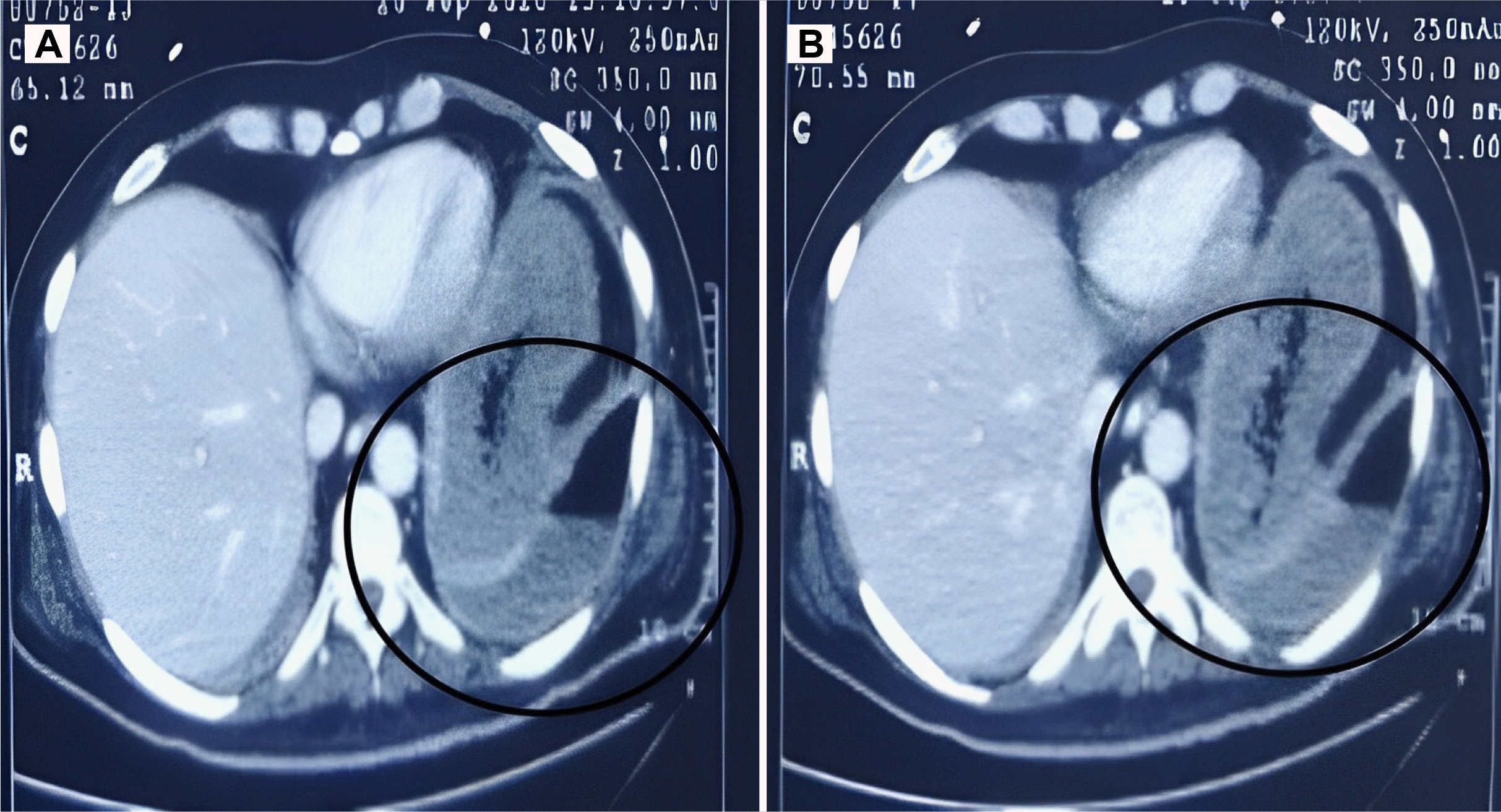
COVID-19 was discovered in Wuhan, China in December 2019 and was declared a pandemic by World Health Organization (WHO) in March 2020. The second wave of this epidemic began in India in March 2021. The sickness was severe during this second wave, and deaths occurred even among the younger population. There was a trend of delayed complications throughout this wave, including mucormycosis and thrombo-embolic events. Mucormycosis typically affects the paranasal sinus, which extends to the orbit, mouth, face, brain, and lungs.
5 Mucormycosis of the gastrointestinal system is uncommon. In India, a few cases of gastrointestinal mucormycosis have been reported.
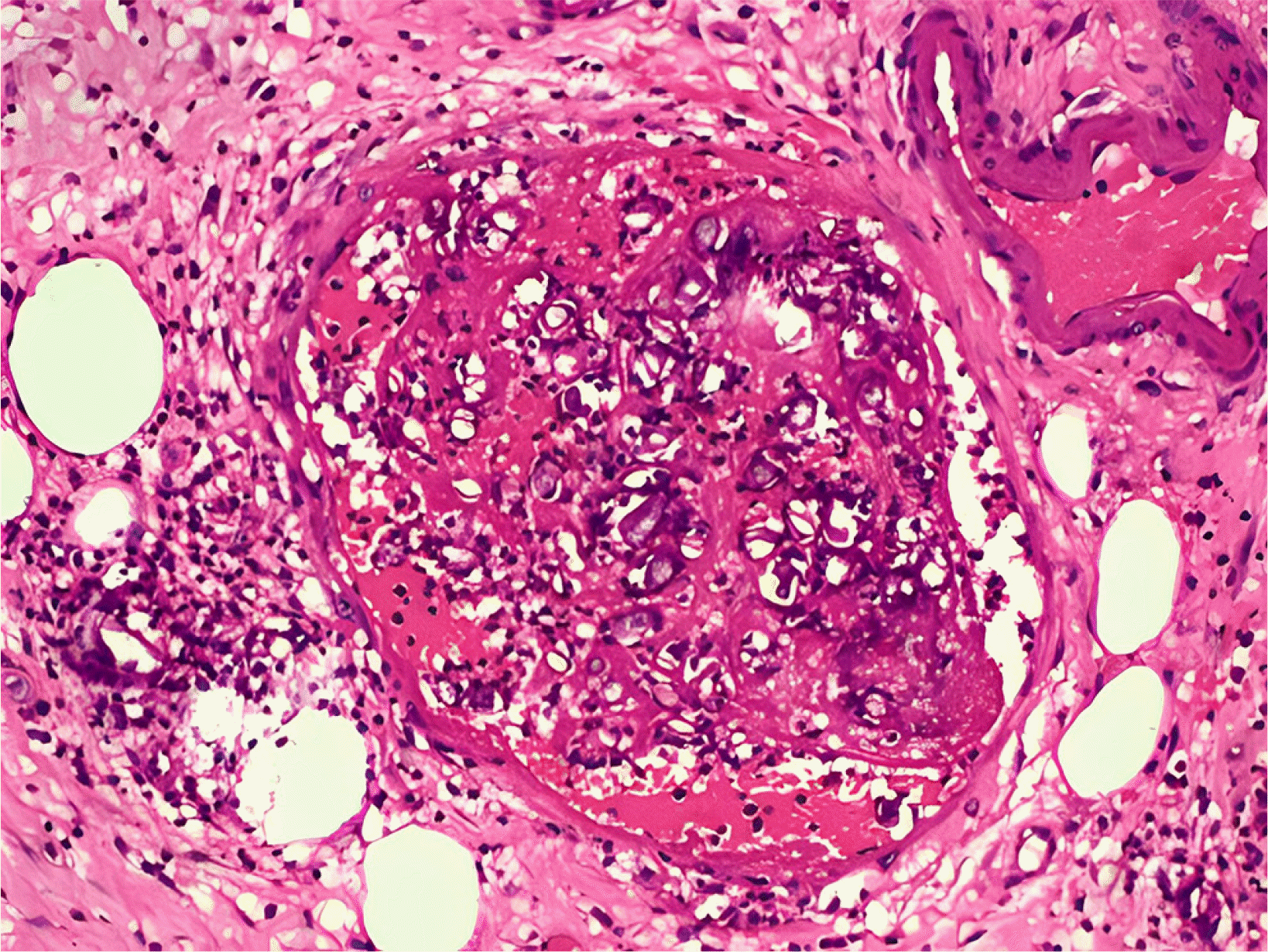
6,7 Mucormycosis is characterized by tissue necrosis because of the inherent capacity of angioinvasion.
8 Most patients are immunocompromised or suffer from poor glycemic control. COVID-19 patients who are immunocompromised due to a viral infection and are receiving steroids concurrently have poor glycemic control.
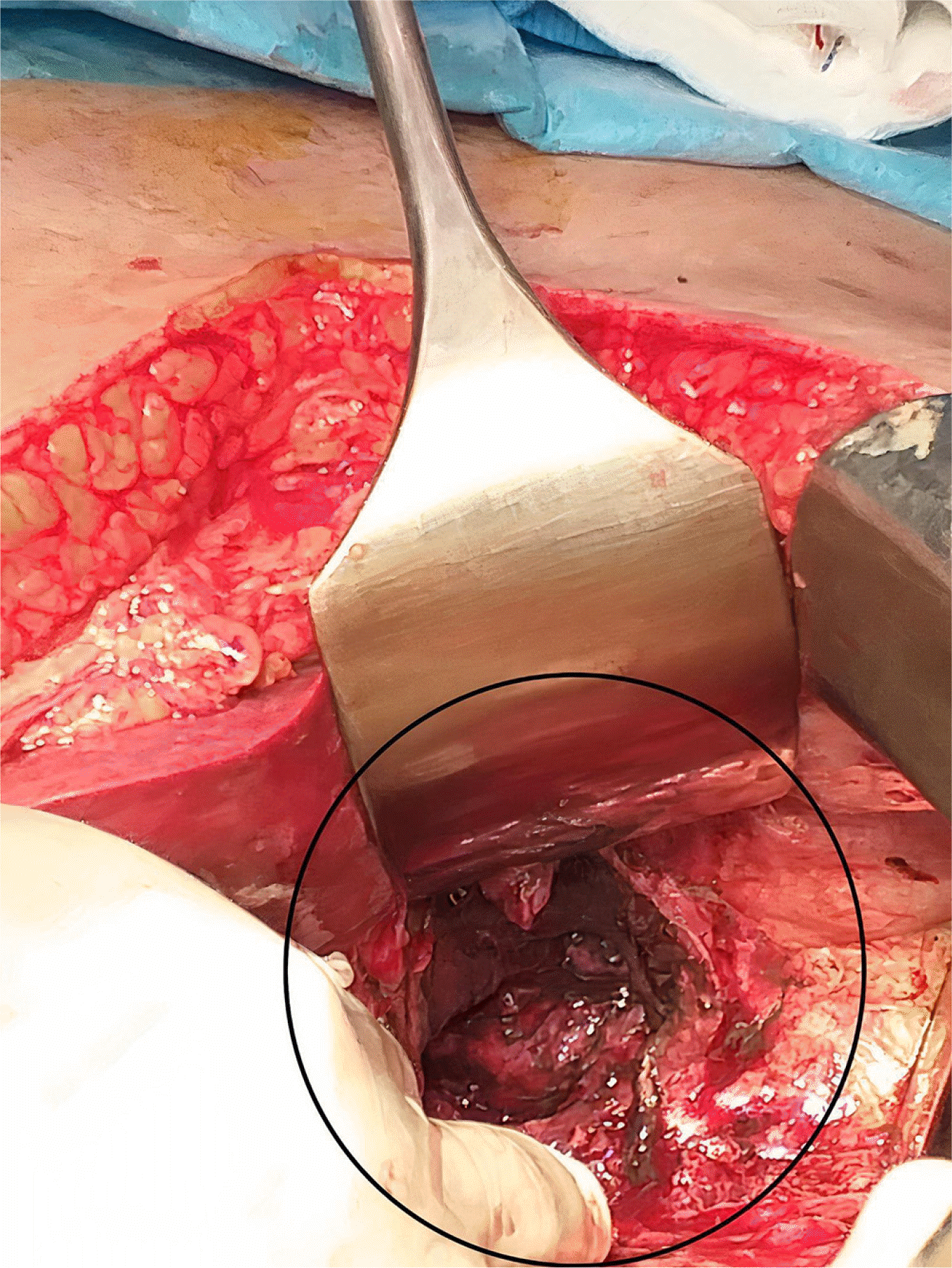
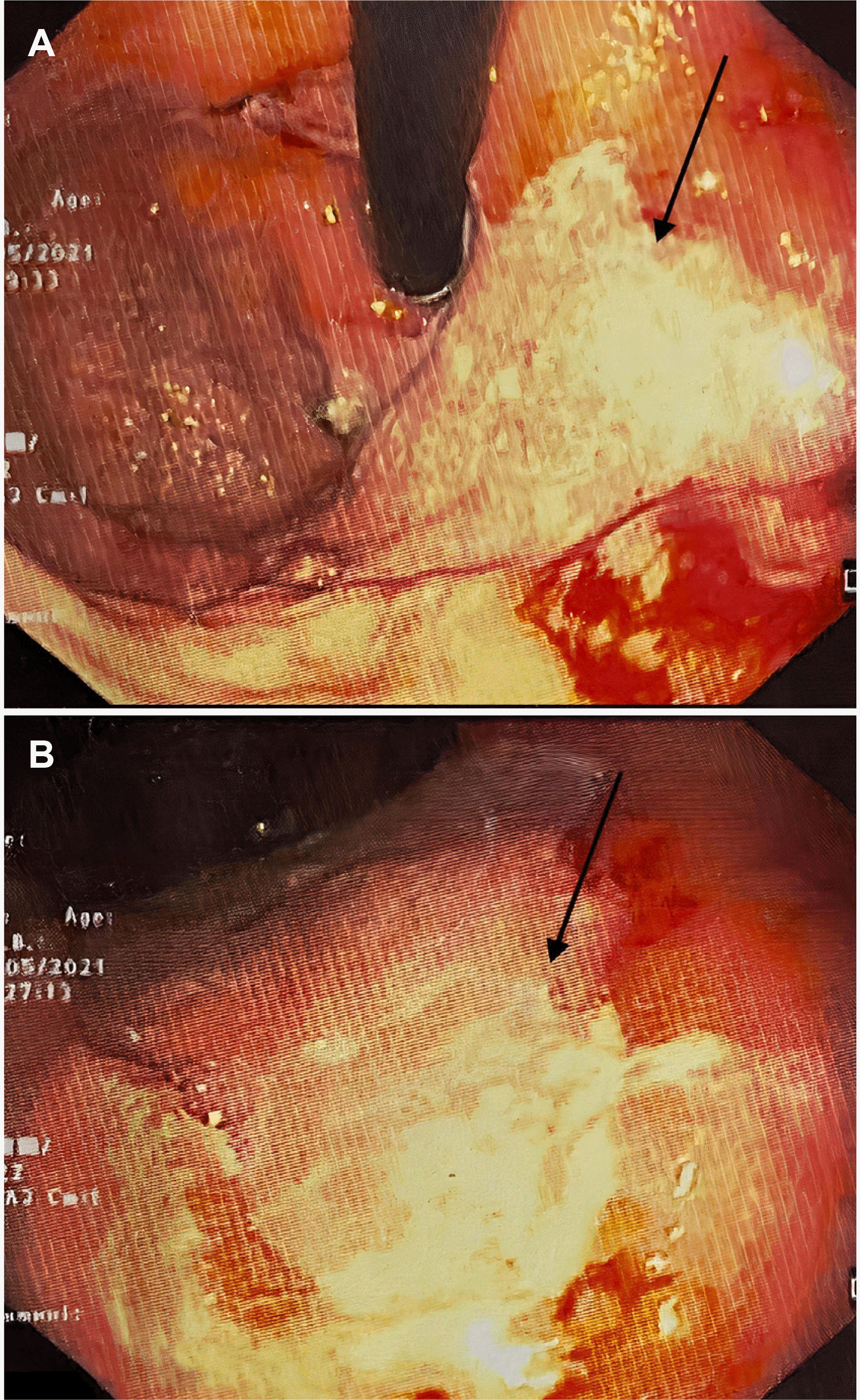
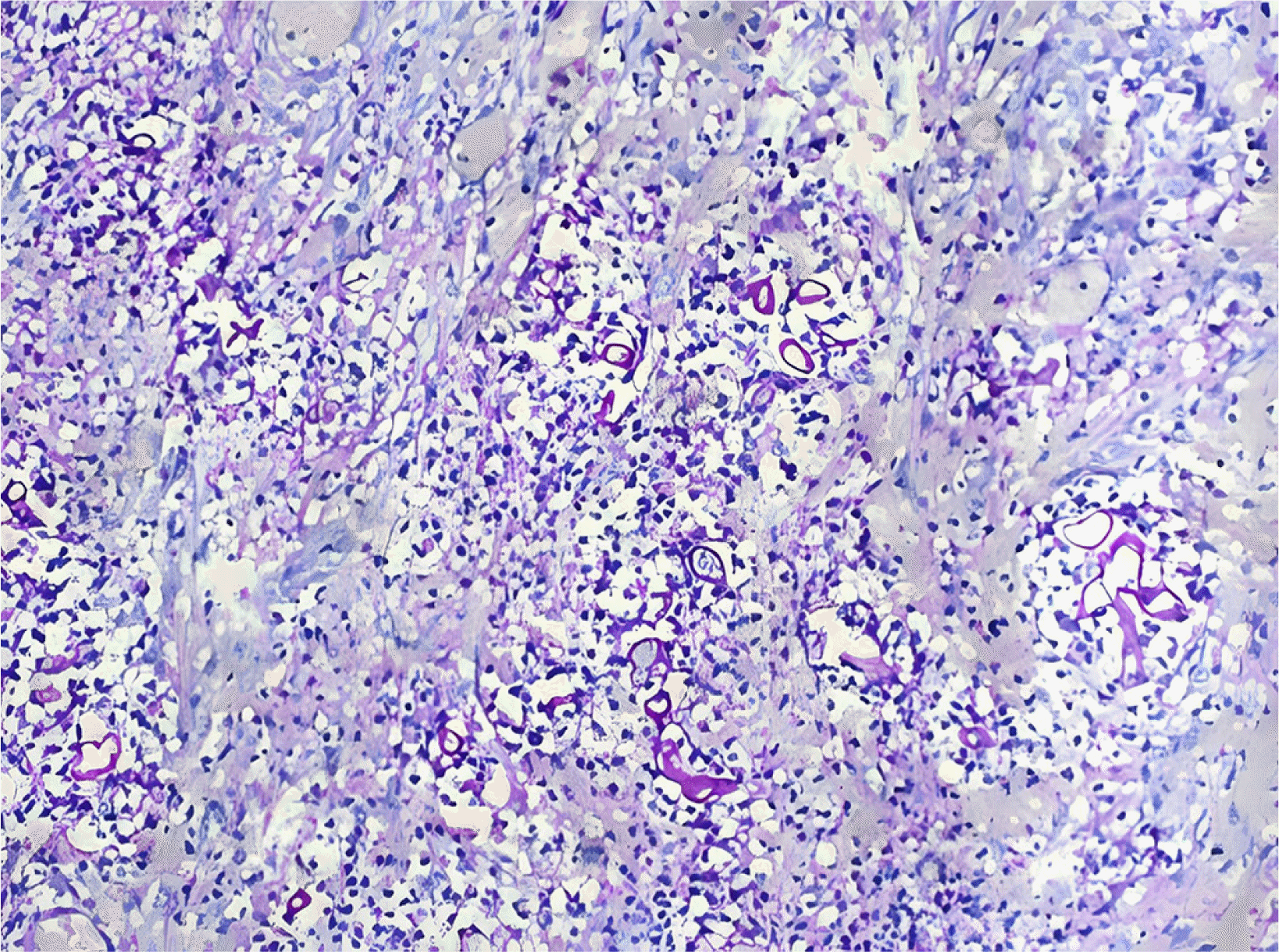
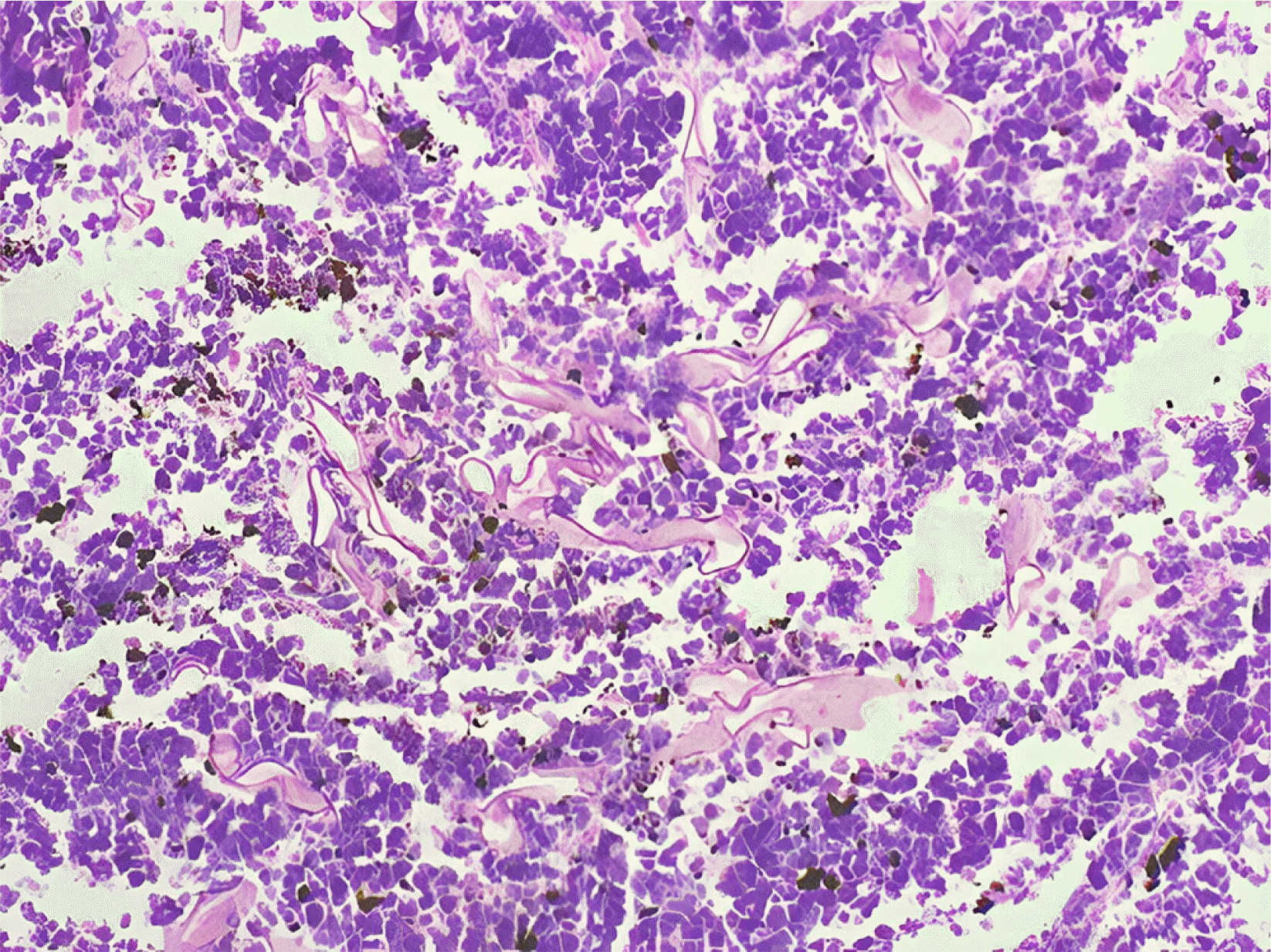
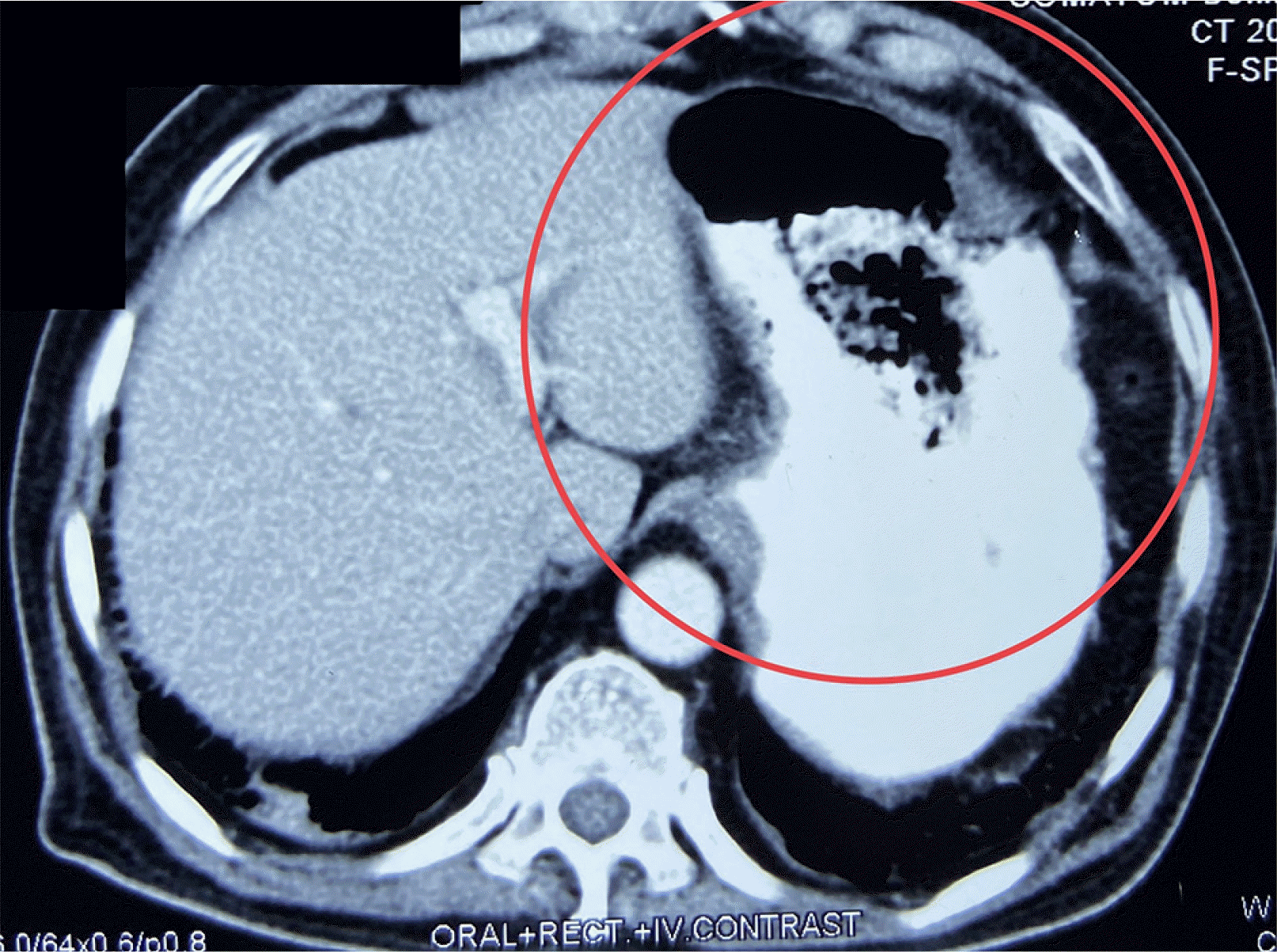
9 Both patients received high doses of steroids and broad-spectrum antibiotics, increasing their susceptibility to fungal infections. The stomach and colon are the most commonly affected organs in GI mucormycosis, and mortality can reach 85%. Non-specific symptoms, GI bleeding, or perforation can also occur. A high level of suspicion is warranted, particularly if there is a history of COVID-19 infection, use of drugs, or diabetes. The identification of fungal hyphae in biopsy specimens is required for further confirmation.
10 Various fungi-specific stains have been used to diagnose mucormycosis, e.g., Hematoxylin and eosin or Periodic acid-Schiff. Antifungals and surgical debridement of the necrosed/involved tissues are necessary for the treatment. Liposomal amphotericin B is the drug of choice for mucormycosis. The recommended dose is 5 mg/kg body weight administered over one hour. The renal function and potassium and magnesium levels in all patients should be monitored for at least once a week because the most prevalent adverse effects are nephrotoxicity, hypokalemia, and hypomagnesemia. Oral posaconazole or isavuconazole can be considered. Mucormycosis is difficult to diagnose and requires prompt treatment. The delays in detection and treatment can increase mortality considerably.
11 Monte Junior et al.
12 reported a case of stomach mucormycosis in a COVID-19 patient. A one-week delay in diagnosis resulted in the patient's death.
12 Even with timely diagnosis and aggressive management, the prognosis is not good. In the first case, the patient could not be saved despite vigorous treatment.
In conclusion, mucormycosis is difficult to diagnose and must be treated immediately. Delays in detection and treatment can result in a twofold increase in mortality. Nevertheless, the prognosis is poor despite quick detection and aggressive treatment. In the second case, prompt diagnosis and treatment saved his life.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download