1. Huang L, Liu B, Cha JY, Yuan G, Kelly M, Singh G, Hyman S, Brunski JB, Li J, Helms JA. 2016; Mechanoresponsive properties of the periodontal ligament. J Dent Res. 95:467–475. DOI:
10.1177/0022034515626102. PMID:
26767771.

5. Wescott DC, Pinkerton MN, Gaffey BJ, Beggs KT, Milne TJ, Meikle MC. 2007; Osteogenic gene expression by human periodontal ligament cells under cyclic tension. J Dent Res. 86:1212–1216. DOI:
10.1177/154405910708601214. PMID:
18037658.

6. Garlet TP, Coelho U, Repeke CE, Silva JS, Cunha Fde Q, Garlet GP. 2008; Differential expression of osteoblast and osteoclast chemmoatractants in compression and tension sides during orthodontic movement. Cytokine. 42:330–335. DOI:
10.1016/j.cyto.2008.03.003. PMID:
18406624.

7. Tomida M, Tsujigiwa H, Nakano K, Muraoka R, Naka-mura T, Okafuji N, Nagatsuka H, Kawakami T. 2013; Promotion of transplanted bone marrow-derived cell migration into the periodontal tissues due to orthodontic mechanical stress. Int J Med Sci. 10:1321–1326. DOI:
10.7150/ijms.6631. PMID:
23983592. PMCID:
PMC3753415.

8. Takimoto A, Kawatsu M, Yoshimoto Y, Kawamoto T, Seiryu M, Takano-Yamamoto T, Hiraki Y, Shukunami C. 2015; Scleraxis and osterix antagonistically regulate tensile force-responsive remodeling of the periodontal ligament and alveolar bone. Development. 142:787–796. DOI:
10.1242/dev.116228. PMID:
25670797.

9. Chang M, Lin H, Fu H, Wang J, Yang Y, Wan Z, Han G. 2020; CREB activation affects mesenchymal stem cell migration and differentiation in periodontal tissues due to orthodontic force. Int J Biochem Cell Biol. 129:105862. DOI:
10.1016/j.biocel.2020.105862. PMID:
33045372.

10. Marquez-Curtis LA, Janowska-Wieczorek A. 2013; Enhancing the migration ability of mesenchymal stromal cells by targeting the SDF-1/CXCR4 axis. Biomed Res Int. 2013:561098. DOI:
10.1155/2013/561098. PMID:
24381939. PMCID:
PMC3870125.

14. Liu T, Hu W, Zou X, Xu J, He S, Chang L, Li X, Yin Y, Tian M, Li Z, Zhou J, Jiang X, Chen S. 2020; Human periodontal ligament stem cell-derived exosomes promote bone regeneration by altering microRNA profiles. Stem Cells Int. 2020:8852307. DOI:
10.1155/2020/8852307. PMID:
33293963. PMCID:
PMC7691010. PMID:
f312b2e7a21d4b62aad0a12a893d9c88.

15. Zhang Z, Shuai Y, Zhou F, Yin J, Hu J, Guo S, Wang Y, Liu W. 2020; PDLSCs regulate angiogenesis of periodontal ligaments via VEGF transferred by exosomes in periodontitis. Int J Med Sci. 17:558–567. Erratum in: Int J Med Sci 2022;19:833. DOI:
10.7150/ijms.74583. PMID:
35693750. PMCID:
PMC9149635.

16. Peinado H, Alečković M, Lavotshkin S, Matei I, Costa-Silva B, Moreno-Bueno G, Hergueta-Redondo M, Williams C, García-Santos G, Ghajar C, Nitadori-Hoshino A, Hoffman C, Badal K, Garcia BA, Callahan MK, Yuan J, Martins VR, Skog J, Kaplan RN, Brady MS, Wolchok JD, Chapman PB, Kang Y, Bromberg J, Lyden D. 2012; Melanoma exosomes educate bone marrow progenitor cells toward a pro-metastatic phenotype through MET. Nat Med. 18:883–891. Erratum in: Nat Med 2016;22:1502. DOI:
10.1038/nm1216-1502b. PMID:
27923027. PMCID:
PMC3645291.

17. Klopp AH, Spaeth EL, Dembinski JL, Woodward WA, Munshi A, Meyn RE, Cox JD, Andreeff M, Marini FC. 2007; Tumor irradiation increases the recruitment of circulating mesenchymal stem cells into the tumor microenvironment. Cancer Res. 67:11687–11695. DOI:
10.1158/0008-5472.CAN-07-1406. PMID:
18089798. PMCID:
PMC4329784.

18. Wu Z, Pu P, Su Z, Zhang X, Nie L, Chang Y. 2020; Schwann Cell-derived exosomes promote bone regeneration and repair by enhancing the biological activity of porous Ti6Al4V scaffolds. Biochem Biophys Res Commun. 531:559–565. DOI:
10.1016/j.bbrc.2020.07.094. PMID:
32811642.

20. Khraiwesh B, Arif MA, Seumel GI, Ossowski S, Weigel D, Reski R, Frank W. 2010; Transcriptional control of gene expression by microRNAs. Cell. 140:111–122. DOI:
10.1016/j.cell.2009.12.023. PMID:
20085706.

21. Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. 2007; Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 9:654–659. DOI:
10.1038/ncb1596. PMID:
17486113.

22. Vyas N, Dhawan J. 2017; Exosomes: mobile platforms for targeted and synergistic signaling across cell boundaries. Cell Mol Life Sci. 74:1567–1576. DOI:
10.1007/s00018-016-2413-9. PMID:
27826642.

23. Tkach M, Théry C. 2016; Communication by extracellular vesicles: where we are and where we need to go. Cell. 164:1226–1232. DOI:
10.1016/j.cell.2016.01.043. PMID:
26967288.

24. Alexander M, Hu R, Runtsch MC, Kagele DA, Mosbruger TL, Tolmachova T, Seabra MC, Round JL, Ward DM, O'Connell RM. 2015; Exosome-delivered microRNAs modulate the inflam-matory response to endotoxin. Nat Commun. 6:7321. DOI:
10.1038/ncomms8321. PMID:
26084661. PMCID:
PMC4557301.

25. Li X, Chen C, Wei L, Li Q, Niu X, Xu Y, Wang Y, Zhao J. 2016; Exosomes derived from endothelial progenitor cells attenuate vascular repair and accelerate reendothelialization by enhancing endothelial function. Cytotherapy. 18:253–262. DOI:
10.1016/j.jcyt.2015.11.009. PMID:
26794715.

26. Chang M, Lin H, Luo M, Wang J, Han G. 2015; Integrated miRNA and mRNA expression profiling of tension force-induced bone formation in periodontal ligament cells. In Vitro Cell Dev Biol Anim. 51:797–807. DOI:
10.1007/s11626-015-9892-0. PMID:
26091625.

27. Li M, Zhang C, Yang Y. 2019; Effects of mechanical forces on osteogenesis and osteoclastogenesis in human periodontal ligament fibroblasts: a systematic review of in vitro studies. Bone Joint Res. 8:19–31. DOI:
10.1302/2046-3758.81.BJR-2018-0060.R1. PMID:
30800296. PMCID:
PMC6359886.

28. Chang M, Lin H, Fu H, Wang B, Han G, Fan M. 2017; Micro-RNA-195-5p regulates osteogenic differentiation of periodontal ligament cells under mechanical loading. J Cell Phy-siol. 232:3762–3774. DOI:
10.1002/jcp.25856. PMID:
28181691.

30. Saludas L, Garbayo E, Ruiz-Villalba A, Hernández S, Vader P, Prósper F, Blanco-Prieto MJ. 2022; Isolation methods of large and small extracellular vesicles derived from cardiovascular progenitors: a comparative study. Eur J Pharm Biopharm. 170:187–196. DOI:
10.1016/j.ejpb.2021.12.012. PMID:
34968647.

31. Duan DY, Tang J, Tian HT, Shi YY, Jia J. 2021; Adipocyte-secreted microvesicle-derived miR-148a regulates adipogenic and osteogenic differentiation by targeting Wnt5a/Ror2 pathway. Life Sci. 278:119548. DOI:
10.1016/j.lfs.2021.119548. PMID:
33930365.

32. Morel O, Toti F, Hugel B, Freyssinet JM. 2004; Cellular microparticles: a disseminated storage pool of bioactive vascular effectors. Curr Opin Hematol. 11:156–164. DOI:
10.1097/01.moh.0000131441.10020.87. PMID:
15257014.

33. Yan S, Han B, Gao S, Wang X, Wang Z, Wang F, Zhang J, Xu D, Sun B. 2017; Exosome-encapsulated microRNAs as circulating biomarkers for colorectal cancer. Oncotarget. 8:60149–60158. DOI:
10.18632/oncotarget.18557. PMID:
28947960. PMCID:
PMC5601128.

34. Jiang H, Toscano JF, Song SS, Schlick KH, Dumitrascu OM, Pan J, Lyden PD, Saver JL, Gonzalez NR. 2019; Differential expression of circulating exosomal microRNAs in refractory intracranial atherosclerosis associated with antian-giogenesis. Sci Rep. 9:19429. Erratum in: Sci Rep 2021; 11:15266. DOI:
10.1038/s41598-021-94233-1. PMID:
34290283. PMCID:
PMC8295272. PMID:
6e9ef5985866448fb8d794fd866b60cd.

35. Li Z, Wang Y, Xiang S, Zheng Z, Bian Y, Feng B, Weng X. 2020; Chondrocytes-derived exosomal miR-8485 regulated the Wnt/β-catenin pathways to promote chondrogenic differentiation of BMSCs. Biochem Biophys Res Commun. 523:506–513. DOI:
10.1016/j.bbrc.2019.12.065. PMID:
31898972.

36. Li W, Han Y, Zhao Z, Ji X, Wang X, Jin J, Wang Q, Guo X, Cheng Z, Lu M, Wang G, Wang Y, Liu H. 2019; Oral mucosal mesenchymal stem cell-derived exosomes: a potential therapeutic target in oral premalignant lesions. Int J Oncol. 54:1567–1578. DOI:
10.3892/ijo.2019.4756. PMID:
30896790. PMCID:
PMC6438436.

37. Pei B, Li T, Qian Q, Fan W, He X, Zhu Y, Xu L. 2020; Downregulation of microRNA-30c-5p was responsible for cell migration and tumor metastasis via COTL1-mediated microfilament arrangement in breast cancer. Gland Surg. 9:747–758. DOI:
10.21037/gs-20-472. PMID:
32775265. PMCID:
PMC7347814.

38. Zhou Y, Shi H, Du Y, Zhao G, Wang X, Li Q, Liu J, Ye L, Shen Z, Guo Y, Huang Y. 2019; lncRNA DLEU2 modulates cell proliferation and invasion of non-small cell lung cancer by regulating miR-30c-5p/SOX9 axis. Aging (Albany NY). 11:7386–7401. DOI:
10.18632/aging.102226. PMID:
31541993. PMCID:
PMC6781974.

39. Zhang J, Liu X, Li H, Chen C, Hu B, Niu X, Li Q, Zhao B, Xie Z, Wang Y. 2016; Exosomes/tricalcium phosphate combination scaffolds can enhance bone regeneration by activating the PI3K/Akt signaling pathway. Stem Cell Res Ther. 7:136. DOI:
10.1186/s13287-016-0391-3. PMID:
27650895. PMCID:
PMC5028974.

40. Zhu M, Guo J, Xia H, Li W, Lu Y, Dong X, Chen Y, Xie X, Fu S, Li M. 2015; Alpha-fetoprotein activates AKT/mTOR signaling to promote CXCR4 expression and migration of hepatoma cells. Oncoscience. 2:59–70. DOI:
10.18632/oncoscience.115. PMID:
25815363. PMCID:
PMC4341465.

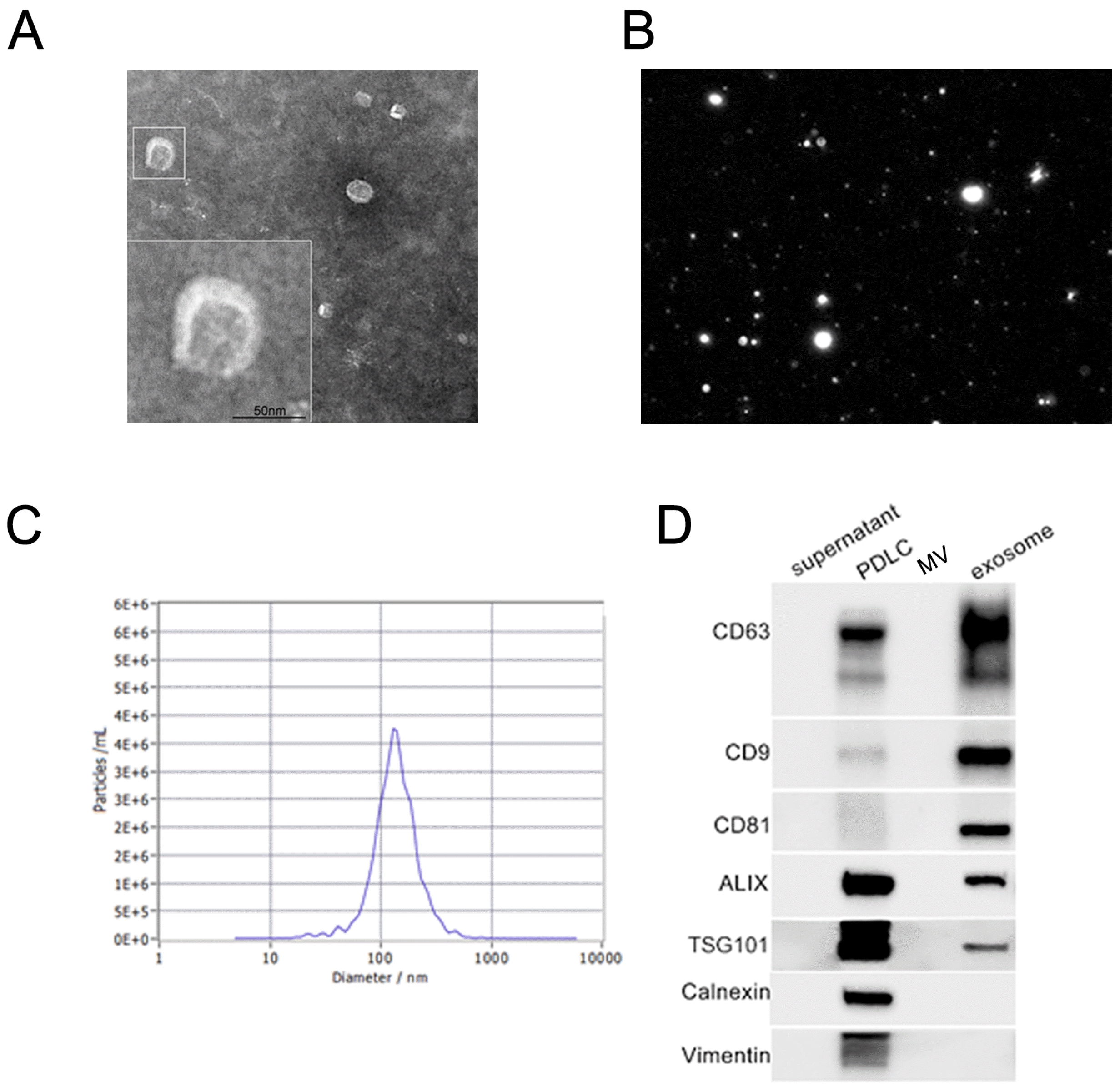
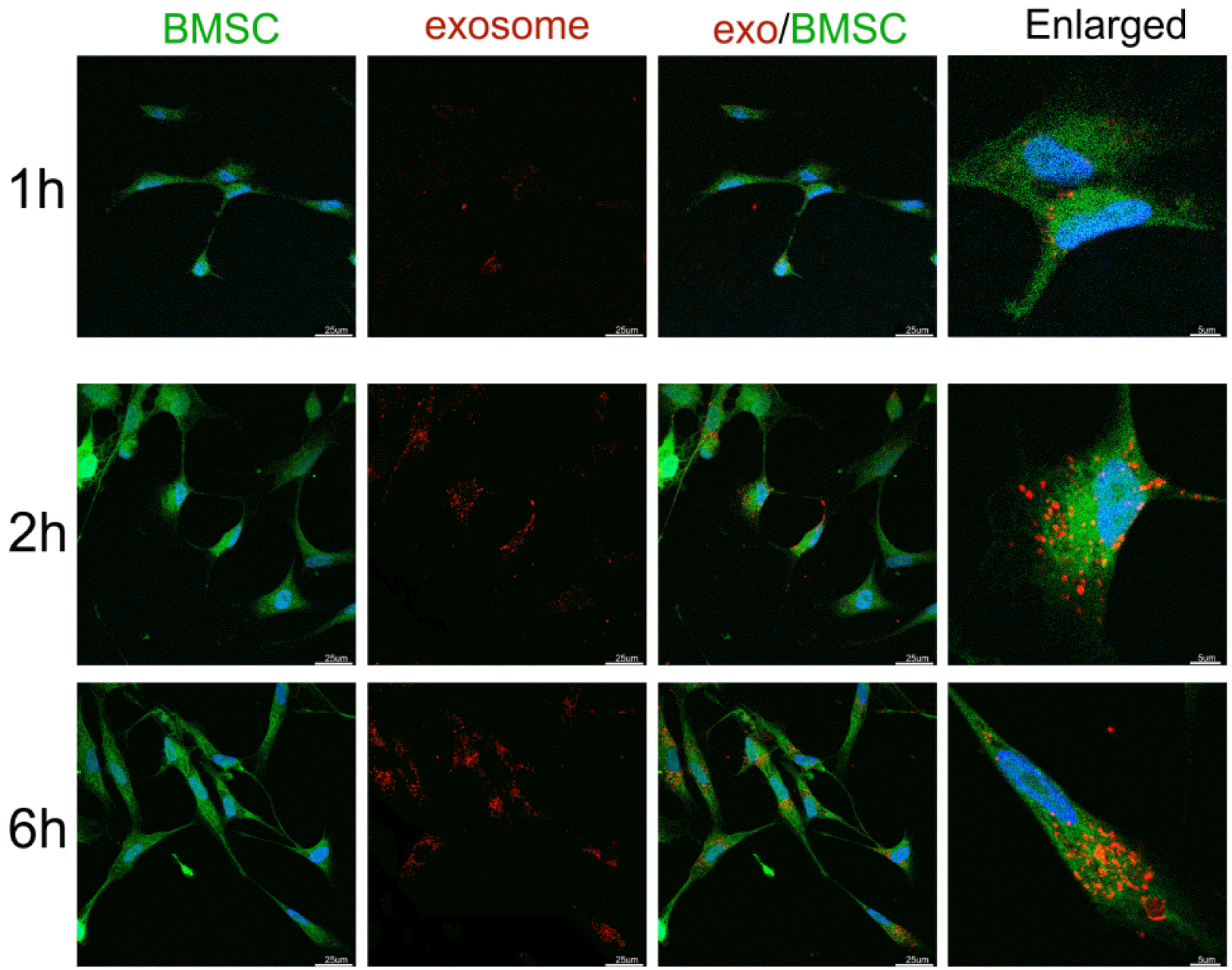
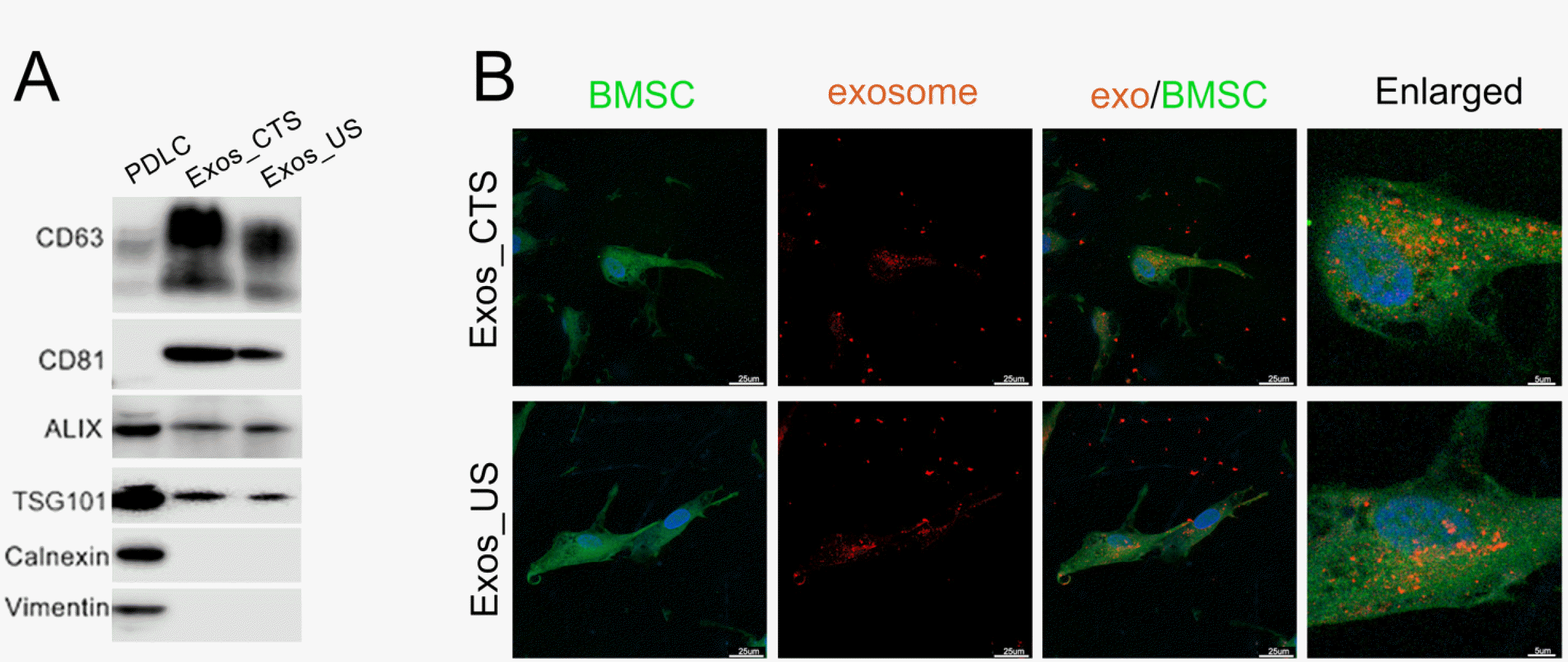
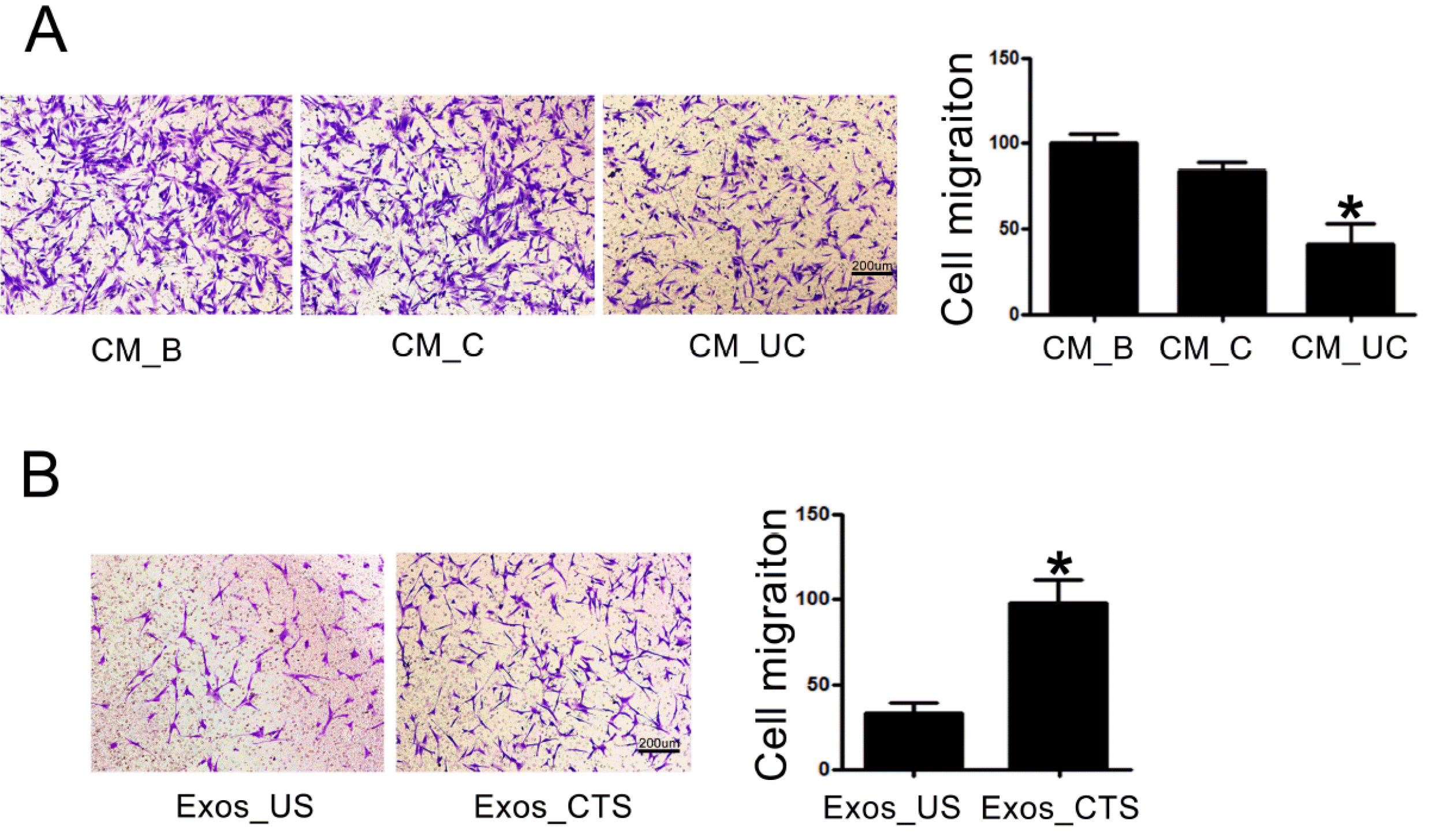




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download