Abstract
Background/Aims
A quick and accurate diagnosis of Helicobacter pylori (H. pylori) infections is vital for effectively managing many upper gastrointestinal tract diseases. Many diagnostic methods have been developed for rapid and accurate diagnosis, including invasive and non-invasive methods, but each tool has some limitations. Among the invasive diagnostic methods, the rapid urease test (RUT) is a relatively time-saving and accurate method, but a variation in the reaction time range causes inconvenience and inefficiency in the clinical field. This study developed a liquid-type medium, Helicotest®, to enable faster detection. This study examined the reaction time of a new liquid-type RUT kit with other commercial kits.
Methods
Two H. pylori strains were cultured (H. pylori ATCC 700392 and 43504), and the urease activity of H. pylori was measured using a urease activity assay kit (MAK120, Sigma Aldrich). Four RUT kits were used to compare the time of H. pylori detection, including Helicotest® (Won Medical, Bucheon, Korea), Hp kit (Chong Kun Dang, Seoul, Korea), CLO kit (Halyard, Alpharetta, GA, USA), and ASAN Helicobacter Test® (ASAN, Seoul, Korea).
Results
The detection of H. pylori was possible in bacterial amounts less than 10 μL. The color change was detected from five minutes with bacterial densities of 5 μL and 10 μL for both strains, whereas 30 minutes and one hour were required for 0.5 μL and a 1 μL bacterial density of ATCC 43504 and 700392 strains, respectively.
Helicobacter pylori (H. pylori) is one of the world’s most common chronic bacterial infections. Previous studies have shown that H. pylori is associated with gastric ulcer disease, chronic gastritis, gastric malignancies, and mucosa-associated lymphoid tissue lymphoma.1 Classified as a human carcinogen, H. pylori eradication therapy reduces the incidence of gastric cancer after endoscopic or surgical resection and that of first relative patients.2-6 The Maastricht V consensus recommends a "test and treat" approach for affected patients; therefore, rapid and accurate diagnosis is essential for appropriate treatment.7,8 Many invasive and non-invasive diagnostic methods have been developed for diagnosing H. pylori infections. Invasive diagnostic methods are performed endoscopically, which include rapid urease test (RUT), histopathological examination, bacterial culture tests, and PCR test. Non-invasive diagnostic methods include urea breath test, fecal antigen test, and serum test.9 Among the invasive methods mentioned above, histopathological examinations, culture examinations, and PCR tests have high accuracy; however, considerable time is needed to confirm the final diagnosis. On the other hand, RUT is a relatively time-saving, inexpensive, and tractable diagnostic method.10 RUT uses a large amount of urease found in H. pylori. Tissue is collected from the gastric antrum and body, mixed with a test medium, and observed for any color change. H. pylori degrades the urea in the medium to form carbon dioxide and ammonia. This increases the pH, changing the reagent color from orange to clear pink, which indicates a positive result.11 The sensitivity and specificity of these tests are high at 90% and 95– 100%, respectively. The Maastricht V consensus recommends RUT as the primary diagnostic test for H. pylori infection, in which a positive result is the basis for immediate eradication therapy.12 RUT is a simple method, but considerable time is sometimes needed to provide accurate test results. The reaction time ranges from five minutes to 24 hours, which sometimes may cause an inconvenient additional number of outpatient department visits for patients to check the final diagnosis.10 Therefore, many test media have been developed to attain rapid results, enabling one-stop diagnosis and treatment. The media used in RUT kits come in three primary forms: gel, paper, and liquid. The kits in gel form include the CLO kit and Hp kit. Paper form kits include the PyloriTek and ProntoDry. Liquid form kits include the UFT300 and EndoscHp. The reaction times vary according to the type of medium used. In particular, a CLO kit takes 24 hours; a PyloriTek takes one hour; a UFT300 takes five minutes.13 Table 1 compares the commercially available RUT kits.13-16 The property of the medium and density of H. pylori in the sample affects the reaction time and accuracy.13,14,16-19 Therefore, a medium that rapidly reacts with samples of low bacterial density would be of greater clinical use. This study developed a new liquid medium, which is not currently commercialized in the Republic of Korea, and the reaction time was evaluated after inoculation with two standard H. pylori strains (H. pylori ATCC 700392 and H. pylori ATCC 43504). The reaction time was measured with various amounts of bacterial density with both strains to identify that it sufficiently reacts with a minimal amount. Finally, research goals were set to evaluate the reaction rate of a new liquid-type medium compared with currently available commercial kits.
A liquid medium, referred to as Helicotest®, was developed by combining sodium dihydrogen phosphate monohydrate (NaH2PO4/H2O), phenol red, sodium acetate (C2H3O2Na), and urea. All experimental tests were conducted at least three times, and urease activity during bacterial culture has shown similar results despite the limitation on taking statistical data due to experimental variation.
This study does not include human or animal experiments and therefore is not applicable to Institutional Review Board approval.
Brucella blood agar was purchased from Synergy Innovation (Seongnam, Korea). NaH2PO4/H2O, phenol red, sodium acetate, and urea were purchased from Sigma-Aldrich (St. Louis, MO, USA).
Two strains of H. pylori, H. pylori ATCC 700392, in which the complete genome sequences have been determined, and H. pylori ATCC 43504, a strain isolated from a gastric cancer patient in Australia, were cultured in Brucella blood agar plates at 37℃ in 10% CO2 incubator under microaerobic conditions.20,21 After 3–5 days, the grown H. pylori strains were suspended in 5 μL of distilled water in various turbidities and centrifuged at 12,000 rpm for five minutes. H. pylori was stored in distilled water at -20℃ and regenerated before adding to Helicotest® solution.
The urease activity of H. pylori was measured using the method described in the MAK120 product manual from Sigma-Aldrich. Detailed instructions can be found in the Technical Bulletin of the Urease Activity Assay Kit. This method used the amount of ammonia produced by the urease to evaluate the urease activity and the Berthelot reagent reaction product, measured from the light absorption at a wavelength of 670 nm. The urease activities of H. pylori ATCC 700392 and ATCC 43504 strains were measured in different amounts of bacterial density in 0.1 μL, 0.5 μL, 1 μL, 5 μL, and 10 μL.
The reaction rate was evaluated from the gradual color change of the rapid urease test kit. As RUT uses large amounts of urease found in H. pylori, the presence of H. pylori can be demonstrated by the change in reagent color from orange to clear pink. Different bacterial amounts (0.1 μL, 0.5 μL, 1 μL, and 10 μL) of H. pylori in both standard strains (e.g., ATCC 700392 and ATCC 43504) were inoculated in Helicotest®, and the presence of color change at zero minutes, five minutes, 10 minutes, 30 mintues, and one hour were evaluated, as shown in Fig. 1.
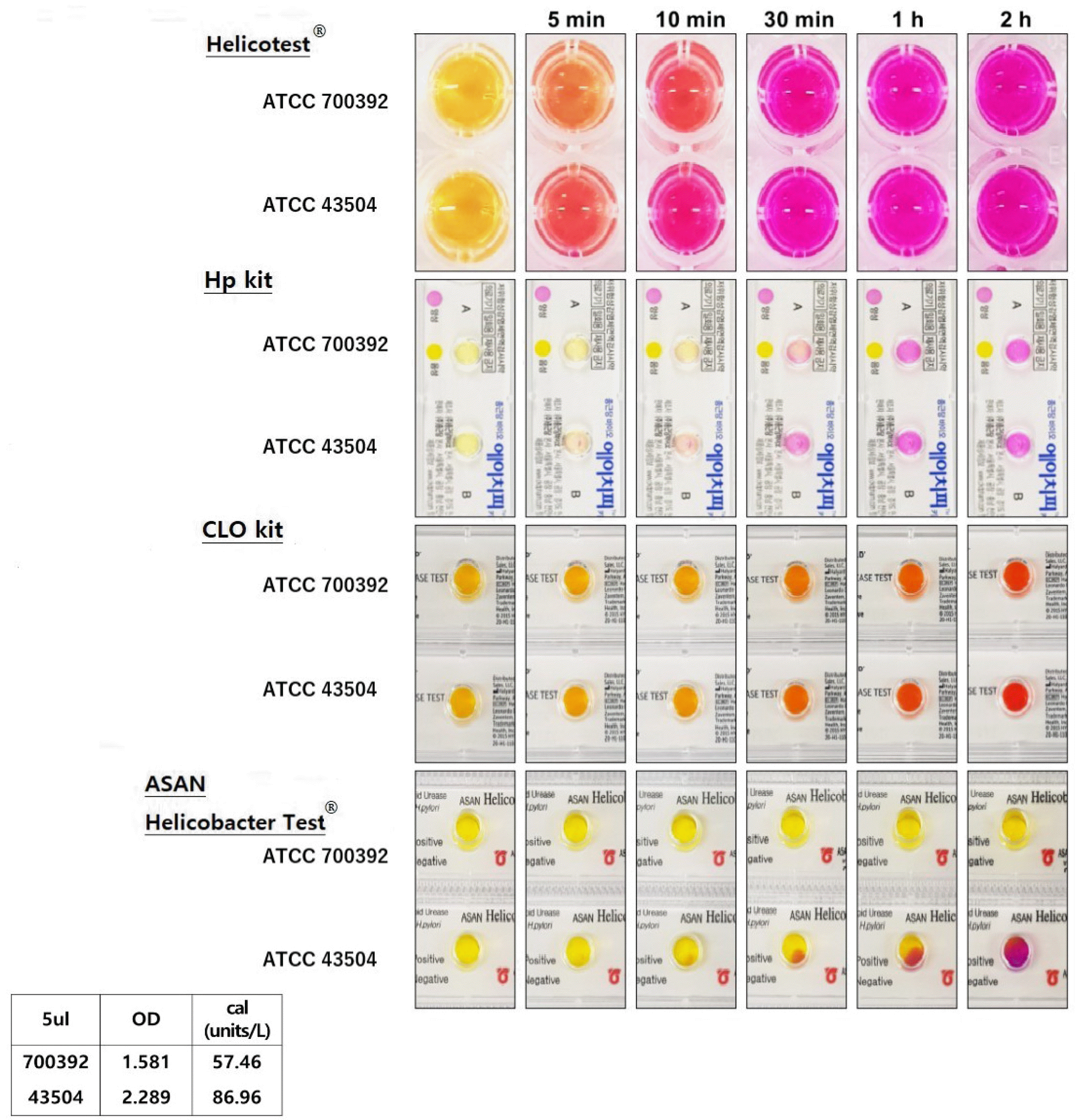
Two H. pylori strains, H. pylori ATCC 700392 and ATCC 43504 strain, were cultured to compare the new liquid type kit, Helicotest® (Won Medical, Bucheon, Korea), with other semi-solid (agar gel) commercial kits: Hp kit (Chong Kun Dang, Seoul, Korea), CLO kit (Halyard, Alpharetta, GA, USA), and ASAN Helicobacter Test® (ASAN, Seoul, Korea) and reaction process. The results were shown by the color change, which was recorded every minute to compare the reaction rate.
This study examined the reaction time of both H. pylori strains using a new liquid-type rapid urease test kit. The rapid urease activities of the H. pylori strains at different bacterial densities using Helicotest® were compared with other commercial kits.
Fig. 1 shows the color change in Helicotest® over time in various amounts of two H. pylori strains. The reaction rate was more rapid, with high bacterial density in both strains. The color change was detected from five minutes with a bacterial density of 5 μL and 10 μL for both strains, whereas 30 minutes and one hour were required for the 0.5 μL and 1 μL bacterial density of ATCC 43504 and 700392 strains, respectively. With a bacterial density of 0.1 μL, no color change was presented within one hour in this study.
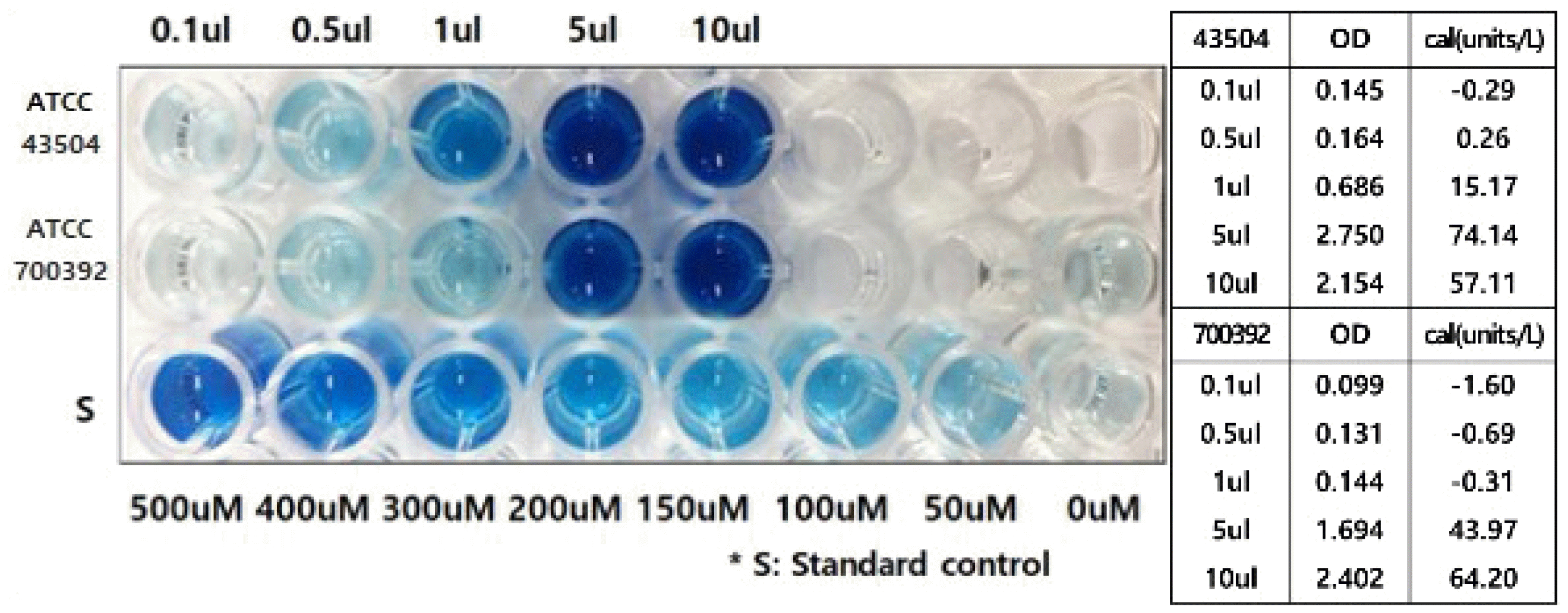
Each volume of H. pylori was matched to evaluate the urease activity in Fig. 2 to estimate pathogen ability rather than the bacterial number. This helps evaluate the actual pathogenic ability rather than the absolute bacterial load, which is more meaningful for developing diagnostic tools in the clinical field.
Fig. 2 presents the urease activity of both strains with different bacterial amounts shown in Fig. 1. The urease activity of 0.1 μL, 0.5 μL, 1 μL, and 10 μL bacterial amounts of H. pylori in both standard strains (e.g., ATCC 700392, ATCC 43504) were evaluated from the optical density at 670 nm. In both strains, the urease activity increased according to the bacterial density. The ATCC 700392 strain showed a positive reaction from one hour after inoculation with a bacterial density of 0.5 μL and 1 μL and from five minutes with more than 5 μL. The urease activity was -0.69 units/L, -0.31 units/L, and 43.97 unit/L at 700392 strain amounts of 0.5 μL, 1 μL, and 5 μL, respectively, with optical densities of 0.131, 0.144, and 1.694. An excessively unmeasurable small bacterial load can produce a negative result. The ATCC 43504 strain showed a positive reaction from 30 minutes with a strain amount of 0.5 μL, and from five minutes with a bacterial density of more than 5 μL. The urease activity was 0.26 unit/L and 74.14 unit/L, with optical densities of 0.164 and 2.75, respectively. An evaluation of the urease activity presents the pathogenic ability of bacteria that can enable an estimation of the reaction rate using rapid urease test kits through the appropriate bacterial concentration for diagnosis.
The reaction rates of Helicotest® and other semi-solid type commercial kits were compared (Fig. 3). The reaction time of both strains was presented at five minutes, 10 minutes, 30 minutes, one hour, and two hours for color change with four different RUT kits. With Helicotest®, the ATCC 700392 and ATCC 43504 strains showed a color change from five minutes, which was the fastest reaction, followed by the Hp kit, CLO kit, and ASAN Helicobacter Test®.
Using the Hp kit, the 43504 strain reacted for 10 minutes, and the 700392 strain reacted for 30 minutes each. Both strains showed a positive sign after 30 minutes using the CLO kit. Using an ASAN Helicobacter Test®, the 700392 strain took more than two hours for detection, and the 43504 strain began to change between 10 and 30 minutes. No further evaluation was conducted after two hours because 5 μL of the 700392 strain showed less urease activity than the 43504 strain (Fig. 3).
As one of the critical infections in the upper GI tract, detecting H. pylori is an alarming issue in managing many GI tract diseases. As a well-known carcinogen that is associated with many gastric diseases, H. pylori eradication is a meaningful outcome of upper GI tract diseases. Considering the test and treat approach recommended by the Maastricht V consensus, a minimal time gap between diagnosis and management of H. pylori infection would enable a better outcome for many upper GI tract diseases. For the greater clinical usage, this study focused on developing a medium that reacts rapidly with samples of low bacterial density.
Therefore, this study developed a liquid-type H. pylori diagnostic medium technology that can shorten reaction time using the liquid phase medium of different compositions compared with commercial kits. Helicotest® is composed of phenol red, urea, sodium acetate, sodium dihydrogen phosphate, and vancomycin. In contrast, other commercial liquid kits are composed of phenol red, urea, gamma valerolactone, sodium azide composition, and buffer based on sodium acetate. In particular, the composition of sodium dihydrogen phosphate (pH 7.0) and sodium acetate (pH 7.3) enabled a faster reaction rate. Helicotest® presented a positive reaction to a low bacterial density and weak urease activity. Furthermore, the reaction rate was highest compared to the current gel-type products. The products selected for comparison in this study are semi-solid type medium, Hp kit, CLO kit, and ASAN Helicobacter test®, which are widely commercialized. The Hp kit contains a gel-based urea substrate, which needs up to two hours for the final result. The sensitivity and specificity were 90% and 97.5%, respectively, and the reaction time was 35.2±21.4 minutes, even at a low bacterial density from a Sydney classification grade 1 infection.16 The CLO kit is a gel-based medium that requires up to 24 hours for diagnosis.
The sensitivity after one hour was 71%, but after 24 hours, the sensitivity and specificity became 93% and 99–100%, respectively.18 The ASAN Helicobacter test® is a product that can also serve as a transport medium. According to the product manual, the results are presented two hours after sample inoculation. If no color develops, it is recommended to check again after 24 hours. One study reported a sensitivity and specificity of 88.7% and 94.0%, respectively.19
In this study, the Helicotest® provided definitive test results within five minutes and up to one hour, significantly reducing the time required for diagnosis and treatment. As presented in the result, with Helicotest®, the ATCC 700392 and ATCC 43504 strains showed the fastest reaction, followed by the Hp kit, CLO kit, and ASAN Helicobacter Test®.
A rapid H. pylori diagnosis is essential for facilitating the prompt initiation of the appropriate treatment. Upon its linkage to many upper GI tract diseases, the effective diagnosis of H. pylori infection is essential for appropriately managing upper GI tract diseases. The success of H. pylori eradication therapy is influenced by many factors, such as patient compliance and the ability of proton pump inhibitors (PPIs) to suppress acid secretion. On the other hand, the most important factor is antibiotic resistance, particularly to clarithromycin.22 The Maastricht V consensus recommends not using the standard three therapies for a H. pylori infection (PPI, amoxicillin, and clarithromycin) in cases where clarithromycin resistance exceeds 15%.12 According to the Evidence-based Guidelines for the Treatment of Helicobacter pylori Infection in the Republic of Korea: 2020 Revised Edition, the clarithromycin resistance rate in the Republic of Korea has increased gradually over the past 10 years. Recent studies confirmed that it was as high as 17.8–31.0%.23,24 The success rate of the standard three therapies from 2007 to 2011 was 72.3%, which decreased to 70.3% from 2012 to 2016.24 Therefore, the necessity of clarithromycin resistance tests has attracted attention. The most common method is culturing H. pylori to perform antibiotic susceptibility tests.26,27 On the other hand, this method is time-consuming because the organisms are difficult to culture, and clinical applications of the test are challenging. Instead, a PCR test can be used to test for the clarithromycin resistance gene,22 but this method is expensive and difficult to apply in bulk. Therefore, a double method approach has emerged where an inexpensive RUT is performed and followed by a PCR test if a positive result is confirmed. Reducing the time to confirm the RUT result would sequentially shorten the time for determining the PCR test. In some cases, both RUT and PCR tests can be performed simultaneously after endoscopy. As Helicotest® enables prompt confirming test results, it would contribute to the flexibility of applying a double method for establishing appropriate treatment methods. Furthermore, as RUT has high specificity, even in post-gastrectomy patients, this advantage also can be applied to post-gastrectomy patients.4-6
No clinical trial has been conducted for Helicotest®, meaning the accuracy of the test is unknown. The sensitivity and specificity need to be confirmed through clinical trials, in which false positives and false negatives are evaluated. In general, RUT may produce a false negative result under conditions that reduce the concentration of strains present in the test sample, such as antibiotics, bismuth-containing preparations, and PPIs.28-31 PPIs can also cause hypochlorhydria, which may induce the colonization of other bacteria and produce false positives. If the test result is evaluated after 24 hours, other bacteria with urease can degrade urea in the test medium, producing a false positive.32 Further studies are needed to determine how these factors and others affect Helicotest® in relation to the accuracy and identification of the optimal detection conditions. Typically, RUT is not highly sensitive. Hence, a negative result, including the possibility of acute infection, cannot be excluded. Therefore, it is not used for the determination of eradication.12,33
Helicotest® is expected to have this limitation, which requires further research. Finally, in contrast to the widely commercialized semi-solid type rapid urease test kit, Helicotest® as a liquid type test kit has to determine optimal storage and transport methods. The properties of the medium affect the reaction time and the method of storage and transport. Therefore a change in the properties of the medium from semi-solid to liquid type would affect the storage and transport methods. In other words, different optimization methods for Helicotest® might be required. In addition, for commercialization, further trials and experimental data will be needed to evaluate the standard maximal usage period that should be suggested to consumers. In conclusion, the newly developed liquid-type rapid urease test kit (Helicotest®) showed the fastest reaction rate compared to the commercialized three semi-solid type RUT kits. Nevertheless, further study and clinical trials will be needed to determine the actual sensitivity and specificity associated with false negative and false positive reactions.
Notes
Financial support
This research was supported by a grant of the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant number: HI16C2319) and supported by the National Reseach Foundation of Korea (NRF), funded by the Ministry of Education (NRF-2020R1F1A1076839).
REFERENCES
1. Chey WD, Wong BC. Practice Parameters Committee of the American College of Gastroenterology. 2007; American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol. 102:1808–1825. DOI: 10.1111/j.1572-0241.2007.01393.x. PMID: 17608775.

2. Choi IJ, Kook MC, Kim YI, et al. 2018; Helicobacter pylori therapy for the prevention of metachronous gastric cancer. N Engl J Med. 378:1085–1095. DOI: 10.1056/NEJMoa1708423. PMID: 29562147.

3. Choi IJ, Kim CG, Lee JY, et al. 2020; Family history of gastric cancer and Helicobacter pylori treatment. N Engl J Med. 382:427–436. DOI: 10.1056/NEJMoa1909666. PMID: 31995688.

4. Choi YI, Chung JW. 2020; Helicobacter pylori eradication in patients undergoing gastrectomy: diagnosis and therapy. Korean J Helicobacter Up Gastrointest Res. 20:204–209. DOI: 10.7704/kjhugr.2019.0037.

5. Lee YC, Chiang TH, Chou CK, et al. 2016; Association between Helicobacter pylori eradication and gastric cancer incidence: a systematic review and meta-analysis. Gastroenterology. 150:1113–1124.e5. DOI: 10.1053/j.gastro.2016.01.028. PMID: 26836587.

6. Kim YH, Shin SW. 2018; Helicobacter pylori and prevention of gastric cancer. N Engl J Med. 378:2244. DOI: 10.1056/NEJMc1805129.
7. Tseng CA, Wang WM, Wu DC. 2005; Comparison of the clinical feasibility of three rapid urease tests in the diagnosis of Helicobacter pylori infection. Dig Dis Sci. 50:449–452. DOI: 10.1007/s10620-005-2456-5. PMID: 15810624.
8. Pohl D, Keller PM, Bordier V, Wagner K. 2019; Review of current diagnostic methods and advances in Helicobacter pylori diagnostics in the era of next generation sequencing. World J Gastroenterol. 25:4629–4660. DOI: 10.3748/wjg.v25.i32.4629. PMID: 31528091. PMCID: PMC6718044.
9. Monteiro L, Mégraud F. 1999; [How to detect Helicobacter pylori before and after eradication treatment?]. Gastroenterol Clin Biol. 23(10 Pt 2):C3–19. French.
10. McNicholl AG, Ducons J, Barrio J, et al. Helicobacter pylori Study Group of the Asociación Española de Gastroenterología (AEG). Accuracy of the ultra-rapid urease test for diagnosis of Helicobacter pylori infection. Gastroenterol Hepatol. 2017; 40:651–657. English, Spanish. DOI: 10.1016/j.gastre.2017.07.012.
11. Uotani T, Graham DY. 2015; Diagnosis of Helicobacter pylori using the rapid urease test. Ann Transl Med. 3:9.
12. Malfertheiner P, Megraud F, O'Morain CA, et al. European Helicobacter and Microbiota Study Group and Consensus panel. Management of Helicobacter pylori infection-the Maastricht V/Florence consensus report. Gut. 2017; 66:6–30. DOI: 10.1136/gutjnl-2016-312288. PMID: 27707777.

13. Wang YK, Kuo FC, Liu CJ, et al. 2015; Diagnosis of Helicobacter pylori infection: current options and developments. World J Gastroenterol. 21:11221–11235. DOI: 10.3748/wjg.v21.i40.11221. PMID: 26523098. PMCID: PMC4616200.

14. Yousfi MM, El-Zimaity HM, Cole RA, Genta RM, Graham DY. 1997; Comparison of agar gel (CLOtest) or reagent strip (PyloriTek) rapid urease tests for detection of Helicobacter pylori infection. Am J Gastroenterol. 92:997–999.
15. Vaira D, Vakil N, Gatta L, et al. 2010; Accuracy of a new ultrafast rapid urease test to diagnose Helicobacter pylori infection in 1000 consecutive dyspeptic patients. Aliment Pharmacol Ther. 31:331–338. DOI: 10.1111/j.1365-2036.2009.04196.x. PMID: 19891666.
16. Hong SJ, Ryu CB, Kim JO, et al. 2001; Diagnostic usefulness of Hp kit test for the detection of Helicobacter pylori infection. Korean J Gastrointest Endosc. 22:8–13.
17. Roe IH, Lee MI, Kim JT, et al. 1999; Comparison of Hp kit test and CLO test for the diagnosis of Helicobacter pylori infection. Korean J Gastroenterol. 34:448–454.
18. Laine L, Lewin D, Naritoku W, Estrada R, Cohen H. 1996; Prospective comparison of commercially available rapid urease tests for the diagnosis of Helicobacter pylori. Gastrointest Endosc. 44:523–526. DOI: 10.1016/S0016-5107(96)70002-0. PMID: 8934155.
19. Lee J, Kim PS, Lee K, Lee JH, Lee JK, Lee CH. 2003; Evaluation of Asan Helicobacter test for diagnosis of Helicobacter pylori Infection. Korean J Clin Microbiol. 6:156–159.
20. Kinoshita-Daitoku R, Ogura Y, Kiga K, et al. 2020; Complete genome sequence of Helicobacter pylori Strain ATCC 43504, a type strain that can infect gerbils. Microbiol Resour Announc. 9:e00105–20. DOI: 10.1128/MRA.00105-20. PMID: 32354967. PMCID: PMC7193922.

21. Cerda O, Rivas A, Toledo H. 2003; Helicobacter pylori strain ATCC700392 encodes a methyl-accepting chemotaxis receptor protein (MCP) for arginine and sodium bicarbonate. FEMS Microbiol Lett. 224:175–181. DOI: 10.1016/S0378-1097(03)00423-3. PMID: 12892880.

22. Jung HK, Kang SJ, Lee YC, et al. Korean College of Helicobacter and Upper Gastrointesinal Research. Evidence based guidelines for the treatment of Helicobacter pylori infection in Korea 2020. Korean J Intern Med. 2021; 36:807–838. DOI: 10.3904/kjim.2020.701. PMID: 34092054. PMCID: PMC8273819.
23. Lee JH, Ahn JY, Choi KD, et al. Korean College of Helicobacter; Upper Gastrointestinal Research. Nationwide antibiotic resistance mapping of Helicobacter pylori in Korea: A prospective multicenter study. Helicobacter. 2019; 24:e12592. DOI: 10.1111/hel.12592. PMID: 31111572.
24. Lee JW, Kim N, Kim JM, et al. 2013; Prevalence of primary and secondary antimicrobial resistance of Helicobacter pylori in Korea from 2003 through 2012. Helicobacter. 18:206–214. DOI: 10.1111/hel.12031. PMID: 23241101.

25. Lee JY, Kim N, Nam RH, Choi SI, Lee JW, Lee DH. 2019; Primary and secondary antibiotic resistance of Helicobacter pylori in Korea from 2003 to 2018. Helicobacter. 24:e12660. DOI: 10.1111/hel.12660. PMCID: PMC6790945.

26. Wenzhen Y, Yumin L, Quanlin G, et al. 2010; Is antimicrobial susceptibility testing necessary before first-line treatment for Helicobacter pylori infection? Meta-analysis of randomized controlled trials. Intern Med. 49:1103–1109. DOI: 10.2169/internalmedicine.49.3031. PMID: 20558925.

27. López-Góngora S, Puig I, Calvet X, et al. 2015; Systematic review and meta-analysis: susceptibility-guided versus empirical antibiotic treatment for Helicobacter pylori infection. J Antimicrob Chemother. 70:2447–2455. DOI: 10.1093/jac/dkv155. PMID: 26078393.

28. Graham DY, Lew GM, Malaty HM, et al. 1992; Factors influencing the eradication of Helicobacter pylori with triple therapy. Gastroenterology. 102:493–496. DOI: 10.1016/0016-5085(92)90095-G. PMID: 1732120.

29. Graham DY, Opekun AR, Hammoud F, et al. 2003; Studies regarding the mechanism of false negative urea breath tests with proton pump inhibitors. Am J Gastroenterol. 98:1005–1009. DOI: 10.1111/j.1572-0241.2003.07426.x. PMID: 12809820.

30. Chey WD, Chathadi KV, Montague J, Ahmed F, Murthy U. 2001; Intragastric acidification reduces the occurrence of false-negative urea breath test results in patients taking a proton pump inhibitor. Am J Gastroenterol. 96:1028–1032. DOI: 10.1111/j.1572-0241.2001.03687.x. PMID: 11316142.

31. Forné M, Viver JM, Espinós JC, Coll I, Tresserra F, Garau J. 1995; Impact of colloidal bismuth subnitrate in the eradication rates of Helicobacter pylori infection-associated duodenal ulcer using a short treatment regimen with omeprazole and clarithromycin: a randomized study. Am J Gastroenterol. 90:718–721.
32. Vaira D, Holton J, Cairns S, et al. 1988; Urease tests for Campylobacter pylori: care in interpretation. J Clin Pathol. 41:812–813. DOI: 10.1136/jcp.41.7.812. PMID: 3410977. PMCID: PMC1141596.

33. Attumi TA, Graham DY. 2011; Follow-up testing after treatment of Helicobacter pylori infections: cautions, caveats, and recommendations. Clin Gastroenterol Hepatol. 9:373–375. DOI: 10.1016/j.cgh.2010.12.025. PMID: 21195791.

Fig. 1
Color change of Helicotest® over time inoculated with ATCC 700392 and ATCC 43504 strain in various amounts.
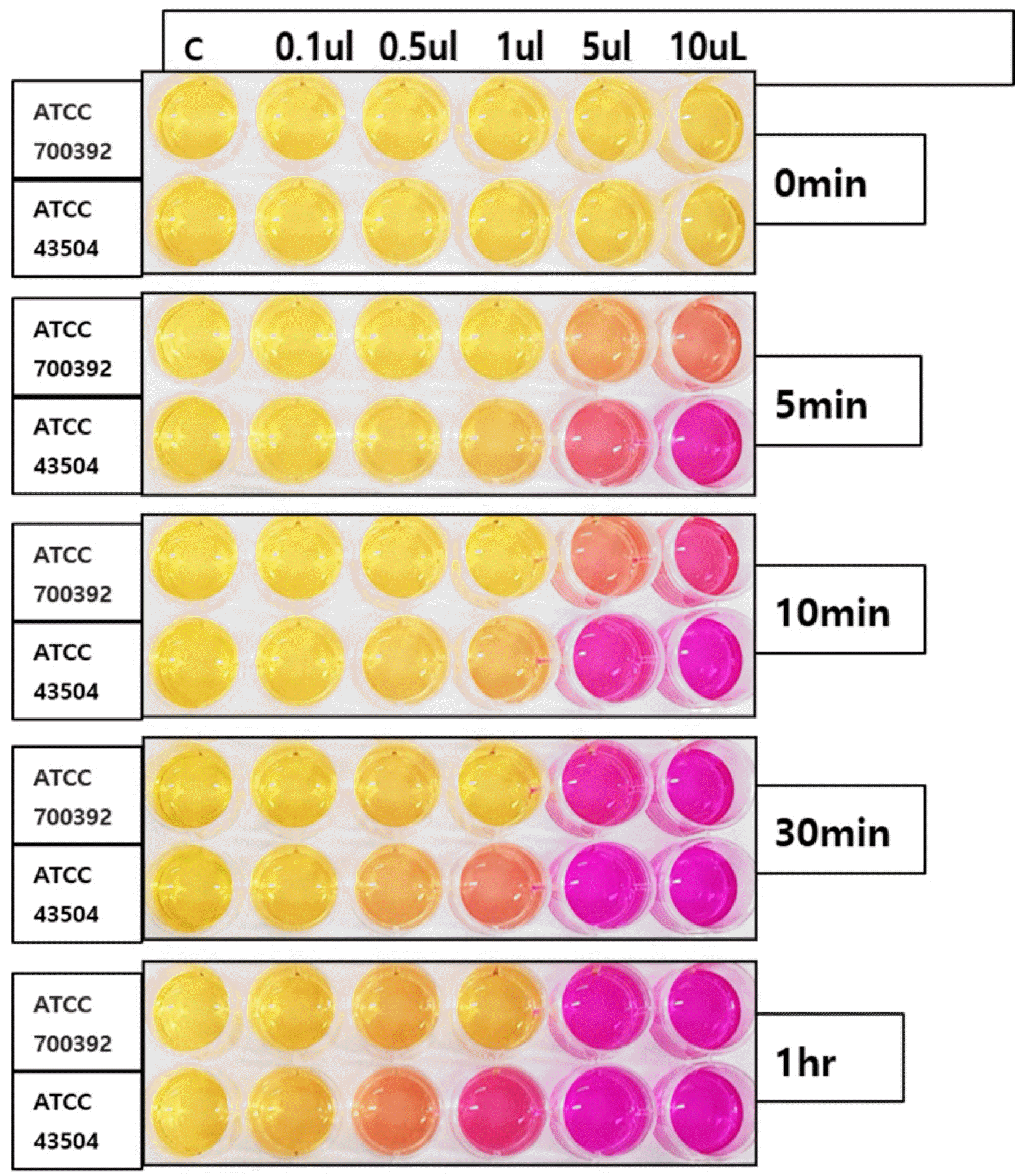
Table 1
Comparison between Commercially Available Rapid Urease Test Kits




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download