Abstract
Background and Objectives
Embryologically, mesodermal development is closely related to the development of various organs such as muscles, blood vessels, and hearts, which are the main organs that make up the body. However, treatment for mesoderm developmental disorders caused by congenital or acquired factors has so far relied on surgery and drug treatment for symptom relief, and more fundamentally, treatment for mesoderm developmental disorders is needed.
Methods and Results
In our study, microRNA (miRNA), which plays an important role in the mesoderm development process, was identified and the developmental function was evaluated. miRNAs consist of small nucleotides, which act as transcription factors that bind to the 3’ untranslated region and suppressed target gene expression. We constructed the human embryonic stem cell (hESC) knockout cell line and analyzed the function and characteristics of miR-5739, which plays an important role in mesoderm lineage. miR-5739 acts as a transcription factor targeting SMA, Brachyury T, Hand1, which controls muscle proliferation and differentiation, and KDR gene, which regulates vessel formation in vitro. In vivo results suggest a role in regulating muscle proliferation and differentiation. Gene ontology analysis confirmed that the miR-5739 is closely related to genes that regulate muscle and vessel proliferation and differentiation. Importantly, abnormal expression of miR-5739 was detected in somatic cells derived from patients with congenital muscle disease.
MicroRNAs (miRNAs) are composed of about 20 nucleotides and regulates expression by binding the AU-rich element located at 3’UTR of the target genes. miRNAs are controlled during stem cell culture, and stem cell-associated miRNA expression is regulated by transcriptional, epigenetic, and post-transcriptional regulation. The expression, proliferation, and differentiation of miRNA and miRNA-mediated gene regulation affect iPSC generation, and differentiation and self-renewal of human pluripotent stem cells (1).
The miR-200 family is known to regulate ZEB2, which is a gene related to the mesenchymal-to-epithelial transition (MET) process, which is an important factor in the production of induced pluripotent stem cells. The miR-302 and miR-372 regulate the epithelial-to-mesenchymal transition (EMT) in the epigenetic state (2). The miR-200 family together with the miR-302 and miR-362/367 clusters have been used to induced pluripotent stem cells production or self-renewal. These miRNAs regulate the expression of Oct3/4, SOX2, Rex1 and Lin-28 are known to increase the expression of let-7a and downregulate self-renewal-related genes as transcription factors (3, 4).
Our concern, miR-5739 has been reported to play an important role in angiogenesis, diffusion, migration, and morphogenesis regulation in mesenchymal stem cells, bin-ding to the target location of the endoglin protein, a transmembrane protein of the transforming growth factor-beta (TGF-β) receptors. This was revealed by controlling the function of the gene and its corresponding sub-signals in the process of inducing endothelial cell differentiation with the human embryonic stem cells CHA3 and H9 cell lines (5).
TGF-β and ALK-1 signals required for the differen-tiation of vascular endothelial cells are associated with Activin A and BMP4. These genes influence the formation of other organs in the mesoderm, such as cardiac, muscle, and suggest that miR-5739 regulation is related to the development of other mesoderm organs.
In the present study, the expression of miR-206, miR-486, miR-499 and bi-cistronic miRNA miR-1 and miR-133 was activated by MyoD, myogenin and MYF5 during muscle differentiation (6, 7), Various miRNAs are known to affect mesodermal differentiation.
Therefore, we constructed miR-5739 knockout cell line using human embryonic stem cells and CRISPR-Cas9 to investigate the function of miR-5739 known so far and its function in mesoderm development. In addition, we intend to verify the possibility of using miR-5739 as a cell therapy by applying it to a genetic disease model.
WA09 and miR-5739 knockout cells were cultured in StemMACSTM iPS-Brew XF medium (Miltenyi Biotec, Germany) on Matrigel-coated plates. The cells were maintained at 37℃ in humidified air with 5% CO2. The cells were passaged with 0.5 mM EDTA (Invitrogen, USA) when they were approximately 80% confluent. The study was approved by the institutional review board (IRB) of the Konkuk University (KUH1280080).
First of all, 40 μg of in-vitro transcribed sgRNA and 30 μg of recombinant Cas9 protein were electroporated using a P3 Primary Cell 4D-NucleofectorⓇ X Kit (Lonza). After confirming mutation with the T7E1 assay, the genomic DNA was isolated using a Quick Extract genome isolation kit (Epicentre QE9050). The target region was amplified and subjected to paired-end read sequencing using Illumina MiSeq at LAS (8, 9).
The alkaline phosphatase (AP) staining was performed using Alkaline Phosphatase Kit Ⅱ (Stemgent, USA) according to the manufacturer’s instructions. The cells were photographed using a Nikon Eclipse Ti camera (Nikon, Japan).
To generate hEBs, human embryonic stem cells (hESCs) were detached from Matrigel-coated plates using EDTA 0.5 mM (Invitrogen), and were cultured in a DMEM/F12 suspension supplemented with 10% SR (Gibco), 1% nonessential amino acids (Gibco), 1 mM L-Glutamine, 1% Penicillin-streptomycin and 0.1 mM mercaptoethanol in low-attachment dishes (Corning, ME). The EB medium was changed every day for 10 days.
For definitive endoderm (DE) induction, small clumps of hESCs were incubated in RPMI 1640 medium (Gibco) supplemented with 100 ng/ml Activin A (R&D systems) and 3 μM CHIR99021 (Tocris) on the day one. The next day the medium was changed to RPMI 1,640 medium with 0.2% v/v FBS (Gibco) and 100 ng/ml Activin A, and the cells were incubated for two additional days (10, 11).
Human embryonic stem cells were cultured in Stem-MACSTM iPS-Brew XF medium (Miltenyi Biotec), changed daily for 3 days, until 80∼90% confluency. The cells were treated with 3 to 6 M CHIR99021 for 2 days in RPMI 1640 medium (Gibco) containing B27 supplement, minus insulin (Gibco). After 2 days, the CHIR99021-contaning medium was aspirated and cells were maintained in EGM-2 medium (Lonza) supplemented with 50 ng/ml VEGF (PeproTech), 20 ng/ml BMP4 (PeproTech), and 20 ng/ml bFGF (Sigma-Aldrich) for 3 to 5 additional days.
To induce embryoid body formation, the hESCs were detached from the feeder cells with dispase (Gibco) and transferred to ultra-low attachment plates for suspension in basic FGF-free and BMP4 20 ng/ml in DMEM F/12 with 10% SR for 2 days. The primary EBs were cultured in DMEM supplemented with 5% fetal bovine serum (FBS) (Hyclone) for 12 to 14 days to differentiate into contracting EBs, and plated on 0.1% gelatin-coated plates containing DMEM-high glucose, supplemented with 2% FBS for 8 days. Beating clusters were isolated and dissociated into a single cell suspension by 0.25% trypsin-EDTA treatment for 5 to 10 min at 37℃. Subsequently, the cells were re-plated on a dish that was coated with a mixture of Matrigel and 0.1% gelatin solution at a ratio of 1:100, respectively, in low-glucose DMEM supplemented with 2% FBS for 10 days. Contracting cardiomyocytes were cultured at 37℃ and 5% CO2 in a humidified incubator, and the media was refreshed every 3 to 4 days (12).
Undifferentiated hESCs maintained under the defined culture conditions were treated with 10 μM RA 3 days after seeding for 4∼5 days. These neuroectoderm-differentiated hESCs were transferred to a serum free suspension culture to allow floating neuroblasts to form in the hESC media lacking bFGF for 4∼5 days. For further differentiating into a neuronal phenotype, the neuroblasts were then permitted to attach to a tissue culture plate in a defined medium containing DMEM/F-12, 1% N-2 supplement, 8 μg/ml heparin, 20 ng/ml VEGF, 10 ng/ml NT-3, 10 ng/ml BDNF (13).
Total RNA was extracted from the cells using RNeasy Mini Kit (QIAGEN) according to the manufacturer’s instructions, and cDNA synthesis was performed using High Capacity cDNA Reverse Transcription Kit (Applied Bios-ystem, USA). Reverse transcription polymerase chain reaction (RT-PCR) was performed in triplicate with AccuPower RT-PCR Master Mix (Bioneer, Korea). The primer sequences used in these assays are listed in Supplementary Table S1.
The cells were fixed in 4% (v/v) paraformaldehyde for 30 min and permeabilized with 0.3% bovine serum albumin (BSA) in PBS for 3 min. After treatment with 1% (v/v) normal goat serum for 30 min at room temperature, the cells were incubated with the primary antibody (FOXA2, SOX17, PECAM, VEGF, Tuj1, Map2b) in blocking solution for 12 h. Cells were washed with PBS and incubated with an Alexa Fluor 488-conjugated secondary antibody in blocking solution for 1 h at room temperature. To identify nuclei, cells were stained with 4,6-damidino-2-phenylindole (DAPI) for 5 min. All images were obtained using a fluorescence microscope (14).
Differentiated cells were dissociated with 0.25% Trypsin-EDTA (Gibco) and then was washced with FACS solution (phosphate-buffered saline [PBS] supplement 2% FBS). The single cells were incubated with phycoerythrin (PE)-conjugated mouse anti-human CD 31 (R&D system). After washing, the cells were analyzed using a FACS Calibur flow cytometer equipped with a cell sorter software (SONY, Japan), Flow cytometry data were analyzed using appropriate controls with an iso-type-matched IgG and unstained controls (14).
WA-09 and miR-5739 knockout cells (1.0×107 cells) were injected into the testicular region of 5-week-old Nude mice (Orient, Korea). The resulting teratomas were explanted 12 weeks later. The teratoma samples were histologically examined after staining the gut epithelium with hematoxylin and eosin (H&E) and the following stains: PAS stain for secretory epithelium, Alcian blue stain for cartilage, and Masson’s trichrome stain for muscle fibers. The images were analyzed using inverted microscopy (Nikon).
Gene Ontology (GO) network and Enrichment Pathway analysis of mir5739-39 and mir5739-65 cells was performed using the ClueGO plug-in v2.5.4 on Cytoscape v3.7.0 on Java script v1.8.0_211 (15). Significantly upregulated genes were selected. ClueGo analysis-incorporated GO was used for Biological Process (EBI, UniProt-GOA), KEGG Pathways, and Reactome Pathways. The pathway restriction was set to p<0.05, and a GO tree interval of 4∼6 was used to specify GO terms. The connectivity score (Kappa Score) was set to 0.4.
Human duchenne muscular dystrophy cell lines (DMD) and hereditary hemorrhagic telangiectasia cell lines(HHT) (Coriell, USA) purchased and cultured in Eagle’s minimum essential medium (Gibco) supplemented with 15% heat-inactivated fetal bovine serum and were maintained at 37℃ in a humidified at atmosphere of 5% CO2 (DMD cell line:Male-GM05112, Female-GM01695/HHT cell line:Male-GM13280, Female-GM03419).
Data are represented as mean±standard derivation, Statistical analysis was performed using student t-tests or one-way analysis of variance (ANOVA), with Tukey’s post-hoc multiple comparisons (GraphPad Prism5). Stati-stically significant difference is marked as *(p<0.05),**(p<0.005), ***(p<0.0001). Histograms of the obtained data were generated using Microsoft Excel 2010 software (Microsoft Corporation, USA).
The WA09 cell lines were generated using the CRISPR/Cas9 gene editing system. We generated a cell line (H9-miR-5739 KO), which was a homozygous knockout of miR-5739, using the CRISPR/Cas9 gene editing system. H9 cells were electroporated with preassembled Cas9 protein/single-chain RNA (sgRNA) ribonucleoprotein (RNPs) targeting the transactivation domain of miR-5739 (Fig. 1A). Four days after electroporation, cells were reseeded as single cells to obtain single-cell-derived clones. The sgRNA-induced indel frequency was measured 3 days post-transfection using T7 endonuclease I (T7E1), and the identity of the mutations was confirmed via deep sequencing (Fig. 1B). We found that the miR-5739 gene expression was significantly reduced in the miR-5739 knockout cell line versus control (Fig. 1C).
The mutated cell lines exhibited classical embryonic stem cell morphology. Their pluripotency was identified using AP staining (Fig. 2A). They showed a normal karyotype (46, XX) (Fig. 2B). These cell lines expressed the pluripotency markers pou5f1 and Nanog (Fig. 2C). We found that the miR-5739 knockout cell lines showed colony types similar to control cells (WA09, H9) and maintained pluripotency.
We compared the three-germ layer differentiation using miR-5739 knockout cell lines. The three-germ layer differentiation was confirmed via immunocytochemistry and showed a poor mesoderm differentiation efficiency. However, there was no significant difference in endoderm and ectoderm differentiation (Fig. 3A). We confirmed that miR-5739 knockout affects only mesoderm differentiation in into a particular lineage. We investigated whether a similar pattern was observed during spontaneous differentiation, and found that the three-germ layer inducing pluripotency was expressed (Pluripotency: POU5F1, Nanog/Endoderm: SOX17, Hnf4a, AFP Ectoderm: Nestin, Tuj1, PAX6). Interestingly, the expression of the collagen type-related gene was confirmed in the mesoderm lineage. However, the expression of the muscle and vascular-related genes was not confirmed (Fig. 3B).
In order to analyze the developmental pluripotency of human embryonic stem cells, the miR-5739 knockout cells were inoculated beneath the testicular capsule of nude mice. Histological examination revealed that the teratomas contained representative tissues from the three germ layers. In the control group H9, the differentiated tissues included gut epithelium (endoderm), muscle, cartilage (mesoderm) and secretory epithelium (ectoderm). Consistent with the differentiation of cells, the muscle type could not be confirmed in the histological analysis in the teratoma of miR-5739 knockout group (Fig. 4).
ClueGO, a widely used Cytoscape plug-in, was used to identify the biological functions of upregulated genes in mir5739 #39 and mir5739 #65 cells. As shown in Fig. 5, the results mainly consisted of five groups: muscle contraction, skeletal muscle contraction, positive regulation of cell communication, interferon alpha/beta signaling, and positive regulation of endothelial cell proliferation. The majority of genes were related to muscle contraction, skeletal muscle contraction, and positive regulation of endothelial cell proliferation (Fig. 5).
We confirmed the expression of the related genes by differentiation into specific mesoderm cells based on previous results. Based on our results, we confirmed that miR-5739 was genetically related mesoderm differentia-tion. Gene analysis and GO analysis showed that miR-5739 specifically affected the development of endothelial cells and cardiomyocytes in the mesoderm lineage. Thus, we used miR-5739 knockout cell line to identify the function of miR-5739 via direct cell differentiation.
First, immunocytochemistry results also confirmed the poor differentiation rate from the miR-5739 knockout cell line and the diminished fluorescence expression compared with H9 control (Fig. 6A). In addition, FACS sorting showed that 22.19% of the endothelial cells in H9 controls differentiated compared with 5.09% and 2.95%, in miR-5739 knockout cell lines, respectively (Fig. 6B). The results of FACS and immunocytochemistry were consistent. Similarly, FACS analysis of cardiomyocyte differentiation into cardiomyocytes demonstrated that the differentiation into cardiomyocytes from the H9 control cells was about 62.89%, and 1.59% and 1.06% in the miR-5739 knockout cell lines (Fig. 6B). It was confirmed that the differentiation of the miR-5739 knockout cell line into mesodermal lineage does not lead to differentiation into vascular and muscle type cells.
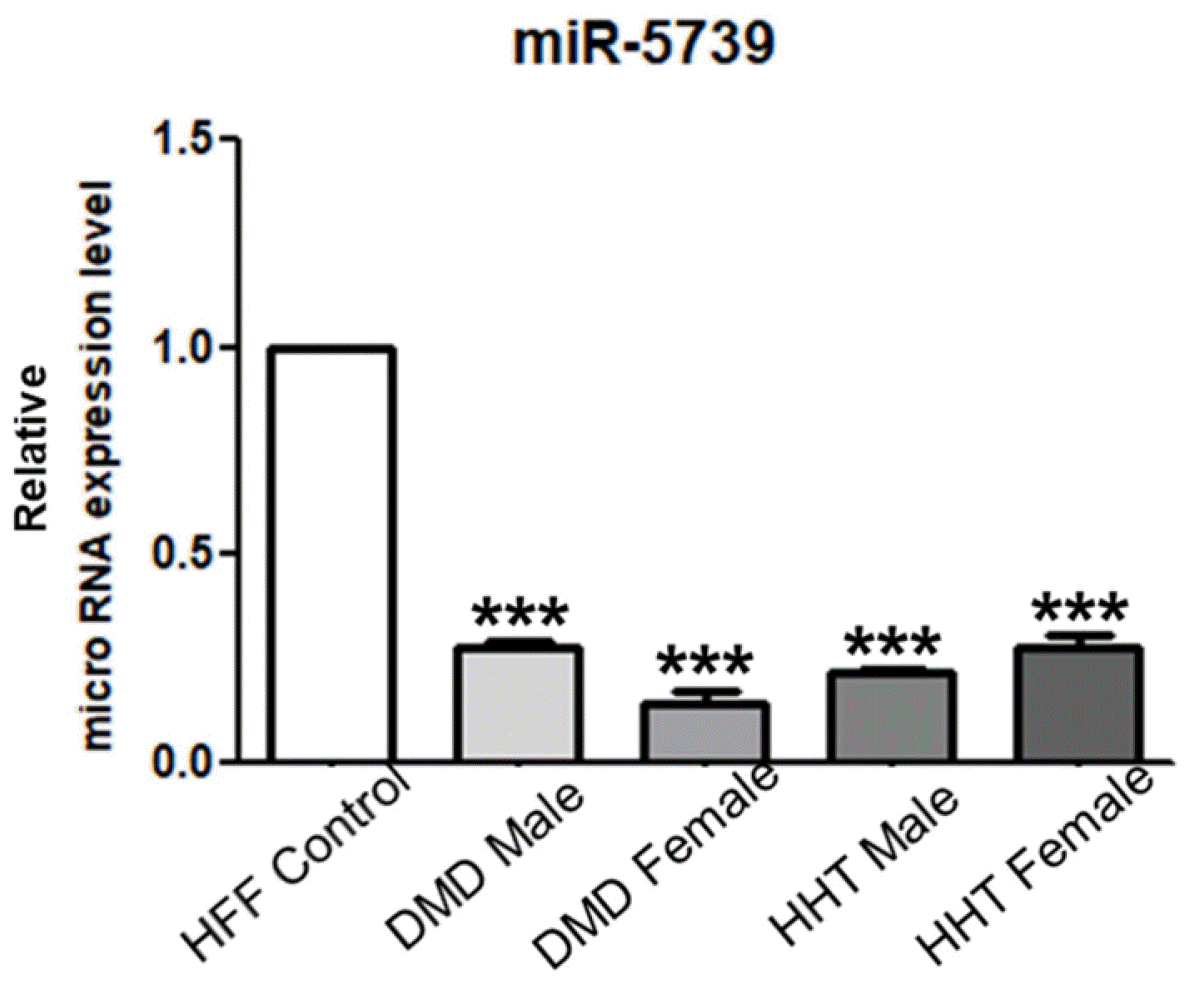
We identified the miR-5739 based on the results of previous experiments. The miR-5739 has been shown to affect mesoderm lineage differentiation, especially in muscle and vascular differentiation. Thus, we have analyzed the miR-5739 expression in muscle and vascular disease. We have studied Duchenne muscular dystrophy (DMD) using cell lines of muscle-related disease and hereditary hemorrhagic telangiectasia (HHT), a vascular abnormality. The expression of miR-5739 was compared and analyzed. Intere-stingly, the miR-5739 expression was decreased in DMD cell lines (27.68% and 14.01%) and HHT cell lines (21.94% and 27.51%) compared with control group human foreskin fibroblasts (Fig. 7). These results implicate the miR-5739 in muscle and vascular genetic diseases, and suggest its utility as a cell therapy agent.
The miR-5739 was first reported in the 2011 BBRC journal (5) and associated with vascular function. Previou-sly, we reported the study of endothelial cell and cardio-myocyte-related differentiation and characterization (14, 16, 17). The miR-5739 knockout cell lines were obtained using the CRISPR/Cas9 gene editing technology based on hESCs (WA09, H9) to further analyze the developmental functions and characteristics of the miR-5739 in relation to mesodermal lineage. The knockout cell lines were constructed with a significantly reduced off-target effect via gene editing (Fig. 1) (10) confirming the retention of pluripotency and self-renewal functions of hESCs (Fig. 2).
In order to analyze the developmental function of the miR-5739, we performed endodermal, mesodermal and ectodermal differentiation in vitro and found significant changes only in mesoderm lineage (Fig. 3). Interestingly, in mesoderm lineage, the collagen type was not affected by differentiation, and only muscle and vessel development were suppressed. The miRNAs such as miR-1, miR-133a, miR-208a/b (18), miR-200c (19), miR-499 (20), (21) and miR-17-92 (22) specifically affect mesoderm and are thought to be developmentally important. Our in vitro results suggest that miR-5739 is a mesoderm-specific regulatory gene.
We performed teratoma experiments to confirm the pluripotency of the two miR-5739 knockout cell line species in vivo, and confirmed the pluripotency of the cells via teratoma formation. Similar to the in vitro results, no muscle shape was observed in teratoma, and no vessel formation was detected. The difference between the injected cells and the original vessels in the mouse could not be established (Fig. 4). Based on these consistent results, we performed GO and signal pathway analysis to investigate the function of miR-5739. We confirmed that the miR-5739 was developmentally related to muscle contraction and endothelial cell proliferation. These results were consistent with our experimental results based on miR-5739 knockout cell lines. We used miR-5739 knockout cell lines to validate the function of miR-5739 by inducing differentiation of endothelial cells and cardiomyocytes to validate the results of pluripotency assays and differentiation, and further GO. Similar to GO results, the miR-5739 was associated with endothelial cells and cardiomyocytes, and the miR-5739 knockout cells showed a drastic reduction in the differentiation efficiency of each cell following induction (Fig. 6).
We found that the miR-5739 affects the development of muscles and blood vessels. We found a correlation between miR-5739 and genetic disorders in the muscle and vessel developmental stages. We first analyzed the role of miR-5739 in diseases related to muscle and vessel formation using the two cell lines representing DMD and HHT. Interestingly, we found that the miR-5739 expression was reduced in both somatic cells. The miR-5739 was associated with Akt, ERK1/2 signaling, transforming growth factor-beta (TGF-β) and Wnt, BMP4, FGF2, Activin A and VEGF signaling pathways mediating muscle and vessel formation (23-25). The miR-5739 and its signal transduction pathway require further investigation.
In summary, we constructed the miR-5739 knockout cell lines and analyzed the developmental function and characteristics of the miR-5739. Based on in vitro and in vivo experiments, the miR-5739 plays an important role in the development of muscle and blood vessels. In addition, based on its developmental role in muscle and vessels, the miR-5739 may be a biomarker in patients with muscle or vessel damage.
Notes
Ethics Statement
All animal experiments were conducted in accordance with the guidelines of the Korean National Agency. As a guideline of Ministry of Agriculture, Food and Rural Affairs-Animal Protection Act (No.12051, 2013) and Ministry of Food and Drug Safety-Act on Laboratory Animals (No.11987, 2013). It was conducted with the experimental procedure and approval of the Konkuk University Animal Experiment Ethics Committee (IACUC No. 18036).
Supplementary Materials
Supplementary data including two tables can be found with this article online at https://doi.org/10.15283/ijsc22187.
References
1. Shim J, Nam JW. 2016; The expression and functional roles of microRNAs in stem cell differentiation. BMB Rep. 49:3–10. DOI: 10.5483/BMBRep.2016.49.1.217. PMID: 26497582. PMCID: PMC4914210.

2. Subramanyam D, Lamouille S, Judson RL, Liu JY, Bucay N, Derynck R, Blelloch R. 2011; Multiple targets of miR-302 and miR-372 promote reprogramming of human fibroblasts to induced pluripotent stem cells. Nat Biotechnol. 29:443–448. DOI: 10.1038/nbt.1862. PMID: 21490602. PMCID: PMC3685579.

3. Chang HM, Martinez NJ, Thornton JE, Hagan JP, Nguyen KD, Gregory RI. 2012; Trim71 cooperates with microRNAs to repress Cdkn1a expression and promote embryonic stem cell proliferation. Nat Commun. 3:923. DOI: 10.1038/ncomms1909. PMID: 22735451. PMCID: PMC3518406.

4. Amador-Arjona A, Cimadamore F, Huang CT, Wright R, Lewis S, Gage FH, Terskikh AV. 2015; SOX2 primes the epigenetic landscape in neural precursors enabling proper gene activation during hippocampal neurogenesis. Proc Natl Acad Sci U S A. 112:E1936–E1945. DOI: 10.1073/pnas.1421480112. PMID: 25825708. PMCID: PMC4403144.

5. Yoo JK, Kim J, Choi SJ, Kim CH, Lee DR, Chung HM, Kim JK. 2011; The hsa-miR-5739 modulates the endoglin network in endothelial cells derived from human embryonic stem cells. Biochem Biophys Res Commun. 415:258–262. DOI: 10.1016/j.bbrc.2011.10.030. PMID: 22020071.

6. Chen JF, Tao Y, Li J, Deng Z, Yan Z, Xiao X, Wang DZ. 2010; microRNA-1 and microRNA-206 regulate skeletal muscle satellite cell proliferation and differentiation by repressing Pax7. J Cell Biol. 190:867–879. DOI: 10.1083/jcb.200911036. PMID: 20819939. PMCID: PMC2935565.

7. Srivastava D, Heidersbach AJ. 2013; Small solutions to big problems: microRNAs for cardiac regeneration. Circ Res. 112:1412–1414. DOI: 10.1161/CIRCRESAHA.113.301409. PMID: 23704215. PMCID: PMC3760376.
8. Kim SJ, Habib O, Kim JS, Han HW, Koo SK, Kim JH. 2017; A homozygous Keap1-knockout human embryonic stem cell line generated using CRISPR/Cas9 mediates gene targeting. Stem Cell Res. 19:52–54. DOI: 10.1016/j.scr.2016.12.028. PMID: 28413007. PMID: 1e2a89c6c8014f008aa1ef8d7725ec5e.

9. Kim S, Kim D, Cho SW, Kim J, Kim JS. 2014; Highly efficient RNA-guided genome editing in human cells via delivery of purified Cas9 ribonucleoproteins. Genome Res. 24:1012–1019. DOI: 10.1101/gr.171322.113. PMID: 24696461. PMCID: PMC4032847.
10. D'Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE. 2005; Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol. 23:1534–1541. DOI: 10.1038/nbt1163. PMID: 16258519.
11. Jiang J, Au M, Lu K, Eshpeter A, Korbutt G, Fisk G, Majumdar AS. 2007; Generation of insulin-producing islet-like clusters from human embryonic stem cells. Stem Cells. 25:1940–1953. DOI: 10.1634/stemcells.2006-0761. PMID: 17510217.

12. Choi SW, Lee HA, Moon SH, Park SJ, Kim HJ, Kim KS, Zhang YH, Youm JB, Kim SJ. 2016; Spontaneous inward currents reflecting oscillatory activation of Na⁺/Ca²⁺ exchangers in human embryonic stem cell-derived cardiomyocytes. Pflugers Arch. 468:609–622. Erratum in: Pflugers Arch 2016;468: 1295. DOI: 10.1007/s00424-015-1769-2. PMID: 26687128.

13. Parsons XH, Parsons JF, Moore DA. 2012; Genome-scale mapping of microRNA signatures in human embryonic stem cell neurogenesis. Mol Med Ther. 1:105. DOI: 10.4172/2324-8769.1000105. PMID: 23543894. PMCID: PMC3609664.

14. Park SJ, Lee JH, Lee SG, Lee JE, Seo J, Choi JJ, Jung TH, Chung EB, Kim HN, Ju J, Song YH, Chung HM, Lee DR, Moon SH. 2019; Functional equivalency in human somatic cell nuclear transfer-derived endothelial cells. Stem Cells. 37:623–630. DOI: 10.1002/stem.2986. PMID: 30721559.

15. Bindea G, Mlecnik B, Hackl H, Charoentong P, Tosolini M, Kirilovsky A, Fridman WH, Pagès F, Trajanoski Z, Galon J. 2009; ClueGO: a Cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics. 25:1091–1093. DOI: 10.1093/bioinformatics/btp101. PMID: 19237447. PMCID: PMC2666812.

16. Yang HM, Choi JJ, Kim HN, Yang SJ, Park SJ, Kang C, Chung HM, Lee MR, Kim SJ, Moon SH. 2019; Reconstituting human cutaneous regeneration in humanized mice under endothelial cell therapy. J Invest Dermatol. 139:692–701. DOI: 10.1016/j.jid.2018.08.031. PMID: 30393080.

17. Han SS, Shim HE, Park SJ, Kim BC, Lee DE, Chung HM, Moon SH, Kang SW. 2018; Safety and optimization of metabolic labeling of endothelial progenitor cells for tracking. Sci Rep. 8:13212. DOI: 10.1038/s41598-018-31594-0. PMID: 30181604. PMCID: PMC6123424.

18. Chistiakov DA, Orekhov AN, Bobryshev YV. 2016; Cardiac-specific miRNA in cardiogenesis, heart function, and cardiac pathology (with focus on myocardial infarction). J Mol Cell Cardiol. 94:107–121. DOI: 10.1016/j.yjmcc.2016.03.015. PMID: 27056419.

19. Poon EN, Hao B, Guan D, Jun Li M, Lu J, Yang Y, Wu B, Wu SC, Webb SE, Liang Y, Miller AL, Yao X, Wang J, Yan B, Boheler KR. 2018; Integrated transcriptomic and regulatory network analyses identify microRNA-200c as a novel repressor of human pluripotent stem cell-derived cardiomyocyte differentiation and maturation. Cardiovasc Res. 114:894–906. DOI: 10.1093/cvr/cvy019. PMID: 29373717.

20. Fu JD, Rushing SN, Lieu DK, Chan CW, Kong CW, Geng L, Wilson KD, Chiamvimonvat N, Boheler KR, Wu JC, Keller G, Hajjar RJ, Li RA. 2011; Distinct roles of microRNA-1 and -499 in ventricular specification and functional maturation of human embryonic stem cell-derived cardiomyo-cytes. PLoS One. 6:e27417. DOI: 10.1371/journal.pone.0027417. PMID: 22110643. PMCID: PMC3217986. PMID: c906e6f1799b400b8bf5c02dcd4e7ff2.

21. Kuppusamy KT, Sperber H, Ruohola-Baker H. 2013; MicroRNA regulation and role in stem cell maintenance, cardiac differentiation and hypertrophy. Curr Mol Med. 13:757–764. DOI: 10.2174/1566524011313050007. PMID: 23642057. PMCID: PMC3898432.

22. Wang J, Bai Y, Li H, Greene SB, Klysik E, Yu W, Schwartz RJ, Williams TJ, Martin JF. 2013; MicroRNA-17-92, a direct Ap-2α transcriptional target, modulates T-box factor activity in orofacial clefting. PLoS Genet. 9:e1003785. DOI: 10.1371/journal.pgen.1003785. PMID: 24068957. PMCID: PMC3777996. PMID: 9a295d38ba574972bbee7701823ca1cd.

23. Bhatnagar S, Kumar A. 2010; Therapeutic targeting of signaling pathways in muscular dystrophy. J Mol Med (Berl). 88:155–166. DOI: 10.1007/s00109-009-0550-4. PMID: 19816663. PMCID: PMC2833214.

24. Botella LM, Albiñana V, Ojeda-Fernandez L, Recio-Poveda L, Bernabéu C. 2015; Research on potential biomarkers in hereditary hemorrhagic telangiectasia. Front Genet. 6:115. DOI: 10.3389/fgene.2015.00115. PMID: 25873934. PMCID: PMC4379940. PMID: 0d24364ca6db49ec87de526b4e919f84.

25. McDonald J, Wooderchak-Donahue W, VanSant Webb C, Whitehead K, Stevenson DA, Bayrak-Toydemir P. 2015; Hereditary hemorrhagic telangiectasia: genetics and molecular diagnostics in a new era. Front Genet. 6:1. DOI: 10.3389/fgene.2015.00001. PMID: 25674101. PMCID: PMC4306304. PMID: 4e0e7895728541ebba65b1b143e8e0f8.

Fig. 1
miR-5739 knockout cell line construction and analysis. (A) Schematic overview of the miR-5739 gene and the Cas9/gRNA target site. (B) miR-5739 specific sgRNA RNP-mediated mutations measured with the T7E1 assay. (C) Real-time PCR for confirming miR-5739 expression reduction in miR-5739 knockout cells.
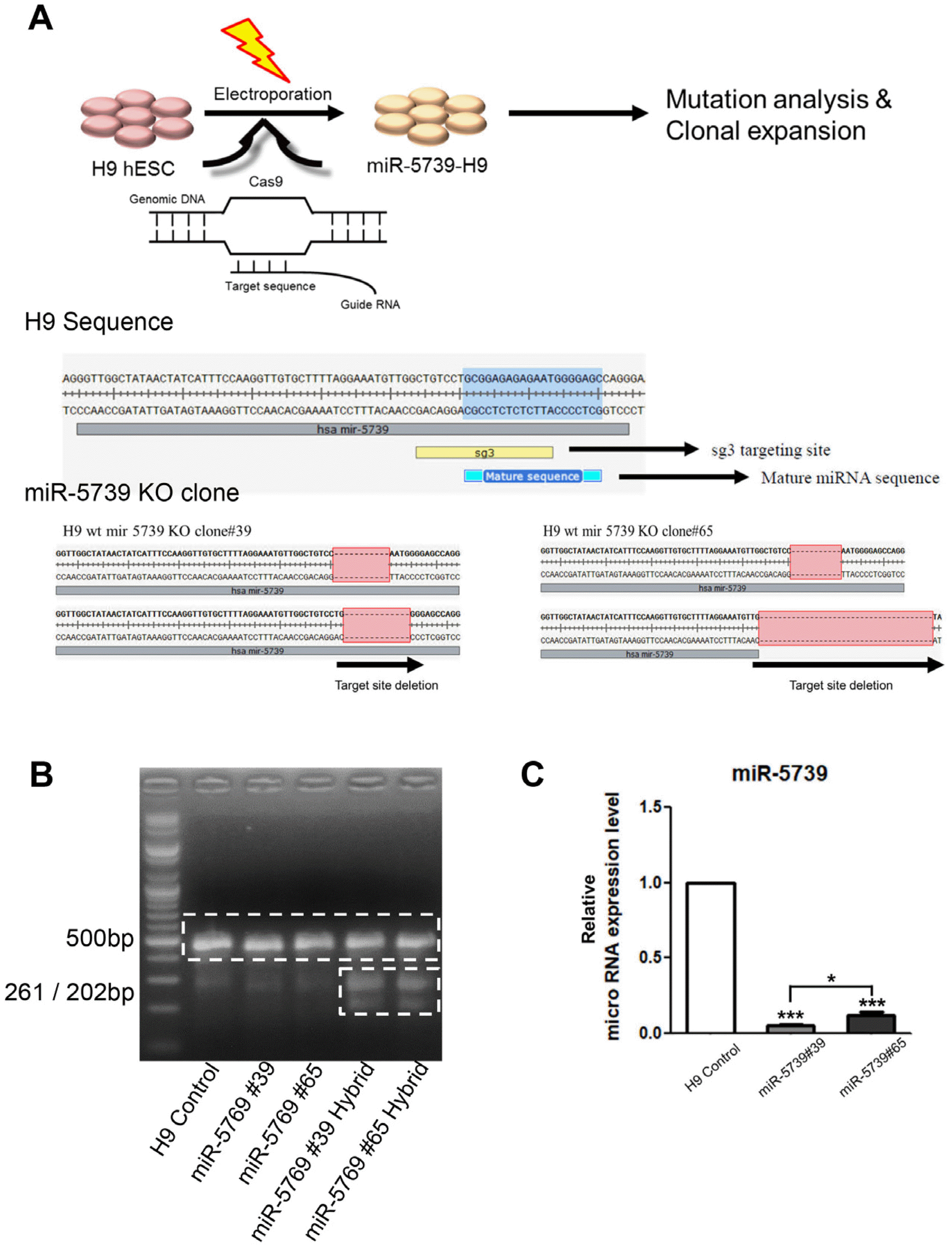
Fig. 2
miR-5739 knockout cells undifferentiated in vitro characterization. (A) H9 and miR-5739 knockout cells colony, AP staining (Scale bar 200 μm). (B) Representative karyotype miR-5739 cell lines showing a normal 48 chromosomes. (C) Pluripotent gene expression profile miR-5739 knockout cell lines compared with the H9.
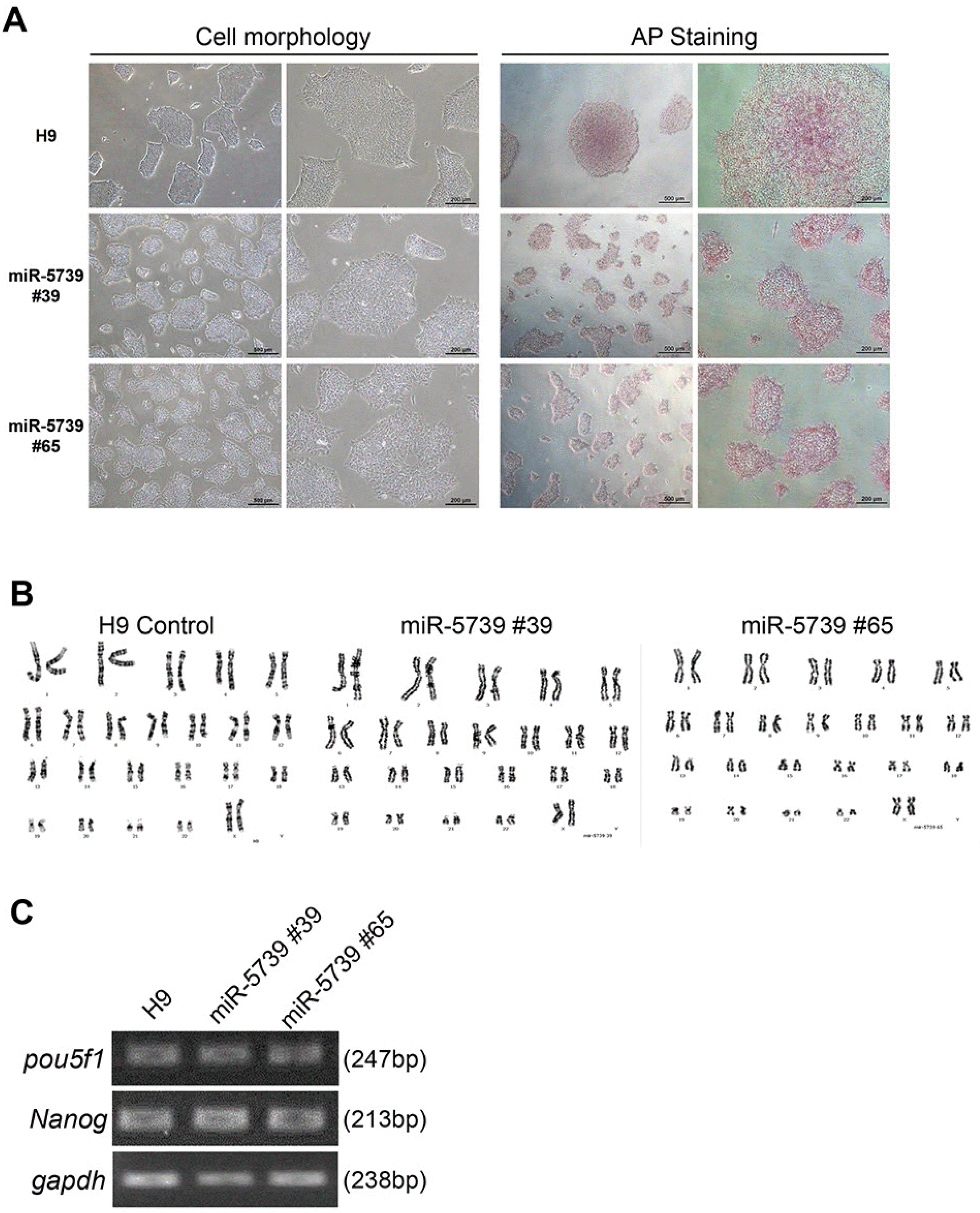
Fig. 3
miR-5739 knockout cells three-germ layer differentiated in vitro cha-racterization. (A) Immunofluoresce-nce was identified using differenti-ation markers in three-germ layer differentiated miR-5739 knockout cells. Top (Endoderm-SOX17 (Green), FOXA2 (Red)), middle (Mesoderm-VEGF (Gre-en), PECAM (Red)), bottom (Ec-toderm-Map2b (Green), Tuj1 (Red), DAPI (blue)), (Scale bar 200 μm). (B) Expression of markers for all three germ layers in 3D culture condition differentiation. GAPDH, Pluri-potent-Nanog, POU5F1, Endoderm-SOX17 (SRY-box transcription factor 17), Hnf4a (Hepatocyte nuclear factor 4 alpha), FOXA2 (Forkhead box protein A2), AFP (Alpha fetoprotein), Mesoderm (Collagen type)-ACAN (Aggrecan), Col1A1 (Collagen type 1 alpha 1 chain), ALP (Alkaline phosphatase), Mesoderm (Muscle type)-KDR (Kinase insert domain receptor), Hand1 (Heart and neural crest derivatives expressed 1), SMA (Smooth muscle actin), T (Brachyury), Ectoderm-Nestin, Tuj1 (Class III beta-tubulin), PAX6.
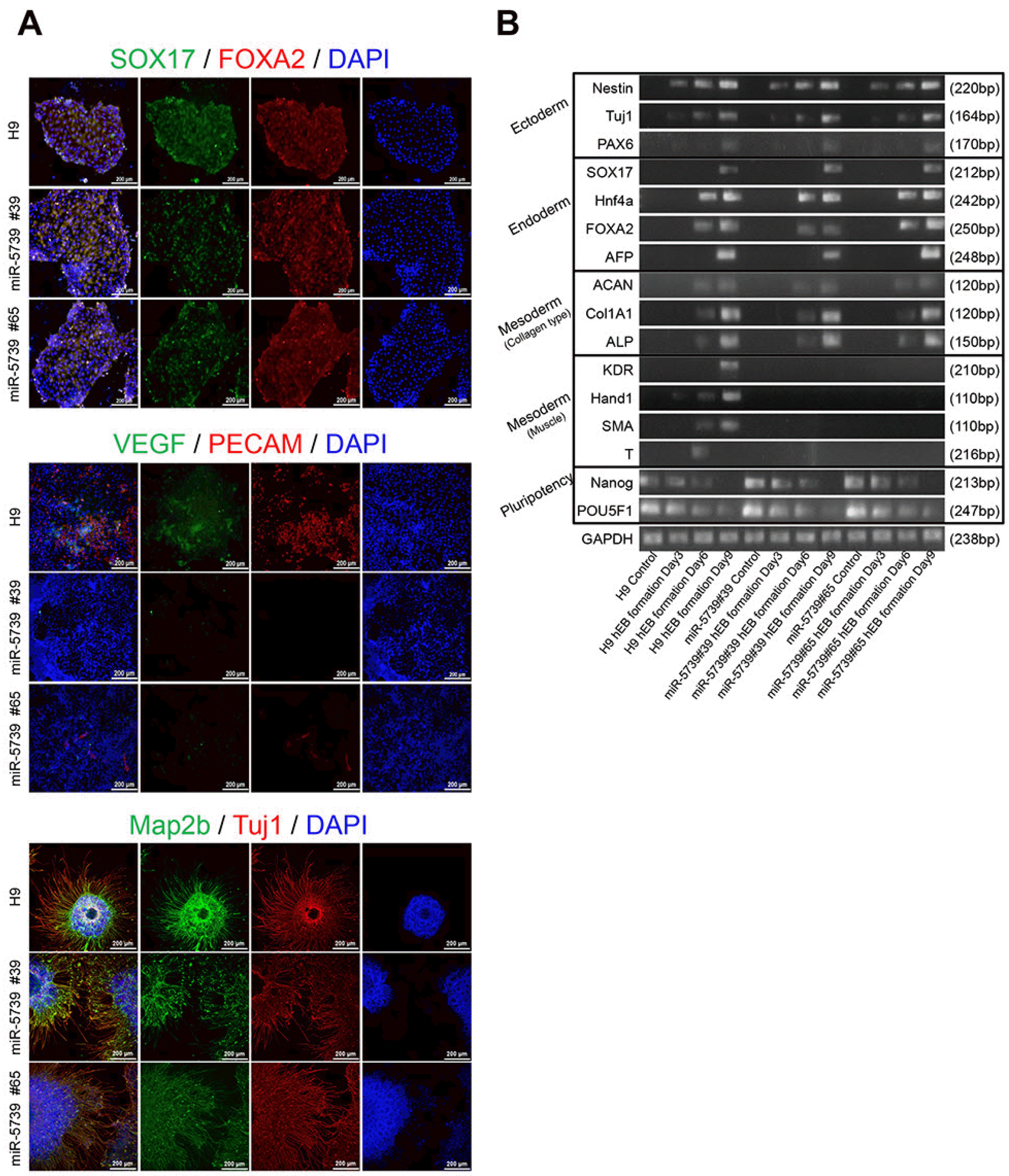
Fig. 4
The effect of miR-5739 knockout cells injection on development in nude mouse. Gut epithelium (Hematoxylin & Eosin staining, magnification ×10, scale bar 100 μm), Muscle (Masson’s trichrome staining, magnification ×10, scale bar 100 μm), Cartilage (Alcian blue staining, magnification ×10, scale bar 100 μm), secretory epithelium (PAS staining, magnification ×10, scale bar 100 μm).
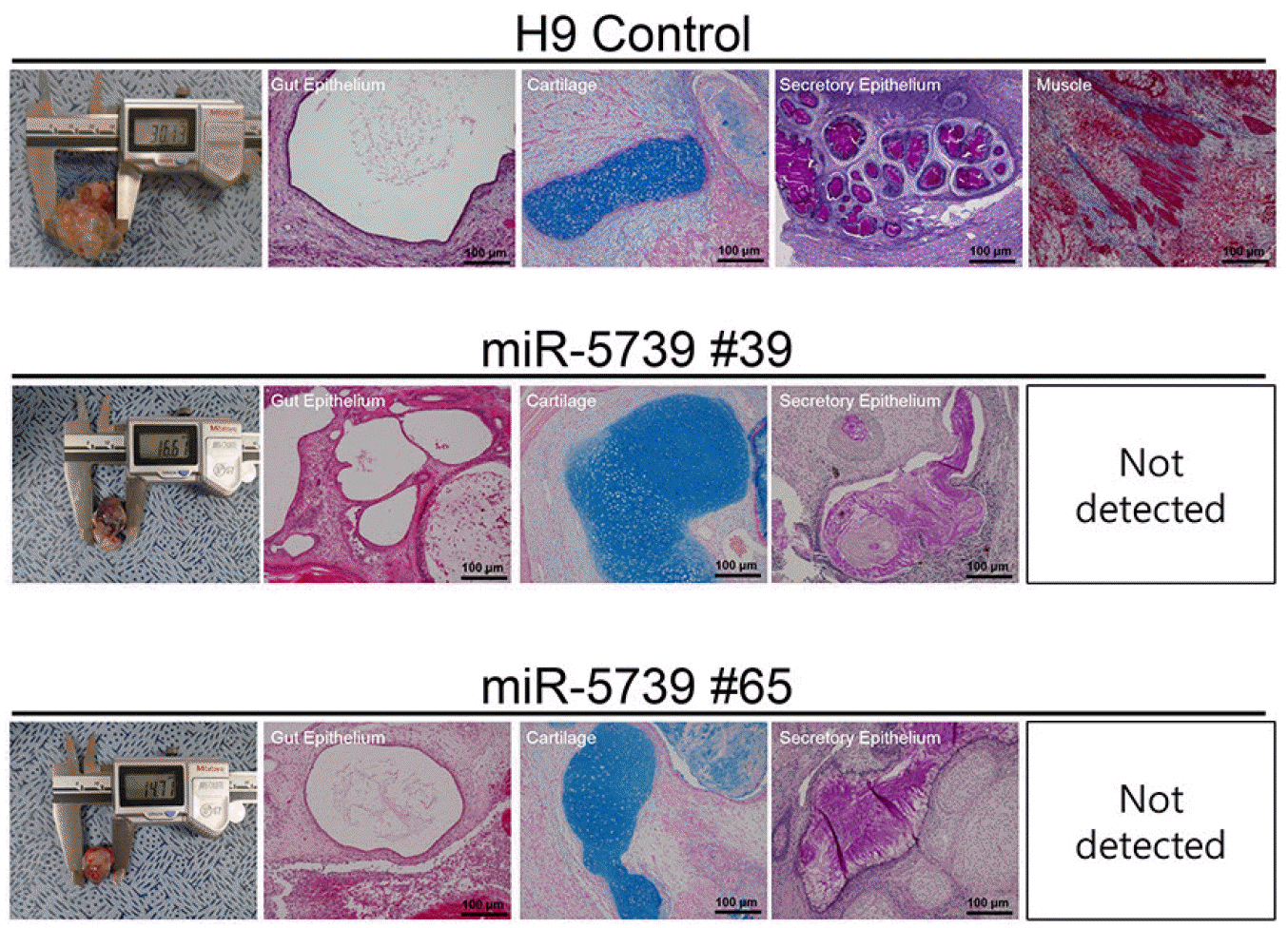
Fig. 5
Clue GO analysis of regulated miR-5739 in human embryonic stem cells. The distribution of four clusters visualized on network. Overview chart with functional groups including specific terms for regulated genes.
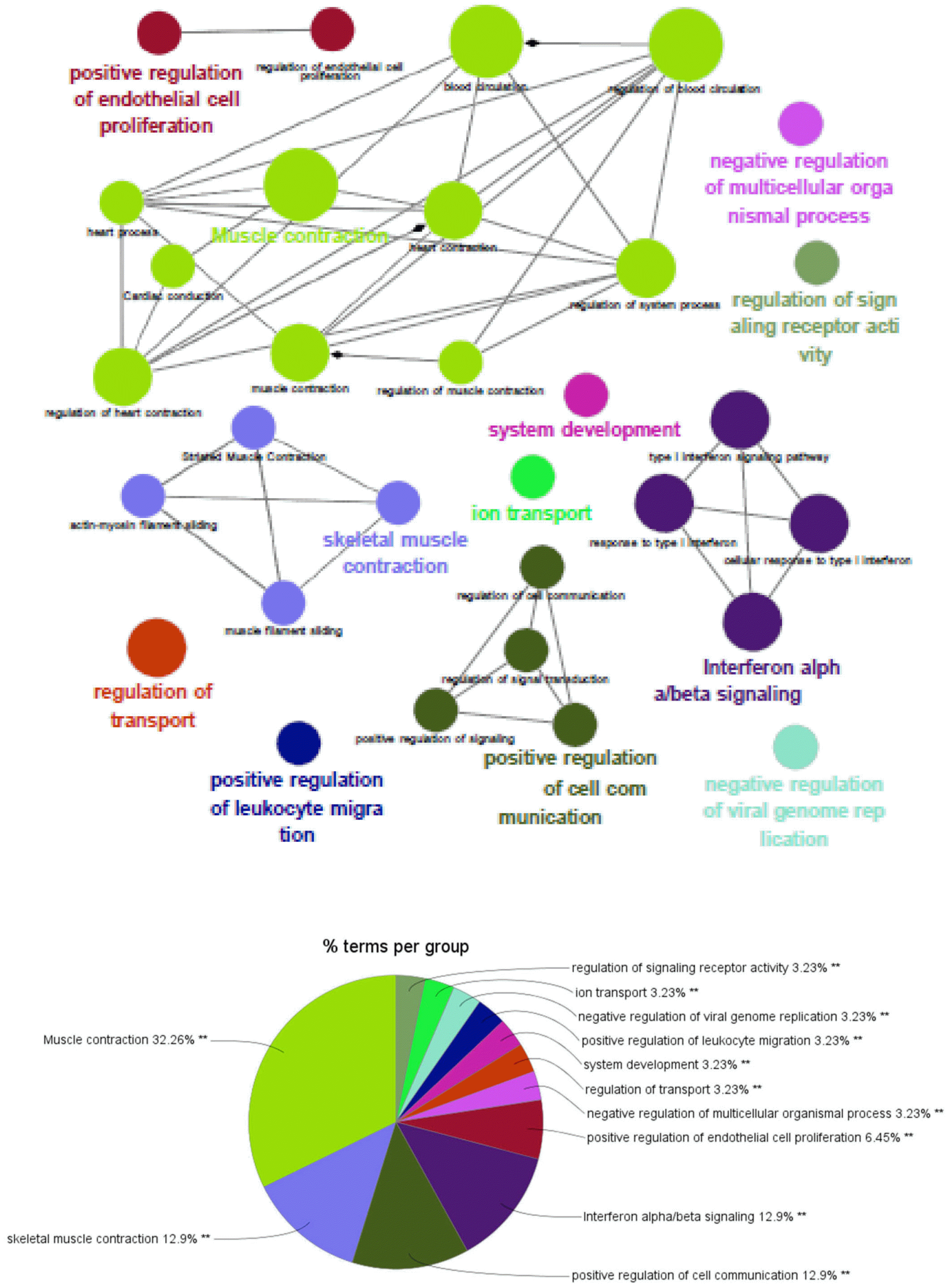
Fig. 6
Muscle and vessel specific differentiated in vitro characterization. (A) Immunofluorescence identified cells differentiated into specific mesoderm at miR-5739 knockout cells. Endothelial cells-VEGF (Green), PECAM (Red), Cardiomyocytes-cTnT(Green), sarcomeric actin(Red), DAPI (blue), (Scale bar 200 μm). (B) FACS analysis of endothelial cells and cardio-myocytes. CD31+H9 cells (22.19%), miR-5739 #39 cells (5.09%), miR-5739 #65 cells (2.95%). cTnT+ H9 cells (62.89%), miR-5739 #39 (1.59%), miR-5739 #65 (1.06%).
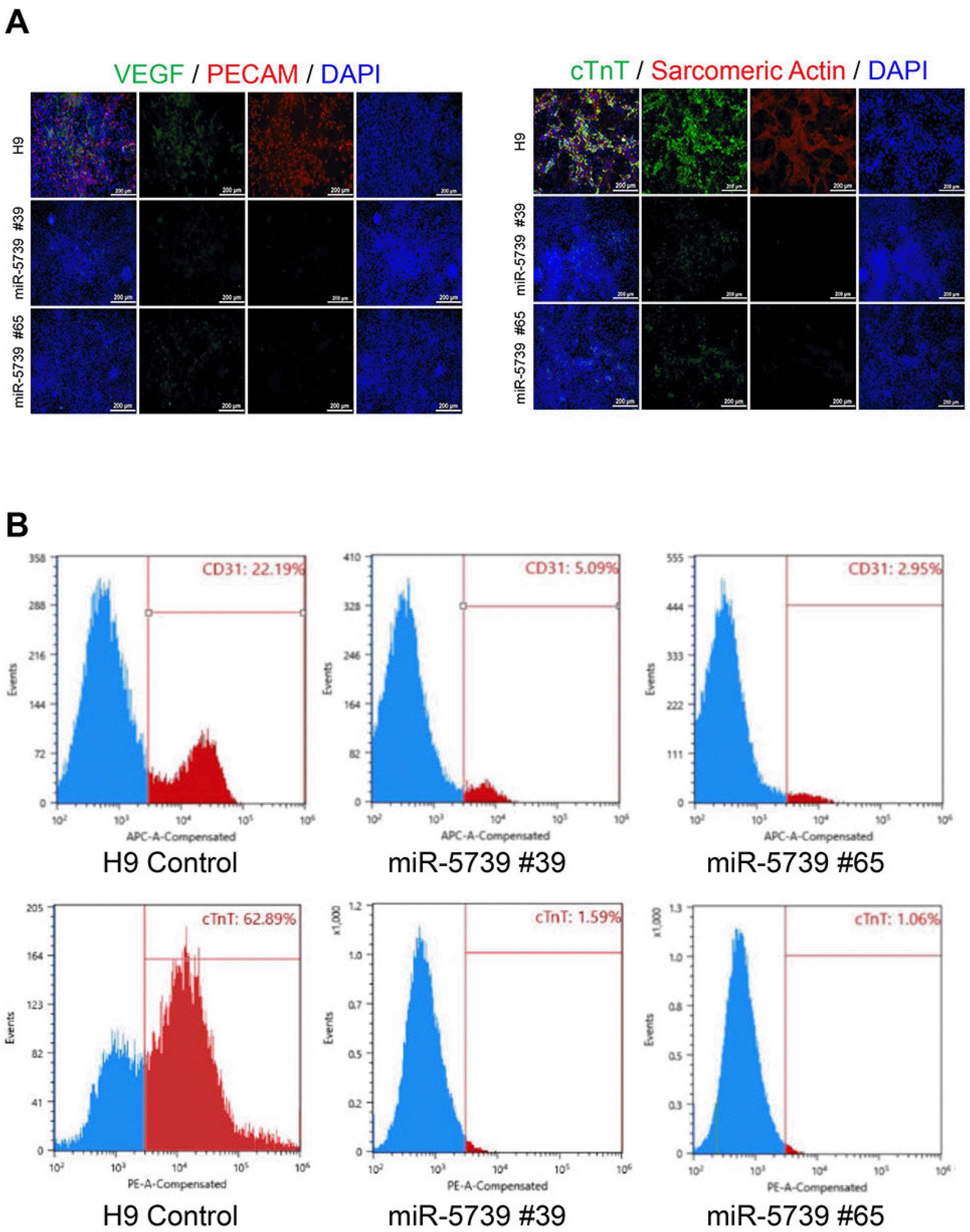
Fig. 7
Verification of miR-5739 expression using congenital muscle and vascular disease cells. Real-time PCR for confirming miR-5739 expression reduction in duchenne muscular dystrophy (DMD) patient’s cell lines and hereditary hemorrhagic telangiectasia patient’s cell lines. DMD cell lines male (27.68%), DMD cell lines female (14.01%) and HHT cell lines male (21.94%), HHT cell lines female (27.51%).




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download