1. Mukherjee B, Morgenbesser SD, DePinho RA. 1992; Myc family oncoproteins function through a common pathway to transform normal cells in culture: cross-interference by Max and trans-acting dominant mutants. Genes Dev. 6:1480–1492. DOI:
10.1101/gad.6.8.1480. PMID:
1644290.

2. Walker W, Zhou ZQ, Ota S, Wynshaw-Boris A, Hurlin PJ. 2005; Mnt-Max to Myc-Max complex switching regulates cell cycle entry. J Cell Biol. 169:405–413. DOI:
10.1083/jcb.200411013. PMID:
15866886. PMCID:
PMC2171929.

3. Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. 2007; Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 131:861–872. DOI:
10.1016/j.cell.2007.11.019. PMID:
18035408.

4. Davis AC, Wims M, Spotts GD, Hann SR, Bradley A. 1993; A null c-myc mutation causes lethality before 10.5 days of gestation in homozygotes and reduced fertility in heterozygous female mice. Genes Dev. 7:671–682. DOI:
10.1101/gad.7.4.671. PMID:
8458579.

5. Stanton BR, Perkins AS, Tessarollo L, Sassoon DA, Parada LF. 1992; Loss of N-myc function results in embryonic lethality and failure of the epithelial component of the embryo to develop. Genes Dev. 6:2235–2247. DOI:
10.1101/gad.6.12a.2235. PMID:
1459449.

6. Moens CB, Auerbach AB, Conlon RA, Joyner AL, Rossant J. 1992; A targeted mutation reveals a role for N-myc in branching morphogenesis in the embryonic mouse lung. Genes Dev. 6:691–704. DOI:
10.1101/gad.6.5.691. PMID:
1577267.

7. Charron J, Malynn BA, Fisher P, Stewart V, Jeannotte L, Goff SP, Robertson EJ, Alt FW. 1992; Embryonic lethality in mice homozygous for a targeted disruption of the N-myc gene. Genes Dev. 6:2248–2257. DOI:
10.1101/gad.6.12a.2248. PMID:
1459450.

8. Hatton KS, Mahon K, Chin L, Chiu FC, Lee HW, Peng D, Morgenbesser SD, Horner J, DePinho RA. 1996; Expression and activity of L-Myc in normal mouse development. Mol Cell Biol. 16:1794–1804. DOI:
10.1128/MCB.16.4.1794. PMID:
8657155. PMCID:
PMC231166.

10. Alitalo K, Bishop JM, Smith DH, Chen EY, Colby WW, Levinson AD. 1983; Nucleotide sequence to the v-myc oncogene of avian retrovirus MC29. Proc Natl Acad Sci U S A. 80:100–104. DOI:
10.1073/pnas.80.1.100. PMID:
6296857. PMCID:
PMC393317.

11. Dalla-Favera R, Bregni M, Erikson J, Patterson D, Gallo RC, Croce CM. 1982; Human c-myc onc gene is located on the region of chromosome 8 that is translocated in Burkitt lymphoma cells. Proc Natl Acad Sci U S A. 79:7824–7827. DOI:
10.1073/pnas.79.24.7824. PMID:
6961453. PMCID:
PMC347441.

12. Sheiness DK, Hughes SH, Varmus HE, Stubblefield E, Bishop JM. 1980; The vertebrate homolog of the putative transforming gene of avian myelocytomatosis virus: characteristics of the DNA locus and its RNA transcript. Virology. 105:415–424. DOI:
10.1016/0042-6822(80)90042-2. PMID:
6158786.

13. Nau MM, Brooks BJ, Battey J, Sausville E, Gazdar AF, Kirsch IR, McBride OW, Bertness V, Hollis GF, Minna JD. 1985; L-myc, a new myc-related gene amplified and expressed in human small cell lung cancer. Nature. 318:69–73. DOI:
10.1038/318069a0. PMID:
2997622.

14. Schwab M, Varmus HE, Bishop JM, Grzeschik KH, Naylor SL, Sakaguchi AY, Brodeur G, Trent J. 1984; Chromosome localization in normal human cells and neuroblastomas of a gene related to c-myc. Nature. 308:288–291. DOI:
10.1038/308288a0. PMID:
6700732.

15. Seeger RC, Brodeur GM, Sather H, Dalton A, Siegel SE, Wong KY, Hammond D. 1985; Association of multiple copies of the N-myc oncogene with rapid progression of neuroblas-tomas. N Engl J Med. 313:1111–1116. DOI:
10.1056/NEJM198510313131802. PMID:
4047115.

16. Nilsson JA, Cleveland JL. 2003; Myc pathways provoking cell suicide and cancer. Oncogene. 22:9007–9021. DOI:
10.1038/sj.onc.1207261. PMID:
14663479.

17. Alarcon-Vargas D, Tansey WP, Ronai Z. 2002; Regulation of c-myc stability by selective stress conditions and by MEKK1 requires aa 127-189 of c-myc. Oncogene. 21:4384–4391. DOI:
10.1038/sj.onc.1205543. PMID:
12080469.

18. Channavajhala P, Seldin DC. 2002; Functional interaction of protein kinase CK2 and c-Myc in lymphomagenesis. Oncogene. 21:5280–5288. DOI:
10.1038/sj.onc.1205640. PMID:
12149649.

19. Noguchi K, Kokubu A, Kitanaka C, Ichijo H, Kuchino Y. 2001; ASK1-signaling promotes c-Myc protein stability during apoptosis. Biochem Biophys Res Commun. 281:1313–1320. DOI:
10.1006/bbrc.2001.4498. PMID:
11243879.

22. Tansey WP. 2014; Mammalian MYC proteins and cancer. New J Sci. 2014:757534. DOI:
10.1155/2014/757534.

23. Rochlitz CF, Herrmann R, de Kant E. 1996; Overexpression and amplification of c-myc during progression of human colorectal cancer. Oncology. 53:448–454. DOI:
10.1159/000227619. PMID:
8960139.

25. Uribesalgo I, Benitah SA, Di Croce L. 2012; From oncogene to tumor suppressor: the dual role of Myc in leukemia. Cell Cycle. 11:1757–1764. DOI:
10.4161/cc.19883. PMID:
22510570.
28. Kohl NE, Kanda N, Schreck RR, Bruns G, Latt SA, Gilbert F, Alt FW. 1983; Transposition and amplification of oncogene-related sequences in human neuroblastomas. Cell. 35(2 Pt 1):359–367. DOI:
10.1016/0092-8674(83)90169-1. PMID:
6197179.

29. Stoneley M, Chappell SA, Jopling CL, Dickens M, MacFarlane M, Willis AE. 2000; c-Myc protein synthesis is initiated from the internal ribosome entry segment during apoptosis. Mol Cell Biol. 20:1162–1169. DOI:
10.1128/MCB.20.4.1162-1169.2000. PMID:
10648601. PMCID:
PMC85234.

30. Hui AB, Lo KW, Yin XL, Poon WS, Ng HK. 2001; Detection of multiple gene amplifications in glioblastoma multiforme using array-based comparative genomic hybridization. Lab Invest. 81:717–723. DOI:
10.1038/labinvest.3780280. PMID:
11351043.

31. Wong AJ, Ruppert JM, Eggleston J, Hamilton SR, Baylin SB, Vogelstein B. 1986; Gene amplification of c-myc and N-myc in small cell carcinoma of the lung. Science. 233:461–464. DOI:
10.1126/science.3014659. PMID:
3014659.

32. Kawashima K, Shikama H, Imoto K, Izawa M, Naruke T, Okabayashi K, Nishimura S. 1988; Close correlation between restriction fragment length polymorphism of the L-MYC gene and metastasis of human lung cancer to the lymph nodes and other organs. Proc Natl Acad Sci U S A. 85:2353–2356. DOI:
10.1073/pnas.85.7.2353. PMID:
2895475. PMCID:
PMC279990.

33. Mosquera JM, Beltran H, Park K, MacDonald TY, Robin-son BD, Tagawa ST, Perner S, Bismar TA, Erbersdobler A, Dhir R, Nelson JB, Nanus DM, Rubin MA. 2013; Concurrent AURKA and MYCN gene amplifications are harbingers of lethal treatment-related neuroendocrine prostate cancer. Neoplasia. 15:1–10. DOI:
10.1593/neo.121550. PMID:
23358695. PMCID:
PMC3556934. PMID:
0d9e077856fb4478855c437db140cc23.

34. Mizukami Y, Nonomura A, Takizawa T, Noguchi M, Michigishi T, Nakamura S, Ishizaki T. 1995; N-myc protein expression in human breast carcinoma: prognostic implications. Anticancer Res. 15:2899–2905. PMID:
8669886.
35. Ahmadiyeh N, Pomerantz MM, Grisanzio C, Herman P, Jia L, Almendro V, He HH, Brown M, Liu XS, Davis M, Caswell JL, Beckwith CA, Hills A, Macconaill L, Coetzee GA, Regan MM, Freedman ML. 2010; 8q24 prostate, breast, and colon cancer risk loci show tissue-specific long-range interaction with MYC. Proc Natl Acad Sci U S A. 107:9742–9746. DOI:
10.1073/pnas.0910668107. PMID:
20453196. PMCID:
PMC2906844.

37. Chen CR, Kang Y, Massagué J. 2001; Defective repression of c-myc in breast cancer cells: a loss at the core of the transforming growth factor beta growth arrest program. Proc Natl Acad Sci U S A. 98:992–999. DOI:
10.1073/pnas.98.3.992. PMID:
11158583. PMCID:
PMC14697.

38. Nutt CL, Mani DR, Betensky RA, Tamayo P, Cairncross JG, Ladd C, Pohl U, Hartmann C, McLaughlin ME, Batchelor TT, Black PM, von Deimling A, Pomeroy SL, Golub TR, Louis DN. 2003; Gene expression-based classification of malignant gliomas correlates better with survival than histological classification. Cancer Res. 63:1602–1607. PMID:
12670911.
39. Nau MM, Brooks BJ Jr, Carney DN, Gazdar AF, Battey JF, Sausville EA, Minna JD. 1986; Human small-cell lung cancers show amplification and expression of the N-myc gene. Proc Natl Acad Sci U S A. 83:1092–1096. DOI:
10.1073/pnas.83.4.1092. PMID:
2869482. PMCID:
PMC323017.

40. Kawagoe H, Kandilci A, Kranenburg TA, Grosveld GC. 2007; Overexpression of N-Myc rapidly causes acute myeloid leukemia in mice. Cancer Res. 67:10677–10685. DOI:
10.1158/0008-5472.CAN-07-1118. PMID:
18006809.

41. Wu R, Lin L, Beer DG, Ellenson LH, Lamb BJ, Rouillard JM, Kuick R, Hanash S, Schwartz DR, Fearon ER, Cho KR. 2003; Amplification and overexpression of the L-MYC proto-oncogene in ovarian carcinomas. Am J Pathol. 162:1603–1610. DOI:
10.1016/S0002-9440(10)64294-0. PMID:
12707044. PMCID:
PMC1851191.

42. Xie S, Shen C, Tan M, Li M, Song X, Wang C. 2017; Systematic analysis of gene expression alterations and clinical outcomes of adenylate cyclase-associated protein in cancer. Oncotarget. 8:27216–27239. DOI:
10.18632/oncotarget.16111. PMID:
28423713. PMCID:
PMC5432330.

43. Cui X, Jing X, Yi Q, Long C, Tan B, Li X, Chen X, Huang Y, Xiang Z, Tian J, Zhu J. 2017; Systematic analysis of gene expression alterations and clinical outcomes of STAT3 in cancer. Oncotarget. 9:3198–3213. DOI:
10.18632/oncotarget.23226. PMID:
29423040. PMCID:
PMC5790457.

44. Matthews L, Gopinath G, Gillespie M, Caudy M, Croft D, de Bono B, Garapati P, Hemish J, Hermjakob H, Jassal B, Kanapin A, Lewis S, Mahajan S, May B, Schmidt E, Vastrik I, Wu G, Birney E, Stein L, D'Eustachio P. 2009; Reactome knowledgebase of human biological pathways and processes. Nucleic Acids Res. 37(Suppl 1):D619–D622. DOI:
10.1093/nar/gkn863. PMID:
18981052. PMCID:
PMC2686536.

46. Xu AG, Li SG, Liu JH, Gan AH. 2001; Function of apoptosis and expression of the proteins Bcl-2, p53 and C-myc in the development of gastric cancer. World J Gastroenterol. 7:403–406. DOI:
10.3748/wjg.v7.i3.403. PMID:
11819799. PMCID:
PMC4688731.

47. Weston A, Caporaso NE, Perrin LS, Sugimura H, Tamai S, Krontiris TG, Trump BF, Hoover RN, Harris CC. 1992; Relationship of H-ras-1, L-myc, and p53 polymorphisms with lung cancer risk and prognosis. Environ Health Pers-pect. 98:61–67. DOI:
10.1289/ehp.929861. PMID:
1486864. PMCID:
PMC1519610.

49. Liu H, Radisky DC, Yang D, Xu R, Radisky ES, Bissell MJ, Bishop JM. 2012; MYC suppresses cancer metastasis by direct transcriptional silencing of αv and β3 integrin subu-nits. Nat Cell Biol. 14:567–574. DOI:
10.1038/ncb2491. PMID:
22581054. PMCID:
PMC3366024.

50. Ma X, Huang J, Tian Y, Chen Y, Yang Y, Zhang X, Zhang F, Xue L. 2017; Myc suppresses tumor invasion and cell migration by inhibiting JNK signaling. Oncogene. 36:3159–3167. DOI:
10.1038/onc.2016.463. PMID:
28068320.

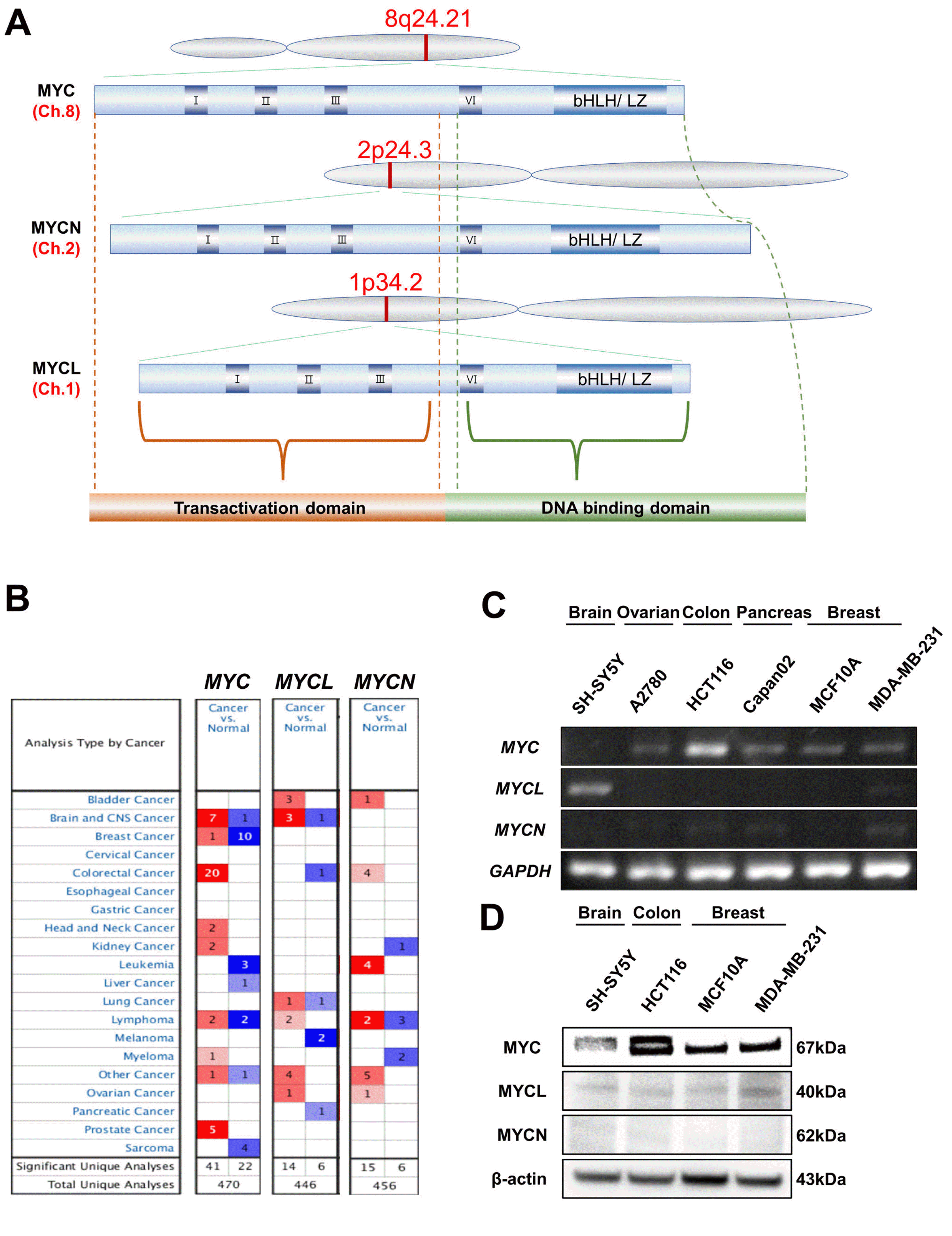
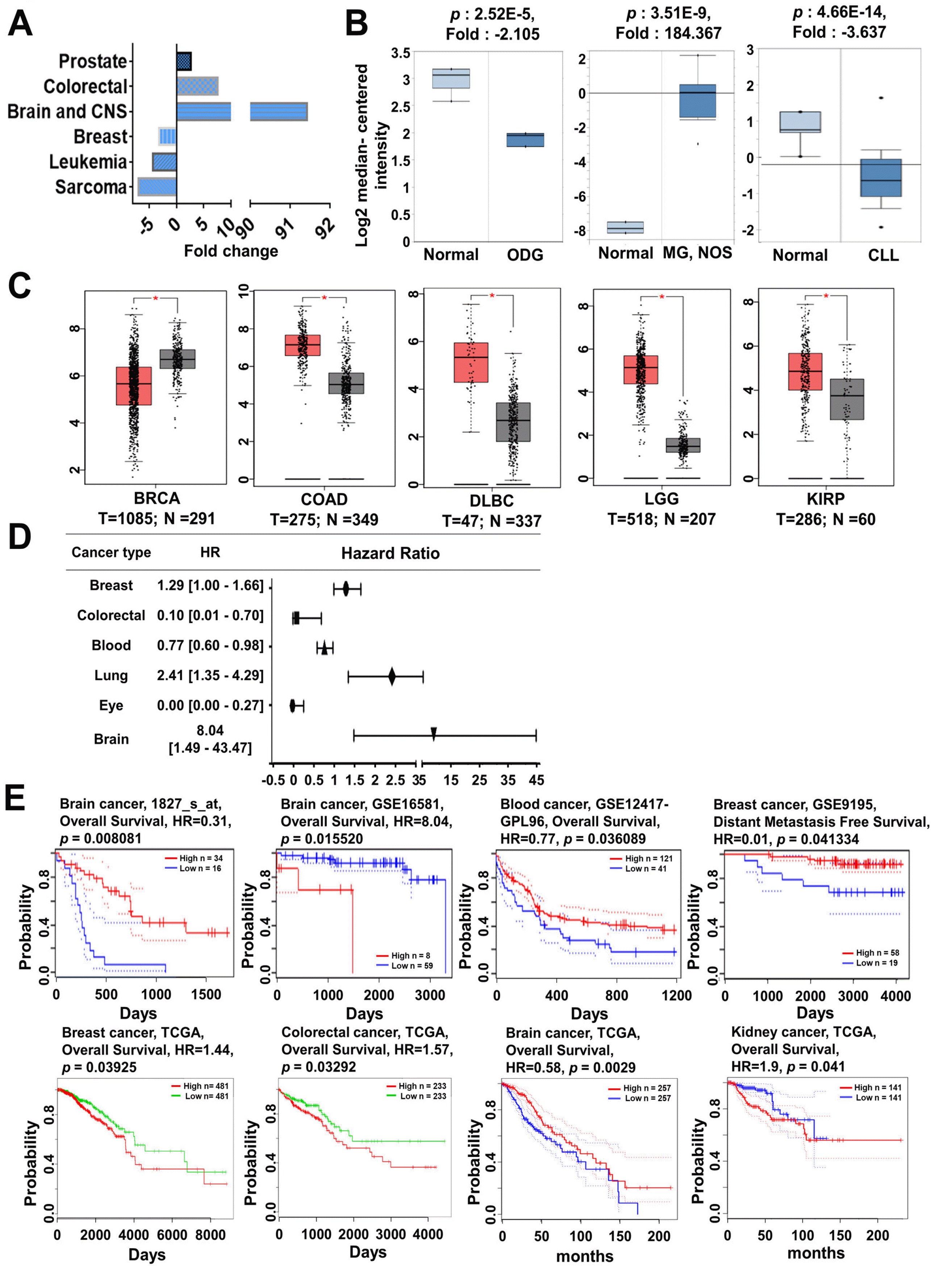
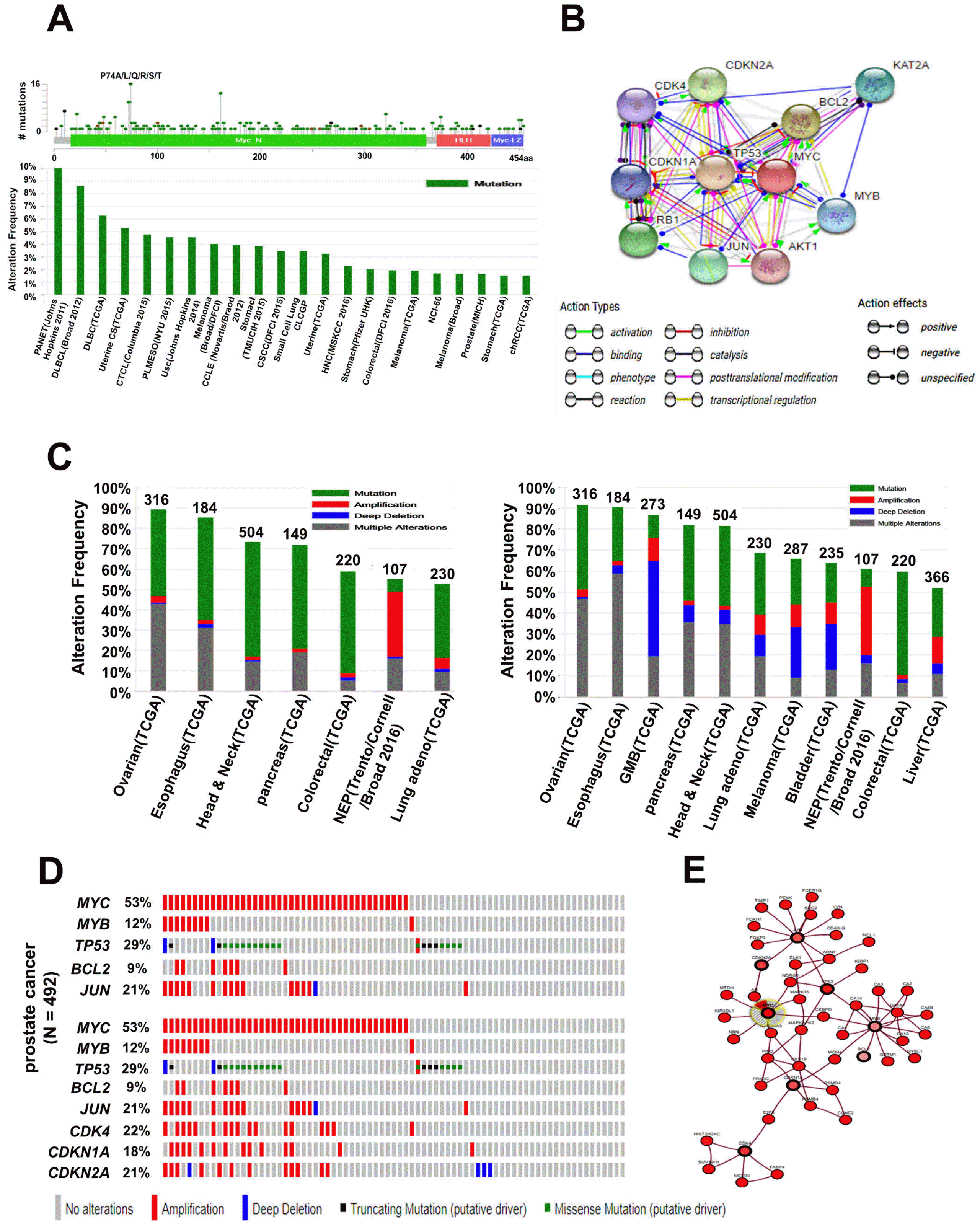
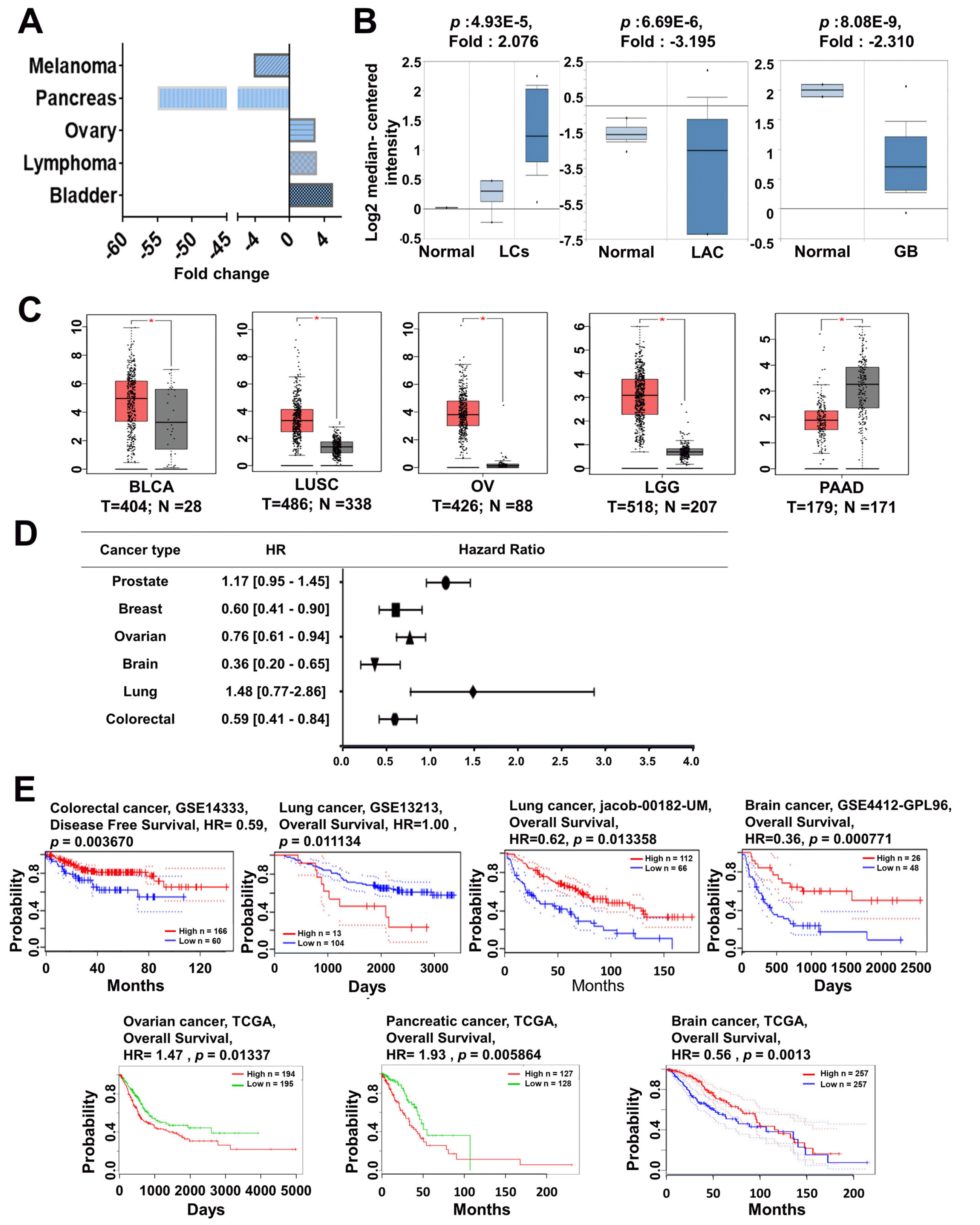
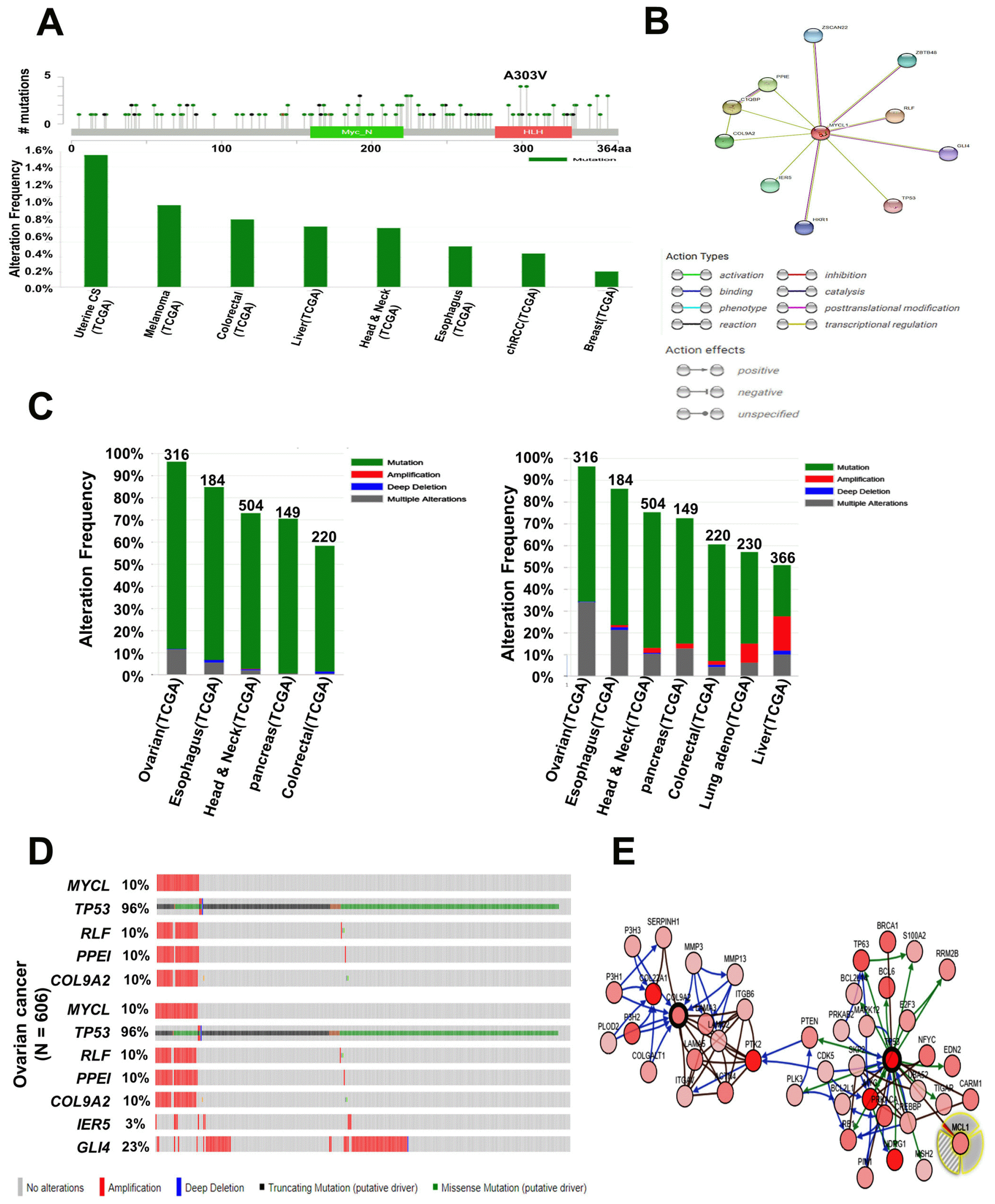
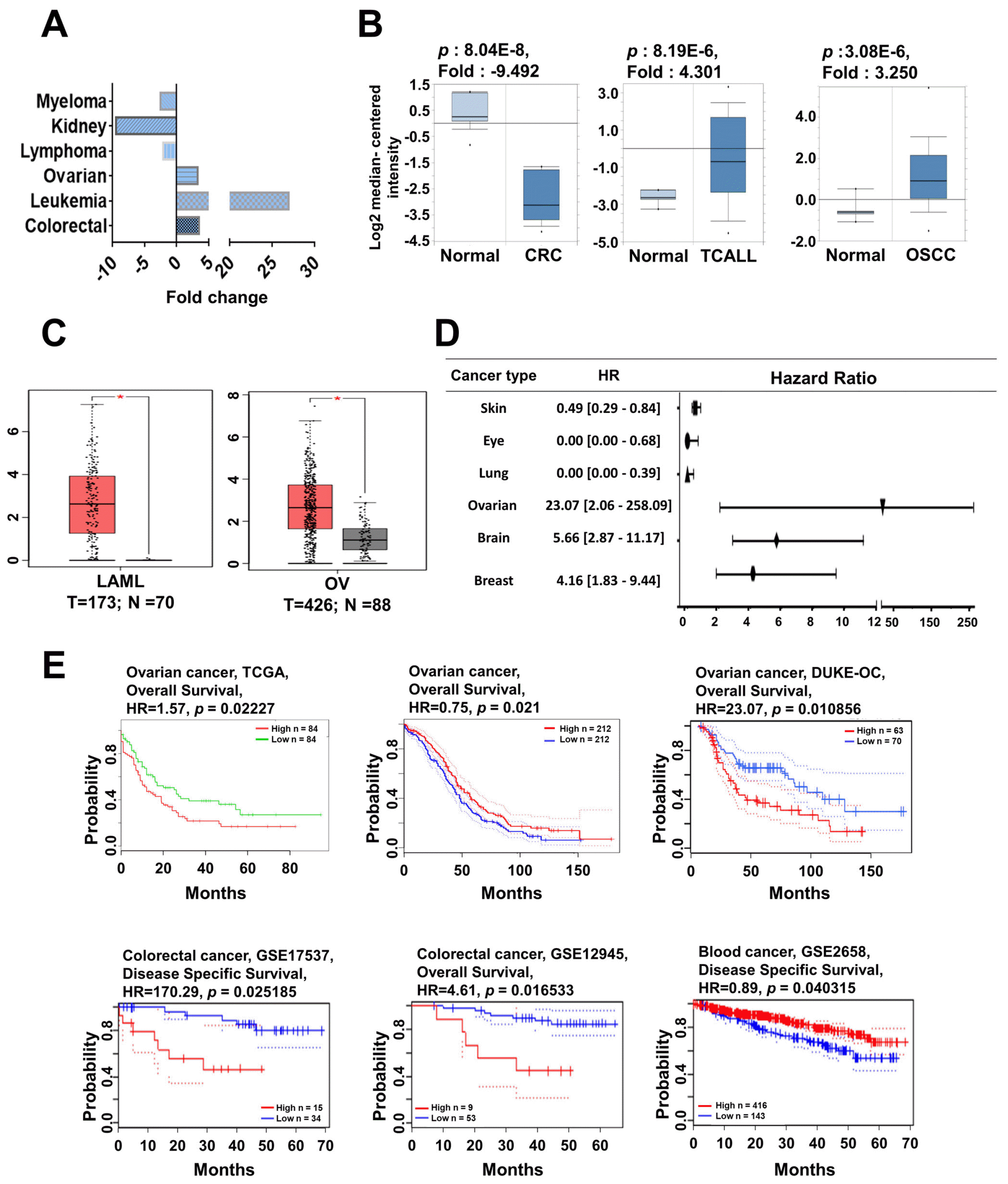
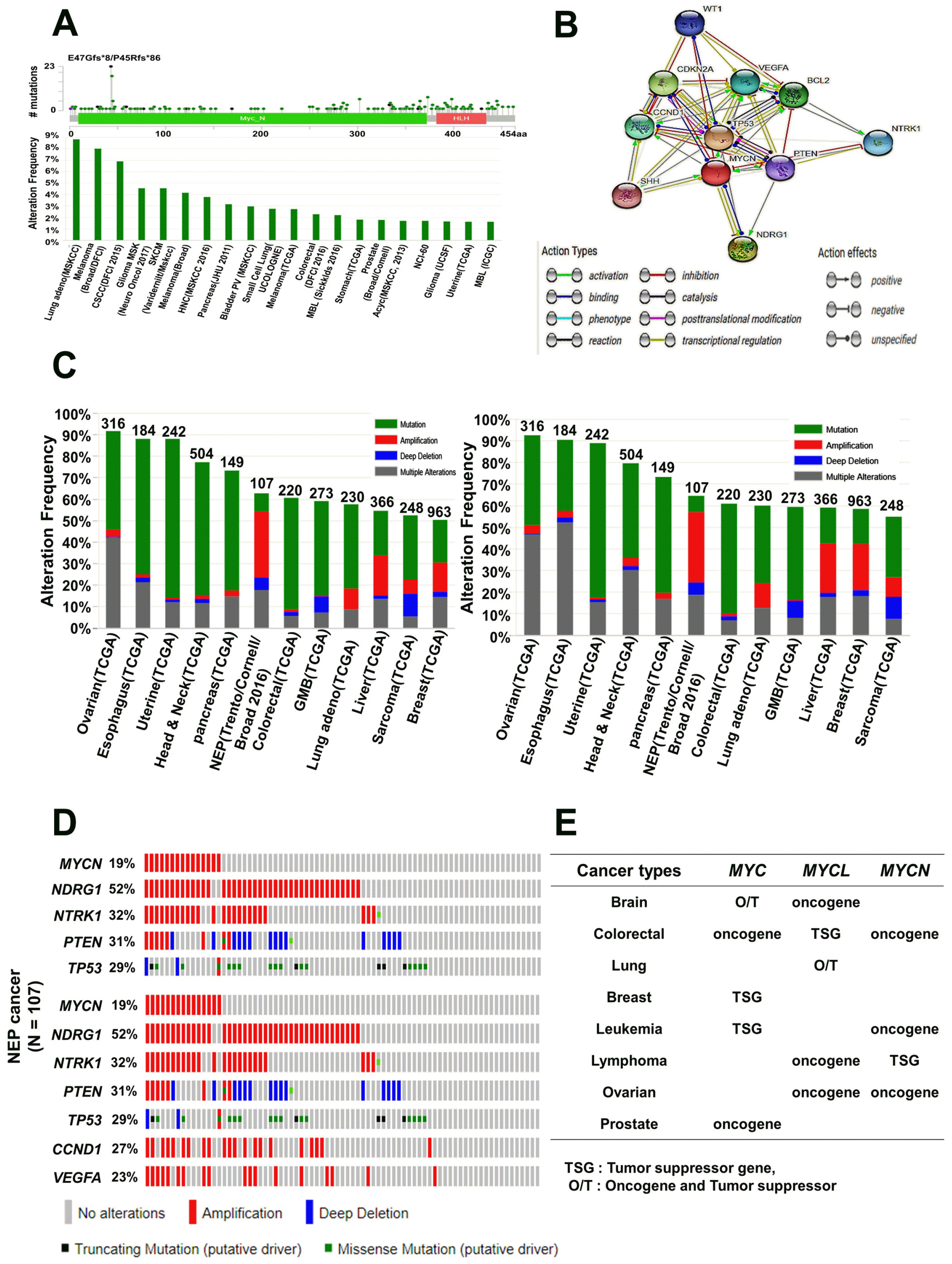
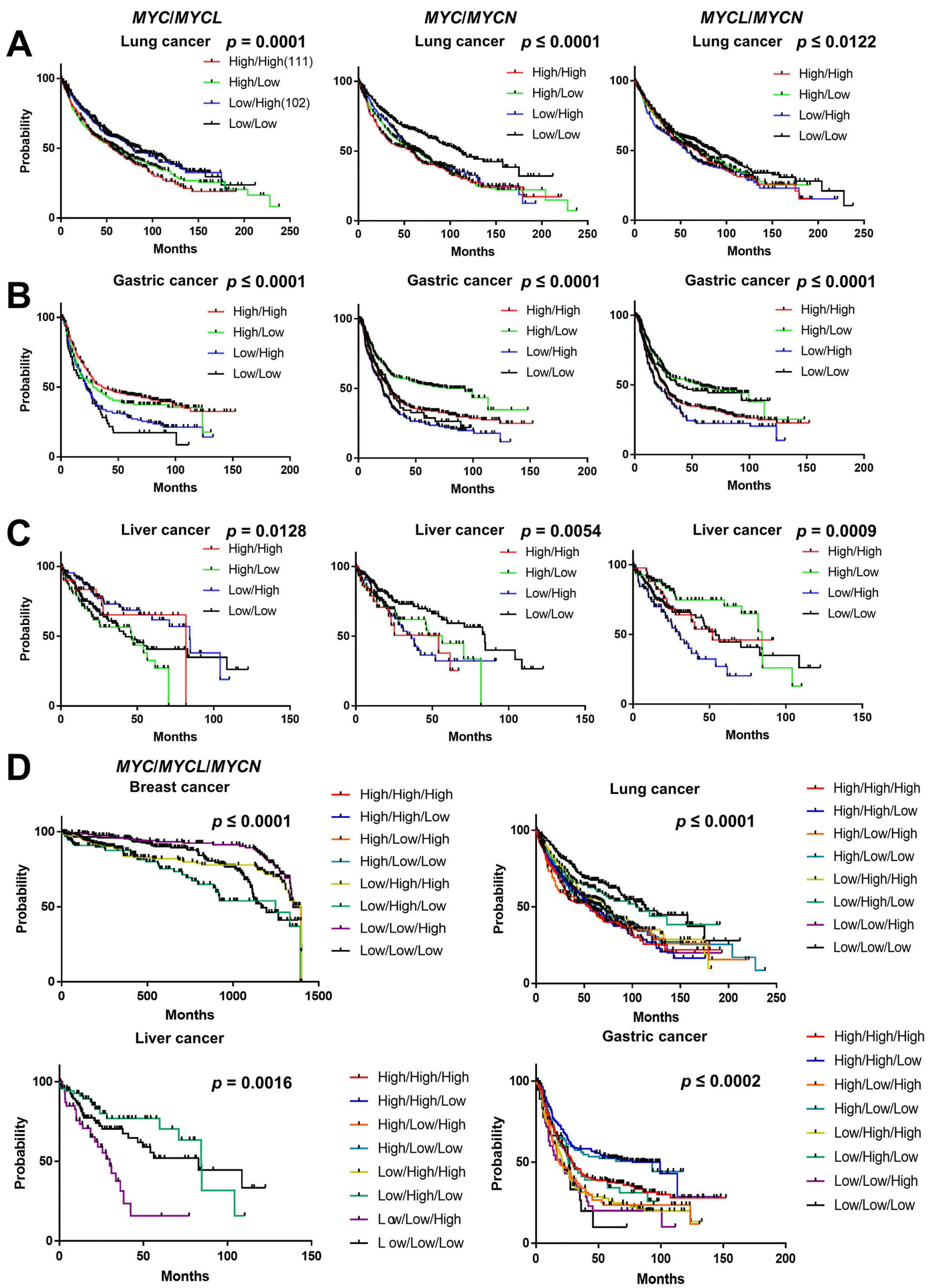




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download