosal cysts [
2]. Although there have been a few rare reports of congenital SGCs, most SGCs occur in premature infants with a history of endotracheal intubation [
3]. The symptoms include stridor, hoarseness, recurrent croup, and failure to thrive as a result of upper airway narrowing.
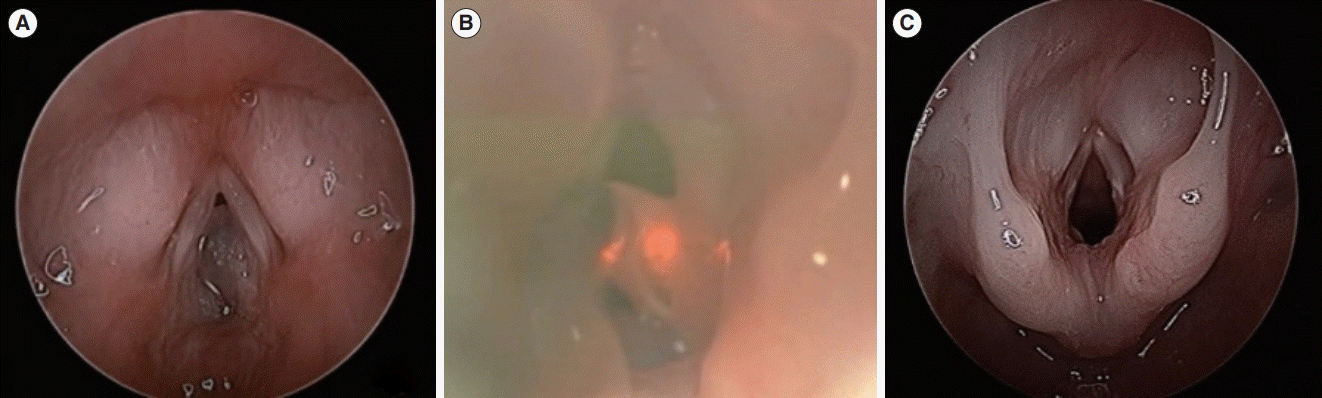
As abnormal breathing sounds are the primary symptom of SGCs, some patients are incorrectly diagnosed with asthma or recurrent croup, and inadequate treatment is provided. In addition, subglottic stenosis, laryngomalacia, vocal cord paralysis, hemangioma, lymphangioma, and foreign bodies that can cause upper airway narrowing should be considered in the differential diagnosis. Although SGCs are often diagnosed using flexible laryngoscopy in outpatient clinics, microlaryngobronchoscopy under general anesthesia can be used to accurately evaluate the extent of the lesion and provide treatment simultaneously.
Marsupialization, the treatment of choice for several types of cysts, is accomplished by a variety of methods, including CO
2 and thulium lasers, cold dissection, and laryngeal microdebriders [
4-
8]. The recurrence rate after treatment has been reported to vary from 0% to 69% [
9], and insufficient marsupialization of the cyst is the primary cause of recurrence [
10]. Because SGCs occur in the narrow subglottic space, it may be difficult to visualize the surgical field while performing endotracheal intubation.
For efficient marsupialization of SGCs, good exposure of the airway is required with an appropriate level of anesthesia and oxygenation. Apneic anesthesia is the most basic tubeless anesthetic technique for achieving a sufficient surgical field of view, but apnea can be maintained for only 3 minutes or less in children; therefore, its use for SGC treatment in children is limited [
11]. Booth et al. [
12] reported performing tubeless airway surgery while providing a high flow of warm and humidified oxygen and maintaining an appropriate level of anesthesia using total intravenous (IV) anesthesia in adult patients. We were able to successfully perform marsupialization of SGCs using a technique called “SponTaneous Respiration using IntraVEnous anesthesia and Hi-flow nasal oxygen” (STRIVE Hi) in children [
13,
14].
As SGCs are a rare cause of stridor in children, only a few studies have reported its clinical features, and the risk factors for SGCs, other than premature birth and history of intubation, are still unknown. Therefore, this study aimed to describe the clinical features and analyze the risk factors of SGCs. We also report our experience of using STRIVE Hi for the marsupialization of SGCs.
Go to :

RESULTS
Of the 11 children, eight (72.7%) were male and 3 (27.3%) were female. All the patients were preterm infants with a history of endotracheal intubation during the neonatal period. The mean duration of intubation was 21.5 days (range, 2–90 days). The average interval from extubation to the onset of symptoms due to SGC was 8.2 months (range, 1–14 months). All patients, except for one who had an abnormal radiologic finding, presented with stridor as a symptom. Seven patients (63.6%) had multiple cysts, and the occurrence rates on the left (54.5%) and right sides (45.5%) of the glottis were similar. All patients were diagnosed as having SGC by transnasal flexible laryngoscopy in the clinic. Under general anesthesia using the STRIVE Hi technique, marsupialization of the cysts was performed. During the follow-up period, which ranged from 3 months to 4 years, no recurrence was observed in any of the patients. Six patients (54.5%) showed coexistence of subglottic stenosis and SGC (
Table 1). No patients underwent tracheostomy.
Table 1.
Summary of the clinical features of 11 children with SGCs
|
No. |
Sex |
Gestational age at birth (wk) |
Duration of previous intubation (day) |
Presentation interval (mo) |
Obstruction grade of the subglottic cyst |
Presenting symptom |
Site |
Management |
Other airway lesion |
|
1 |
M |
34 |
15 |
10 |
1 |
Stridor |
Left 2, right 1 |
L, MMC |
SGS Gr II |
|
2 |
M |
33 |
7 |
5 |
3 |
Stridor, hoarseness |
Right 1 |
L, MMC, B |
SGS Gr II |
|
3 |
M |
32 |
2 |
12 |
2 |
Abnormal radiologic finding |
Left 1 |
C |
SGS Gr I |
|
4 |
M |
24 |
23 |
13 |
2 |
Stridor, dyspnea hoarseness |
Left 2, right 1 |
L, MMC |
- |
|
5 |
M |
33 |
10 |
14 |
3 |
Stridor |
Right 1 |
L |
SGS Gr II |
|
6 |
M |
24 |
37 |
1 |
2 |
Stridor |
Left 1 |
L |
- |
|
7 |
M |
27 |
10 |
3 |
2 |
Stridor, dyspnea, hoarseness |
Left 4, right 3 |
L |
- |
|
8 |
M |
30 |
17 |
9 |
2 |
Stridor |
Left 3, right 3 |
L, MMC, B |
SGS Gr II |
|
9 |
F |
24 |
90 |
4 |
4 |
Stridor, dyspnea, hoarseness |
Left 2, right 2 |
L, MMC, B |
Tracheomalacia |
|
10 |
F |
26 |
5 |
7 |
3 |
Stridor, dyspnea, |
Left 2, right 2 |
L |
SGS Gr I |
|
11 |
F |
24 |
20 |
12 |
4 |
Stridor |
Left 1, right 1 |
L, MMC |
Tracheomalacia |

Gestational age (28.3±4.2 weeks vs. 33.8±4.4 weeks;
P=0.001) and birth weight (1,134.1±515.1 g vs. 2,178.2±910.1 g;
P=0.001) were significantly lower in patients with SGCs than in controls. The duration of endotracheal intubation was significantly longer in patients with SGCs than in controls (21.5±24.8 days vs. 5.3±7.1 days;
P<0.001). There were no significant differences in sex, premature birth, or delivery methods between patients with SGCs and controls. Although patients with SGCs underwent endotracheal intubation more times than the controls, this difference was not statistically significant (1.6±0.8 vs. 1.3±0.8;
P=0.118) (
Table 2).
Table 2.
Comparison of the risk factors between SGC patients and the control group
|
Variable |
SGC patient |
Control |
P-value |
|
Sex |
|
|
0.692 |
|
Male |
8 (72.7) |
26 (78.8) |
|
|
Female |
3 (27.3) |
7 (21.2) |
|
|
Prematurity |
|
|
0.311 |
|
Term |
0 |
6 (18.2) |
|
|
Preterm |
11 (100) |
27 (81.8) |
|
|
Gestational age at birth (wk) |
28.3±4.2 |
33.8±4.4 |
0.001*
|
|
Birth weight (g) |
1,134.1±515.1 |
2,178.2±910.1 |
0.001*
|
|
Delivery method |
|
|
0.461 |
|
Vaginal delivery |
2 (18.2) |
11 (33.3) |
|
|
Cesarean delivery |
9 (81.8) |
22 (66.7) |
|
|
Duration of intubation (day) |
21.5±24.8 |
5.3±7.1 |
<0.001*
|
|
Frequency of intubation |
1.6±0.8 |
1.3±0.8 |
0.118 |

Univariate analysis revealed that gestational age (odds ratio [OR], 0.763;
P=0.003), birth weight (OR, 0.998;
P=0.005), and the intubation period (OR, 1.127;
P=0.021) were significantly associated with SGC development. Multivariate binary logistic regression showed that the intubation period (OR, 1.168;
P=0.043) was a significant independent risk factor for SGC (
Table 3).
Table 3.
Univariate and multivariate analyses of risk factors for SGCs
|
Variable |
Univariate analysis
|
Multivariate analysis
|
|
OR |
95% CI |
P-value |
OR |
95% CI |
P-value |
|
Sex |
|
|
|
|
|
|
|
Male |
1.393 |
0.290–6.679 |
0.679 |
|
|
|
|
Female |
1 (Reference) |
|
|
|
|
|
|
Gestational age at birth (wk) |
0.763 |
0.636–0.915 |
0.003*
|
|
|
|
|
Birth weight |
0.998 |
0.997–0.999 |
0.005*
|
|
|
|
|
Delivery method |
|
|
|
|
|
|
|
Vaginal delivery |
0.444 |
0.082–2.420 |
0.348 |
|
|
|
|
Cesarean delivery |
1 (Reference) |
|
|
|
|
|
|
Duration of intubation (day) |
1.127 |
1.018–1.248 |
0.021*
|
1.168 |
1.005–1.357 |
0.043*
|
|
Frequency of intubation |
1.506 |
0.691–3.281 |
0.303 |
|
|
|

Go to :

DISCUSSION
SGCs are a rare cause of upper airway narrowing or obstruction in children that has been reported to a limited extent in the literature. However, as the survival rate of preterm infants with a history of endotracheal intubation improves, the incidence of SGCs is likely to increase [
6,
16]. Regarding their pathogenesis, it is acknowledged that SGCs develop as a result of endotracheal intubation, and that prematurity and a history of intubation are recognized as contributing factors to their development [
17]. However, not all children who undergo intubation as neonates develop SGCs. Therefore, in this study, the authors analyzed a control group, which underwent intubation at the same time as the SGC patient group, to identify additional risk factors for SGCs beyond only a history of intubation. Gestational age, birth weight, and the intubation duration were found to be associated with SGCs in the present study. In addition, during the marsupialization of SGCs, the STRIVE Hi anesthesia technique was utilized, enabling complete marsupialization to be performed within a wide surgical field, with no need for tracheal intubation. As a result, there was no recurrence and the need for temporary tracheostomy was eliminated.
Children with SGCs usually show symptoms caused by upper airway narrowing, such as stridor, dyspnea, and hoarseness. Because of these symptoms and the low prevalence of SGCs, they are commonly misdiagnosed as bronchitis, asthma, and croup, resulting in inaccurate and unnecessary treatment [
18,
19]. In this study, some patients were treated with antibiotics, nebulizers, and oxygen supplements for respiratory diseases, but no improvement was achieved; therefore, these patients were referred to the otorhinolaryngology department for further evaluation and were ultimately diagnosed with SGC. This suggests that SGC must be considered in the differential diagnosis of pediatric patients with respiratory sound abnormalities if no improvement after treatment is observed or if symptoms of upper airway narrowing are suspected.
There are several causes of upper airway narrowing or obstruction. Therefore, laryngomalacia, vocal cord paralysis, subglottic hemangioma or lymphangioma, and foreign bodies obstructing the upper airway, must also be considered in the differential diagnosis [
18].
Laryngomalacia is marked by symptoms such as inspiratory stridor and choking while feeding, while vocal fold paralysis can lead to aspiration. SGCs do not exhibit any distinctive or specific symptoms except stridor. It is crucial to thoroughly examine the patient’s medical records for a diagnosis of SGC. Similar to previous reports, all of the children diagnosed with SGC in this study were born prematurely and had been intubated at birth [
3]. When an infant with stridor is born prematurely and has a history of intubation during the neonatal period, it is important to consider the possibility of SGC and conduct a laryngoscopy. Laryngoscopy is the most definitive diagnostic method for diagnosing upper airway obstruction. It is important to suspect SGC based on the child’s medical history, physical examination, and imaging tests and perform laryngoscopy as soon as possible. Laryngomalacia, vocal fold paralysis, and foreign objects can be observed relatively easily at the glottis level, but SGCs require a more thorough examination of the dark area below the glottis. Therefore, it is important to perform laryngoscopy carefully and always consider the possibility of SGC in patients with the medical histories described above. In this study, we emphasize that SGC can be accurately and easily diagnosed through transnasal flexible laryngoscopy in a clinical setting, rather than through other methods. Therefore, if upper airway narrowing is suspected or if no improvement is achieved despite conservative treatment for pulmonary abnormalities, transnasal flexible laryngoscopy should be performed without delay, and appropriate management should be implemented.
Marsupialization is the primary treatment of choice for SGC. Previous studies have reported that marsupialization can be performed using various techniques, including cold microinstruments, CO
2 and thulium lasers, microdebriders, and Bugbee fulgurating electrode devices [
8,
18,
20,
21]. Topical MMC was applied to prevent the recurrence of SGCs when a substantial amount of granulation tissue remained after marsupialization, as it is believed to inhibit fibroblast activity and reduce recurrence. However, this phenomenon was not statistically significant because of the small number of cases in this study [
22]. Balloon dilatation is a treatment modality that can be additionally performed after marsupialization. In this study, balloon dilatation was performed when residual soft tissue protruded into the tracheal lumen after marsupialization.
The insufficient marsupialization of SGCs increases the probability of recurrence [
10]. Therefore, adequate exposure of the SGC is essential for effective marsupialization. Because the endotracheal tube prevents sufficient exposure of the SGC, tubeless anesthetic techniques play an important role in preventing recurrence. Apneic anesthesia with intermittent ventilation and jet ventilation is a well-known tubeless anesthetic technique. However, in pediatric patients, the apnea time between intermittent ventilation is only about 3 minutes or shorter; this increases the operation time and can result in laryngeal damage due to repeated re-intubation [
11]. Furthermore, jet ventilation can cause airway barotrauma and subcutaneous emphysema [
23]. The STRIVE Hi anesthesia technique maintains a proper depth of anesthesia with spontaneous respiration using IV anesthesia and provides high-flow, warm, humidified oxygen through the nasal cannula to support breathing [
12]. Booth et al. [
12] reported the advantages of the STRIVE Hi anesthesia technique in adult patients who underwent laryngeal microsurgery. Despite the high recurrence rate of SGCs, which has been reported to range from 0% to 69%, recurrence was not observed in any of the patients in the present study because optimal visualization of the surgical field was obtained using the STRIVE Hi technique, resulting in complete marsupialization of the cyst [
9]. In addition, as sufficient oxygenation was achieved during surgery with the use of the STRIVE Hi technique, no patient required intubation during surgery. This finding is consistent with that of a previous study, which reported that high-flow oxygen could prolong apnea time and avoid repeated intubation during surgery [
24].
According to studies published to date, temporary tracheostomy was performed in 14%–33% of patients with SGCs [
3,
6,
16,
25-
27]. In some cases, tracheostomies were performed before establishing a diagnosis due to worsening dyspnea, but most were temporarily performed when the SGC obstructed the glottis, rendering tracheal intubation difficult or when the cyst obstructed access to the surgical field [
28]. The STRIVE Hi anesthesia technique may be useful for avoiding temporary tracheostomy in order to achieve effective marsupialization of SGC, since it can maintain proper oxygen saturation and thus can overcome the short apnea time in children.
It has been recently reported that the prognosis may differ depending on the depth of the SGC location. Unsaler et al. [
29] reported that subepithelial SGCs with a transparent, thin capsule were more easily marsupialized and did not recur after treatment, unlike those buried in the submucosa. However, in our study, the careful and complete removal of the residual mucosa of the SGC after uncapping of the cyst, regardless of its depth, seems to have contributed to the absence of recurrence. Nevertheless, recurrence could have been underestimated in this study because the recurrence of small cysts might have gone undetected, as only follow-up flexible laryngoscopy after surgery was performed in all patients at the outpatient clinic. Microlaryngobronchoscopy requiring general anesthesia was not performed, since none of the patients had respiratory symptoms suggesting recurrence. However, recurrence has been reported to occur even 4 years after surgery, and the recurrence rate was reported to be up to 56% in a previous study of 105 cases [
3,
6]. In contrast, the present study had a relatively small number of patients with a short follow-up period.
In this study, all patients with SGCs had a history of premature birth. We showed that low gestational age at birth, low birth weight, and a long intubation period were significant risk factors for SGCs. SGCs are retention cysts formed due to the blockage of the ducts of the mucous gland by subepithelial fibrosis, which results from the mucosal damage caused by endotracheal intubation and subsequent healing [
6]. Therefore, it can be assumed that more severe mucosal damage due to intubation is associated with a higher probability of SGC occurrence. An endotracheal tube that is too large for the airway of a premature infant causes more damage to the subglottis. As the airway size in premature infants increases significantly with age, a lower gestational age or weight at the time of intubation may be associated with a higher likelihood of mucosal damage, which results in the formation of SGC. Although a study reported that mucosal necrosis accompanied by inflammation occurred in the subglottis as early as 20 hours after intubation, a longer intubation period results in more mucosal damage due to repeated suctioning, vocal fold adduction, and endotracheal tube movement, which irritate the subglottis mucosa during mechanical ventilation [
30].
Unfortunately, the authors cannot guarantee that no SGC occurred in the children in the control group, as laryngoscopy was not performed after extubation in all cases, especially if no symptoms or only mild symptoms related to airway obstruction were present. It is possible that some SGCs may have gone unnoticed in these cases.
Our study has several limitations. First, this was a retrospective review with only 11 pediatric patients and a relatively short follow-up period at a single center. Further studies with a larger sample size, patients with various severities of SGC, and a longer follow-up period are needed to confirm our results. Patients who underwent neonatal endotracheal intubation during the same period as patients with SGCs were included in the control group. This process might have introduced selection bias, as the controls selected might not have been representative of their population. In addition, there have been reports of patients with SGCs without a history of tracheal intubation; therefore, other risk factors may exist in addition to the factors described in this study.
Go to :





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download