Abstract
Mature cystic teratomas (MCTs) of the ovary can occur at any age; however, the most common period is childbearing age, accounting for 10-20% of ovarian tumors and 85-97% of germ cell tumors. Differentiated thyroid cancers from MCTs in pregnant women are rare. A 32-year-old pregnant women presented with left ovarian mass revealed following a transabdominal ultrasonography performed to confirm pregnancy. After delivery, left ovarian cystectomy was performed and mass was examined by pathologists. The result of pathological examination was a combined papillary thyroid carcinoma (PTC) and stromal carcinoid from a mature teratoma. Thyroid ultrasonography was performed to check for accompanying thyroid cancer. Two nodules with no suspected malignancy were observed in both lobes and no other adjuvant therapy was administered. We would like to report this extremely rare case along with a literature review.
Struma ovarii (SO) is an uncommon form of ovarian germ cell tumor, in which thyroid tissue makes up more than 50% of the tumor.1) Malignant transformation is rare, but occurs in approximately 1-3% of cases,2) and papillary thyroid carcinoma (PTC) is the most common type of neoplasm which could be found in SO. Strumal carcinoid of the ovary is also an extraordinary form of primary germ cell tumor, and consists of an intimate mixture of thyroid tissue and carcinoid tissue.3,4) PTC and strumal carcinoid coexistence in patients with mature teratoma are very rare. Here, we present a rare case of combined PTC and strumal carcinoid arising from a mature cystic teratoma (MCT) in a 32-year-old pregnant woman.
A 32-year-old woman, primipara, visited our out-patient clinic with an ovarian mass suspected of malignancy. Although she had spasmodic dysmenorrhea before pregnancy, she did not experience abdominal pain or mass effect. A left ovarian mass measuring 7.42×7.51×7.46 cm was found inciden-tally during a transabdominal ultrasound for her routine pregnancy checkup (Fig. 1A, B). A left ovarian cystectomy was performed after delivery and mass was examined by pathologists.
A 9.0×6.0×4.0 cm mass with a smooth, glistening, wrinkled, and yellowish-tan external surface was observed on gross pathological examination. The walls of the mass were thin, fibrous, and 0.1 cm thick. The resected tumor was found to be SO with PTC and strumal carcinoid. The tumor cells were positive for pan- cytokeratin (PanCK) and synaptophysin, indicating a carcinoid tumor component, and emphasizing the difference between carcinoid tumors and PTC. Immuno-histochemical staining was positive for PanCK, thyroglobulin (Tg), and synaptophysin, and negative for p53. The Ki-67 proliferation index was 2%.
Microscopically, the tumor was composed of mature tissue representing the three embryonic layers (ectoderm, mesoderm and endoderm). The Cystic component had squamous epithelium with skin adnexal tissue and cartilage (Fig. 2A). Additionally, the thyroid tissue had PTC, which showed papillary structures with nuclear grooves and inclusions (Fig. 2B). Furthermore, the tumor was carcinoid tumor with an organoid pattern of small round cells with minimal atypia and no mitosis (Fig. 2C). Immunohistochemistry revealed that the carcinoid component was positive for synaptophysin (Fig. 2D). Overall, the final histologic diagnosis was a combined PTC and strumal type carcinoid arising from a mature teratoma (Fig. 2E, F).
Positron emission tomography/computed tomography (PET/CT) and pelvic magnetic resonance imaging (MRI) were performed postoperatively (Fig. 1C, D) to evaluate local metastasis or residual disease. Neither metastasis nor residual lesions were observed; however, a 3.23×2.73 cm sized ovarian teratoma was newly observed on right ovary (Fig. 1E). The patient underwent right laparoscopic oophorectomy, and pathological examination confirmed MCT of the right ovary without any malignant transfor-mation. Thyroid ultrasonography was performed to evaluate the accompanying cervical thyroid cancer, and two benign-looking nodules were observed in both lobes, and thyroid function tests were normal.
Currently, she has regular follow-up appointments in the gynecology and endocrinology departments for post-operative care and monitoring of the thyroid nodules, respectively.
MCTs accounts for 10-20% of all ovarian neoplasms; most of them unilateral and found in patients of reproductive age. SO is a rare type of MCT comprised of at least 50% thyroid tissue,5,6) and 95% of SOs are benign.7) Malignant transformation occurs 0.3-10% of all cases of SO and can be divided into three types. Papillary carcinoma is the most common type of malignant thyroid tumor, followed by follicular thyroid carcinoma and follicular variant of papillary carcinoma.8)
Ovarian carcinoid tumors are rare neuroendocrine tumors, accounting for only 0.1% of all ovarian tumors.9) According to Bidzinski et al.,10) ovarian carcinoid tumors are classified into four types based on histopathological characteristics: insular, strumal, trabecular and mucinous carcinoids. In our study, the patient had a strumal type of ovarian carcinoid tumor, the second most common subtype, a combination of thyroid and carcinoid tissue. While insular carcinoids are associated with carcinoid syndrome (flushing, sweating, and diarrhea), strumal carcinoids are not, so our patient had no symptoms of carcinoid syndrome. Carcinoid tumors typically react to unique immunohis-tochemical markers such as chromogranin, synaptophysin, and CD 56.11) In this case, the immunohisto-chemical staining was positive for synaptophysin.
There is no standardized approach for diagnosing, managing, or monitoring malignant transformations. Although surgery is the mainstay of treatment in the literature, therapeutic approaches vary from conservative surgery to total hysterectomy with bilateral removal of the uterine appendages, and chemotherapy and radiation.12-14) Depending on the patient’s menopausal status, the surgical approach may be conservative (to preserve fertility) or aggressive. The prognosis depends on the stage of the disease, with patients without distant metastases having a relatively good prognosis; however, if the disease has spread beyond the ovary, the outcome is very poor.15) Organ-confined tumors have excellent prognosis with a 10-year survival rate of 100%; In contrast, the advanced disease has a 5-year survival rate of 33%.16)
The management of PTC arising from SO after the surgical removal of the ovarian mass is controversial. DeSimone et al.17) proposed total thyroidectomy followed by radioactive iodine as an adjuvant therapy. While aggressive treatment can rule out primary thyroid cancer and prevent the recurrence with a higher probability, total thyroidectomy can cause unnecessary complications in patients at low-risk of recurrence, such as hypocalcemia, recurrent laryngeal nerve damage, and hypothyroidism requiring thyroid hormone replacement. Therefore, individual risk stratification of the malignancy seems ideal for determining appropriate thyroid-targeted therapy. Recently, a risk stratification schema in malignant SO was proposed using pathological variables, similar to the American Thyroid Association (ATA) guideline18) for cervical thyroid carcinoma. The variables were tumor size, extra- ovarian extension, lympho-vascular invasion, surgical margin status and distant metastasis.19) This stratification schema can help identifying high-risk patients, who needs aggressive treatment, and can be used as a guide for PTC treatment.
In this case, the patient had both ovarian tumors, one with concurrent malignant PTC and carcinoid tumor, and another with a benign MCT. Only two cases of PTC and carcinoid tumors arising from MCT have been reported in the literature. The details of these cases are described in Table 1. After bilateral oophorectomy, the patient is currently undergoing postoperative surveillance and has shown no evidence of recurrence to date.
In conclusion, both papillary thyroid carcinoma and carcinoid tumor from an MCT are extremely rare. Due to their low prevalence, preoperative diagnosis is almost impossible, and there is no standard guideline for postoperative management. Further research is needed to understand this rare disease and develop the standardized guideline.
Notes
References
1. Serov SF. 1973. S. F. Serov, R. E. Scully, in collaboration with L. H. Sobin and pathologists in ten countries. World Health Organization;Geneva:
2. Kim JY. 2016; A carcinoid tumor arising from a mature cystic teratoma in a 25-year-old patient: a case study. World J Surg Oncol. 14:120. DOI: 10.1186/s12957-016-0867-8. PMID: 27098182. PMCID: PMC4839142.

3. Antovska VS, Trajanova M, Krstevska I, Gosheva I, Chelebieva J, Prodanova I. 2018; Ovarian strumal carcinoid tumour: case report. Open Access Maced J Med Sci. 6(3):540–3. DOI: 10.3889/oamjms.2018.138. PMID: 29610616. PMCID: PMC5874381.

4. Robboy SJ, Scully RE. 1980; Strumal carcinoid of the ovary: an analysis of 50 cases of a distinctive tumor composed of thyroid tissue and carcinoid. Cancer. 46(9):2019–34. DOI: 10.1002/1097-0142(19801101)46:9<2019::AID-CNCR2820460921>3.0.CO;2-W. PMID: 7427909.
5. Ioannidis A, Kourtidis L, Zatagias A, Chorti A, Gourvas V, Efthimiadis C, et al. 2021; Papillary thyroid carcinoma arising in struma ovarii. Med Case Rep Study Protoc. 2(6):e0112. DOI: 10.1097/MD9.0000000000000112.

6. Yoo SC, Chang KH, Lyu MO, Chang SJ, Ryu HS, Kim HS. 2008; Clinical characteristics of struma ovarii. J Gynecol Oncol. 19(2):135–8. DOI: 10.3802/jgo.2008.19.2.135. PMID: 19471561. PMCID: PMC2676458.
7. Salman WD, Singh M, Twaij Z. 2010; A case of papillary thyroid carcinoma in struma ovarii and review of the literature. Patholog Res Int. 2010:352476. DOI: 10.4061/2010/352476. PMID: 21151690. PMCID: PMC2991076.

8. Devi P, Aghighi M, Mikhail N. 2020; Papillary thyroid carcinoma in struma ovarii. Cureus. 12(4):e7582. DOI: 10.7759/cureus.7582.
9. Lopes Dias J, Cunha TM, Gomes FV, Calle C, Felix A. 2015; Neuroendocrine tumours of the female genital tract: a case-based imaging review with pathological correlation. Insights Imaging. 6(1):43–52. DOI: 10.1007/s13244-014-0378-5. PMID: 25592289. PMCID: PMC4330232.

10. Bidzinski M, Piatek S, Cwikla J, Nasierowska-Guttmejer A, Danska-Bidzinska A. 2021; Contemporary principles of diagnostic and therapeutic management in cervical and ovarian neuroendocrine tumors. Ginekol Pol. 92(4):312–7. DOI: 10.5603/GP.a2020.0189. PMID: 33751517.

11. Chun YK. 2015; Neuroendocrine tumors of the female reproductive tract: a literature review. J Pathol Transl Med. 49(6):450–61. DOI: 10.4132/jptm.2015.09.20. PMID: 26459408. PMCID: PMC4696532.

12. Devaney K, Snyder R, Norris HJ, Tavassoli FA. 1993; Proliferative and histologically malignant struma ovarii: a clinicopathologic study of 54 cases. Int J Gynecol Pathol. 12(4):333–43. DOI: 10.1097/00004347-199310000-00008. PMID: 8253550.
13. O'Connell ME, Fisher C, Harmer CL. 1990; Malignant struma ovarii: presentation and management. Br J Radiol. 63(749):360–3. DOI: 10.1259/0007-1285-63-749-360. PMID: 2379061.
14. Berghella V, Ngadiman S, Rosenberg H, Hoda S, Zuna RE. 1997; Malignant struma ovarii. A case report and review of the literature. Gynecol Obstet Invest. 43(1):68–72. DOI: 10.1159/000291823. PMID: 9015705.
15. Gainford MC, Tinker A, Carter J, Petru E, Nicklin J, Quinn M, et al. 2010; Malignant transformation within ovarian dermoid cysts: an audit of treatment received and patient outcomes. an Australia New Zealand gynaecological oncology group (ANZGOG) and gynaecologic cancer intergroup (GCIG) study. Int J Gynecol Cancer. 20(1):75–81. DOI: 10.1111/IGC.0b013e3181c7fccf. PMID: 20130506.
16. Kopf B, Rosti G, Lanzanova G, Marangolo M. 2005; Locally advanced ovarian carcinoid. J Exp Clin Cancer Res. 24(2):313–6.
17. DeSimone CP, Lele SM, Modesitt SC. 2003; Malignant struma ovarii: a case report and analysis of cases reported in the literature with focus on survival and I131 therapy. Gynecol Oncol. 89(3):543–8. DOI: 10.1016/S0090-8258(03)00141-0. PMID: 12798728.

18. Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, et al. 2016; 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid. 26(1):1–133. DOI: 10.1089/thy.2015.0020. PMID: 26462967. PMCID: PMC4739132.

19. Egan C, Stefanova D, Thiesmeyer JW, Lee YJ, Greenberg J, Beninato T, et al. 2022; Proposed risk stratification and patterns of radioactive iodine therapy in malignant struma ovarii. Thyroid. 32(9):1101–8. DOI: 10.1089/thy.2022.0145. PMID: 35765923.
Fig. 1
Representative images before and after the surgery. (A, B) Preoperative transabdominal ultrasonography revealed a left ovarian mass measuring 7.42×7.51×7.46 cm. (C, D) Postoperative Positron emission tomography/computed tomography (PET/CT) showed no metastasis or residual tumor. (E) Postoperative pelvic magnetic resonance imaging (MRI) showed 3.23×2.73 cm sized right ovarian teratoma.

Fig. 2
Mixed papillary thyroid carcinoma and strumal carcinoid arising from mature cystic teratoma. (A) Mature teratoma showing seromucinous glands, skin and cartilage. (B) Papillary thyroid carcinoma with nuclear grooves and inclusions. (C) Carcinoid component showing organoid structures with minimal cytologic atypia. (D) Positive immunohistochemistry for synaptophysin to carcinoid component (lower part). (E) Upper part showing papillary carcinoma and lower part with carcinoid component. Benign mucinous epithelium between them. (F) Left part showing papillary carcinoma and Right part with carcinoid component. Benign mucinous epithelium between them.
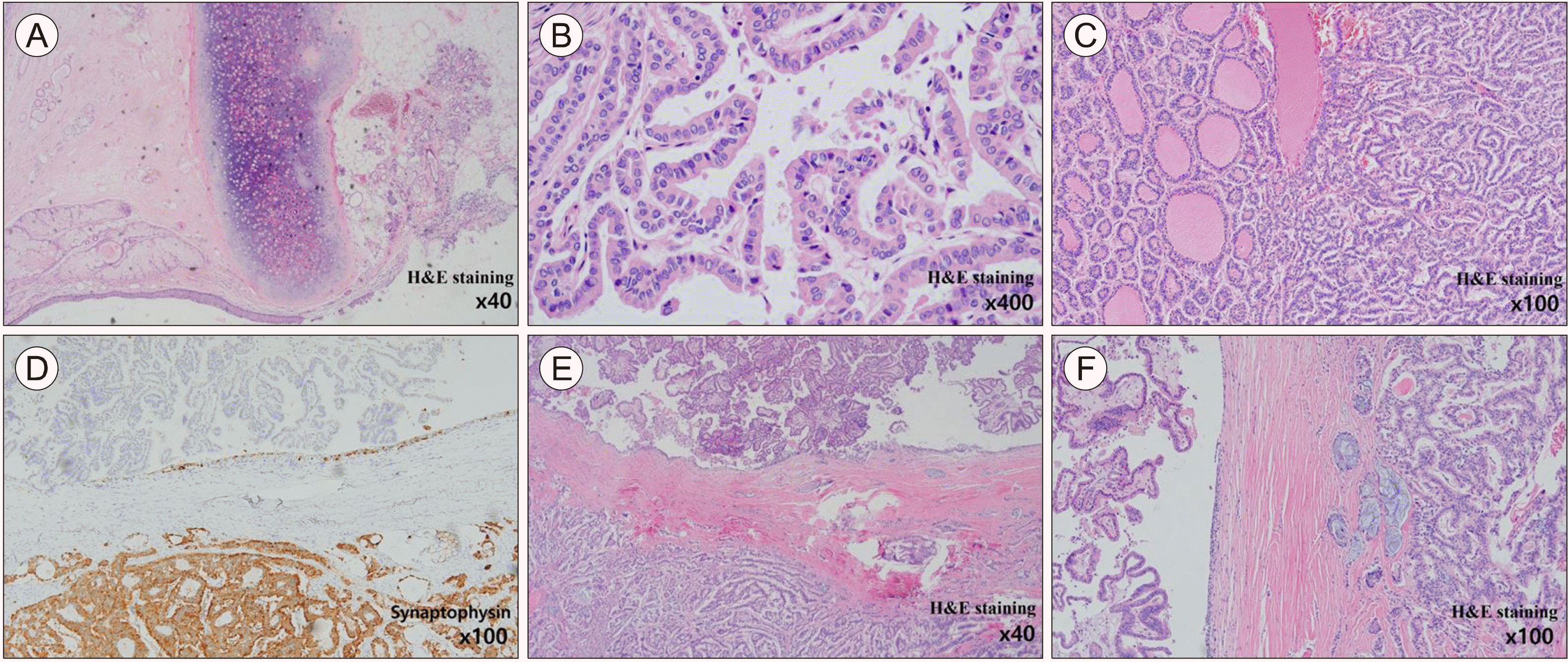
Table 1
Cases of papillary thyroid carcinoma and carcinoid tumor arising from a struma ovarii




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download