Abstract
Gastrointestinal arteriovenous malformations (AVMs) are a rare disease. Sigmoid-anorectal AVM has only been reported in a few cases. The condition is usually detected when patients have gastrointestinal bleeding complications. The diagnosis and treatment of colorectal AVMs are still challenging. This paper presents a case of an Asian 32-year-old female patient admitted to hospital because of lower gastrointestinal bleeding lasting 17 years. The patient was diagnosed with sigmoid-rectal arteriovenous malformation and failed with other medical treatments. The damaged gastrointestinal tract was removed by a laparoscopic low anterior resection. The results were positive after a three-month follow-up; the bleeding was resolved, and the anal sphincter function was intact. Laparoscopic low anterior resection is a safe, less invasive, and effective approach for managing patients with digestive tract bleeding due to extensive colorectal AVM and preservation of the anal sphincter.
Margolis et al.1 described gastrointestinal (GI) arteriovenous malformations (AVMs), which are characterized by aberrant, thin-walled arteriovenous communications within the submucosa and mucosa of the gastrointestinal wall. Despite the advances in assistive tools and the ability to diagnose intestinal AVMs, it is still a rare disease with an unconfirmed overall prevalence, and the etiology is still poorly understood. Few cases of gastrointestinal AVMs have been reported, and colorectal AVMs are even rarer. Most GI AVMs are asymptomatic and discovered incidentally through endoscopy. On the other hand, they can cause severe painless gastrointestinal bleeding and subsequent chronic anemia. The diagnosis of GI AVMs is prominent in gastrointestinal endoscopy and angiography.2 In addition to the risks of misdiagnosis, the choice of treatment is also a challenging problem, depending on the clinical manifestation, extensive lesions, and location of vascular malformations of the gastrointestinal tract and the potential of the medical facility. This paper presents a case of sigmoid-anorectal AVM causing prolonged lower gastrointestinal bleeding treated successfully with a laparoscopic low anterior resection with preservation of the anal sphincter.
We obtained individual written informed consents for the procedure and for publication.
An Asian 32-year-old female patient without a history of hematologic diseases presented with symptoms of hematochezia persistently from age 15, with a frequency every two– four weeks. The patient was treated several times in other hospitals with blood transfusion and endoscopic hemostasis. The patient was referred to the authors’ hospital because of bloody stools eight times a day, accompanied by symptoms of dizziness and lightheadedness when changing positions. She reported no abdominal pain or vomiting and showed no fever. On admission, a physical examination identified severe anemia with pallor, orthostatic hypotension, and hemoglobin of 5.9 g/dL. No other abnormalities were observed. A colonoscopy revealed extensive and dilated vascular structures and congested and erythematous mucosa of approximately 25 cm from the very low rectum to the sigmoid colon, with active bleeding spots in some vascular malformations (Fig. 1). A gastroduodenoscopy showed no remarkable abnormalities. An abdominal ultrasound showed no evidence of portal hypertension. An abdominal and pelvic CT scan resulted in an image of varicose in the wall of the rectum and the sigmoid colon. The patient was diagnosed initially with lower gastrointestinal bleeding suspected of sigmoid and rectal arteriovenous malformation. She was transfused with a total of four units of packed red blood cells. The day after admission, the patient underwent endoscopic hemostasis with argon plasma coagulation (APC). On the other hand, endoscopic hemostasis was not thorough because of the scattered bleeding lesions in the colon and the fragile rectosigmoid mucosa.
Although the patient was stable hemodynamically, the bleeding continued for several days after admission. Therefore, surgical treatment was considered. The patient underwent a laparoscopic low anterior resection with primary anastomosis to resect the damaged bowel. The intraoperative findings revealed several extensively tangled vascular lesions that covered the entire sigmoid colon and rectum from the intestinal wall to the corresponding mesentery (Fig. 2). After mobilizing the necessary intestinal segment, the lesion was resected based on the macroscopic observation of damage, with the proximal margin at the descending-sigmoid colon flexure and the distal margin at the rectum just above the levator ani muscle. The vascular malformation lesion extended to almost the end of the lower third of the rectum. A decision was made not to resect the entire rectum to preserve the anal sphincter. A transanal mucosectomy was performed to eliminate the remaining lesion. The colorectal anastomosis was performed for bowel continuity by the circular stapler approximately 4 cm from the anal margin.
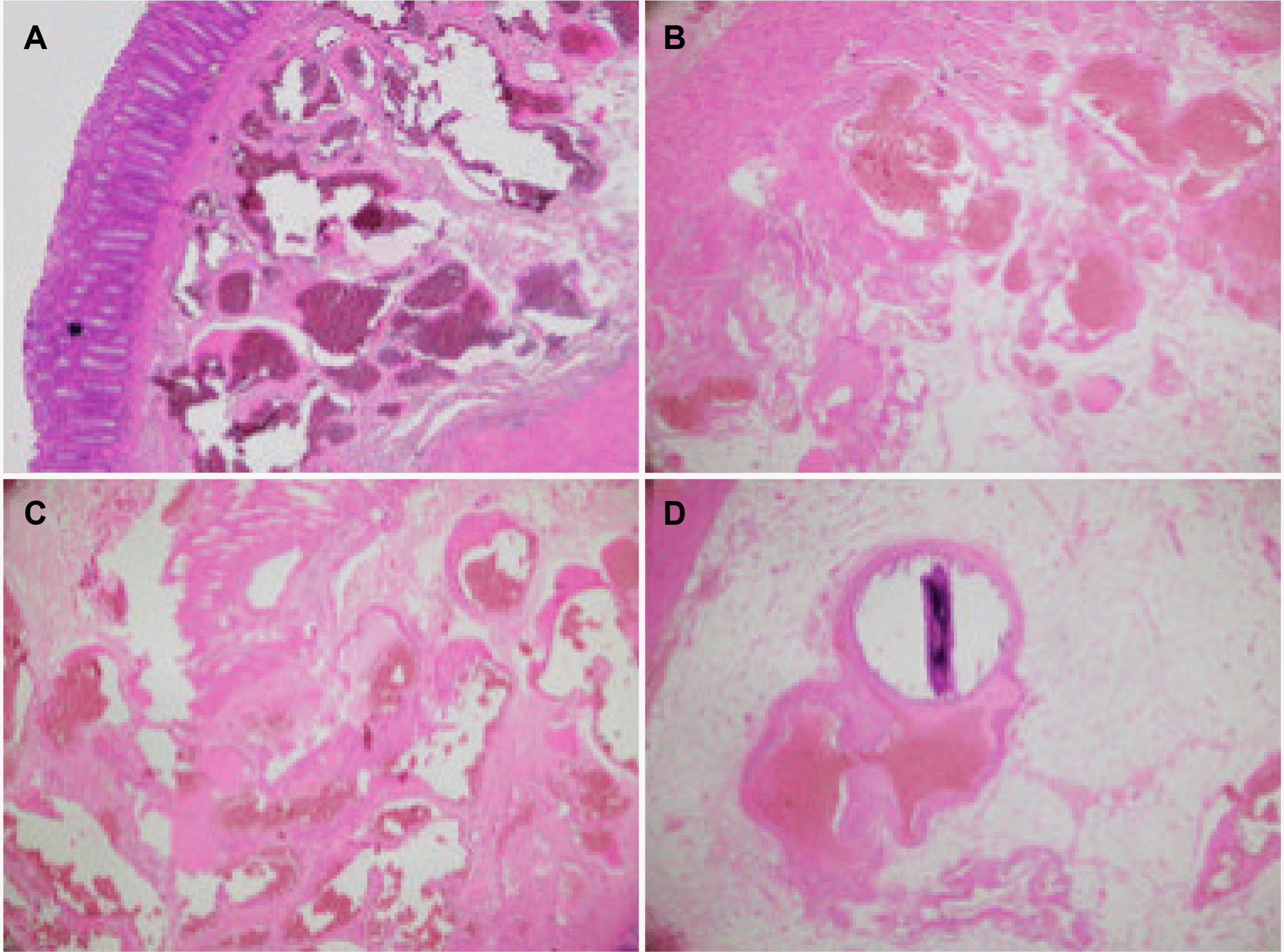
The gross specimen showed multiple intraluminal bleeding points from the corresponding extensive vascular lesions on the sigmoid-rectal wall (Fig. 3). The histopathological results confirmed that this was an AVM of the colonic submucosal layers and pericolic fat, described by angiogenesis and the findings of the arteriovenous shunts (Fig. 4).
The patient was discharged on postoperative day 6. At the three-month follow-up, she was doing well and denied any signs and symptoms of re-bleeding and a good anal sphincter function.
Different terms are used interchangeably to describe GI vascular lesions. There is no standardization in classifying these lesions, contributing to the confusion. Yano et al.3 proposed a classification that included four categories to clarify these issues. Type 1 lesions are angioectasias, the most common type. Types 2 and 3 are arterial lesions, such as Dieulafoy lesions and AVMs, which are distinguished by their pulsatile character. Type 4 is an unclassifiable vascular lesion with an unusual morphology.3 AVMs of the GI tract are characterized by thick-walled blood vessels that extend through the mucosa and submucosa into the muscular layer and may distort the adjacent tissues.4
The patient presented with GI bleeding caused by colorectal AVM. The major causes of lower GI bleeding include diverticular disease, anorectal disease, ischemia, and neoplasia.5 Arteriovenous malformations of the lower GI tract are uncommon, with an unclear prevalence in the general population. In a review of three prospective studies of GI endoscopic cancer screening in asymptomatic healthy patients, only eight of 964 patients (0.8%) had vascular malformations, with the most common location being the right colon (62%).6 The precise etiology of AVM is not well understood. Most of the literature divided them into two main categories, congenital and acquired. Congenital cases are believed to be related to genetic abnormalities that alter the formation of transforming growth factor beta, which is involved in the integrity of vascular endothelial cells.7 The acquired cases were explained by the hypothesis of a precapillary sphincteric dysfunction and degeneration of the postcapillary venules caused by vascular dilatation and obstruction of venous outflow in the submuscular due to dilation of the lumen of the digestive tract.8
The detection and accurate diagnosis of vascular malformations of the GI tract remains a complex problem. Most of these abnormalities are detected when the patient presents with GI bleeding, particularly intermittent GI bleeding, and chronic anemia complications. On the other hand, there are still cases where the incidental discovery of the disease through GI endoscopy is indicated for other reasons.2 Many studies have examined the diagnosis, prognosis, and treatment of GI AVMs. Among them, GI endoscopy and angiography play the most important roles in diagnosis and treatment. For colonic AVMs, standard angiography is considered the gold standard for diagnosis, with the presence of contrast extravasation or “blush,” indicating the location of bleeding, combined with early venous filling such that both the artery and the vein are visualized simultaneously.9 Computed tomography angiography (CTA) has played an increasing role in the diagnosis, with a sensitivity, specificity, and positive predictive value of CTA for detecting colonic vascular malformations was 70%, 100%, and 100%, respectively, as demonstrated by vascular accumulation in the colonic wall, early venous filling, and enlarged feeding vessels.10 GI endoscopy methods in diagnosing GI AVMs include upper GI endoscopy, deep small bowel enteroscopy, capsule endoscopy, and colonoscopy. Combining multiple endoscopic modalities is essential because this anomaly can be found anywhere in the GI tract. In diagnosing colonic AVMs, colonoscopy has an estimated sensitivity of more than 80%, with limitations related to the length of the examination, colon preparation, and the presence of small bowel lesions.2 The GI AVMs usually present as a lesion approximately 10 mm in size, with a flat, cherry-red surface consisting of disorganized blood vessels radiating from a central feeding vessel.
One of the main challenges with malformations of the GI tract is the treatment choice. In addition to standard resuscitation for each case of GI bleeding, several radical treatments for vascular malformations have been proposed, including endoscopic gastrointestinal hemostasis using APC, electrocoagulation, mechanical hemostasis, injection sclerotherapy, or selective embolization. GI endoscopy reported a relatively high rate of initial hemostasis, with an 85% rate of achieving patient stabilization and not requiring blood transfusion. On the other hand, cases of procedure-related complications were still reported. Nevertheless, this method is less effective for extensive and submucosal lesions, particularly for lesions in the right colon because of the thin colonic wall.11 Vasopressin embolization is up to 91% effective in patients with less GI bleeding caused by diverticulosis and vascular disease. The rate of recurrent bleeding is up to 50% after intervention.12 The complications related to intestinal ischemia after an embolism were also recorded at a rate of up to 13%, of which 5.2% of patients presented with shock and required surgery to remove the necrotic bowel.13
Surgical intervention is necessary for 18–25% of patients with lower GI bleeding requiring blood transfusion. The indications for surgical intervention for lower GI bleeding include critical bleeding, shock, hypotension, failure of nonsurgical interventions, or the patient without contraindicating comorbidity and with a reasonable life expectancy. On the other hand, this is also a dangerous decision because the mortality rate in emergency surgery to resolve lower GI bleeding is up to 10%.12 The surgical treatment for GI AVMs is not always curative because the AVM may not be removed entirely or unrecognized at surgery. In addition, new lesions may develop after an incomplete resection.14 Nevertheless, with lesions extending from the sigmoid colon to near the end of the rectum, a more radical surgical resection in the rectal region can lead to anal sphincter dysfunction, reducing the quality of life. Therefore, the recommended treatment for rectosigmoid malformations has changed from an abdominoperineal resection before 197115 to a low anterior resection (LAR) with a mucosal resection as the current standard,16 with the goal being sphincter preservation.
In the present case, a long lesion from the rectum to the sigmoid colon was observed, and the patient was symptomatic for 17 years. The initial treatment of endoscopic mucosal hemostasis has also failed. The transarterial embolism method was not used because of the risk of intestinal necrosis and the recurrence rate. Therefore, a riskier and more radical treatment, surgery, was decided. On the other hand, surgery was challenging because of the bleeding risks from the AVM. The AVM extended longitudinally from the sigmoid colon to the rectum, the colon wall to the mesentery, and even to the adjacent fat tissue in the pelvic region. Dissecting and identifying the margin with the normal bowel wall was challenging, and the risk of an anastomotic leak was high because the damage was difficult to investigate during surgery and the blood supply for the margins. On the other hand, preserving the anal sphincter and performing an intestinal reconstruction without a stoma is one of the utmost objectives in a young female patient. Therefore, a decision was made to resect the colon and rectum as much as possible and combine it with mucosectomy for the remaining involved lower rectum. With a follow-up period of three months, this patient showed a complete recovery after surgery, as demonstrated by good intestinal peristalsis and cessation of gastrointestinal bleeding with successful anal sphincter preservation.
In conclusion, AVMs rarely involve the gastrointestinal tract, and the sigmoid–anorectal AVM is even rarer. This paper described a case of extensive colorectal AVM involving the sigmoid colon and rectum that was treated successfully by a laparoscopic LAR.
ACKNOWLEDGEMENTS
The authors thank the medical staffs of the Department of Colon and Rectal Surgery, 108 Military Central Hospital, Hanoi, Vietnam for their participation in this study. We could not have completed this study without their diligence and support.
REFERENCES
1. Margulis AR, Heinbecker P, Bernard HR. 1960; Operative mesenteric arteriography in the search for the site of bleeding in unexplained gastrointestinal hemorrhage: a preliminary report. Surgery. 48:534–539.
2. Richter JM, Hedberg SE, Athanasoulis CA, Schapiro RH. 1984; Angiodysplasia. Clinical presentation and colonoscopic diagnosis. Dig Dis Sci. 29:481–485. DOI: 10.1007/BF01296266. PMID: 6609803.
3. Yano T, Yamamoto H, Sunada K, et al. 2008; Endoscopic classification of vascular lesions of the small intestine (with videos). Gastrointest Endosc. 67:169–172. DOI: 10.1016/j.gie.2007.08.005. PMID: 18155439.

4. Mindelzun RE, Beaulieu CF. 1997; Using biphasic CT to reveal gastrointestinal arteriovenous malformations. AJR Am J Roentgenol. 168:437–438. DOI: 10.2214/ajr.168.2.9016222. PMID: 9016222.

5. Townsend CM, Beauchamp RD, Evers BM, Mattox KL, Sabiston DC. 2021. Sabiston textbook of surgery: the biological basis of modern surgical practice. Elsevier;St. Louis, MO: 21st ed.
6. Foutch PG, Rex DK, Lieberman DA. 1995; Prevalence and natural history of colonic angiodysplasia among healthy asymptomatic people. Am J Gastroenterol. 90:564–567.
7. McAllister KA, Grogg KM, Johnson DW, et al. 1994; Endoglin, a TGF-beta binding protein of endothelial cells, is the gene for hereditary haemorrhagic telangiectasia type 1. Nat Genet. 8:345–351. DOI: 10.1038/ng1294-345. PMID: 7894484.

8. Boley SJ, DiBiase A, Brandt LJ, Sammartano RJ. 1979; Lower intestinal bleeding in the elderly. Am J Surg. 137:57–64. DOI: 10.1016/0002-9610(79)90011-4. PMID: 310250.

9. Gordon FH, Watkinson A, Hodgson H. 2001; Vascular malformations of the gastrointestinal tract. Best Pract Res Clin Gastroenterol. 15:41–58. DOI: 10.1053/bega.2000.0155. PMID: 11355900.

10. Junquera F, Quiroga S, Saperas E, et al. 2000; Accuracy of helical computed tomographic angiography for the diagnosis of colonic angiodysplasia. Gastroenterology. 119:293–299. DOI: 10.1053/gast.2000.9346. PMID: 10930363.

11. Su CC, Yang TW, Tsai MC. 2021; A rare cause of gastrointestinal bleeding: Duodenal arteriovenous malformation. Advances in Digestive Medicine. 8:55–58. DOI: 10.1002/aid2.13198.

12. Farrell JJ, Friedman LS. 2005; Review article: the management of lower gastrointestinal bleeding. Aliment Pharmacol Ther. 21:1281–1298. DOI: 10.1111/j.1365-2036.2005.02485.x. PMID: 15932359.

13. Bua-Ngam C, Norasetsingh J, Treesit T, et al. 2017; Efficacy of emergency transarterial embolization in acute lower gastrointestinal bleeding: A single-center experience. Diagn Interv Imaging. 98:499–505. DOI: 10.1016/j.diii.2017.02.005. PMID: 28341118.

14. Defreyne L, Meersschaut V, van Damme S, Berrevoet F, Robberecht E, Praet M. 2003; Colonic arteriovenous malformation in a child misinterpreted as an idiopathic colonic varicosis on angiography: remarks on current classification of childhood intestinal vascular malformations. Eur Radiol. 13 Suppl 4:L138–L141. DOI: 10.1007/s00330-003-1967-8. PMID: 15018179.

15. Hellstrom J, Hultborn KA, Engstedt L. 1955; Diffuse cavernous hemangioma of the rectum. Acta Chir Scand. 109:277–283.
16. Wang CH. 1985; Sphincter-saving procedure for treatment of diffuse cavernous hemangioma of the rectum and sigmoid colon. Dis Colon Rectum. 28:604–607. DOI: 10.1007/BF02554157. PMID: 4017824.

Fig. 1
Colonoscopy revealed extensive and dilated vascular structures and congested and erythematous mucosa with active bleeding spots in some vascular malformations.
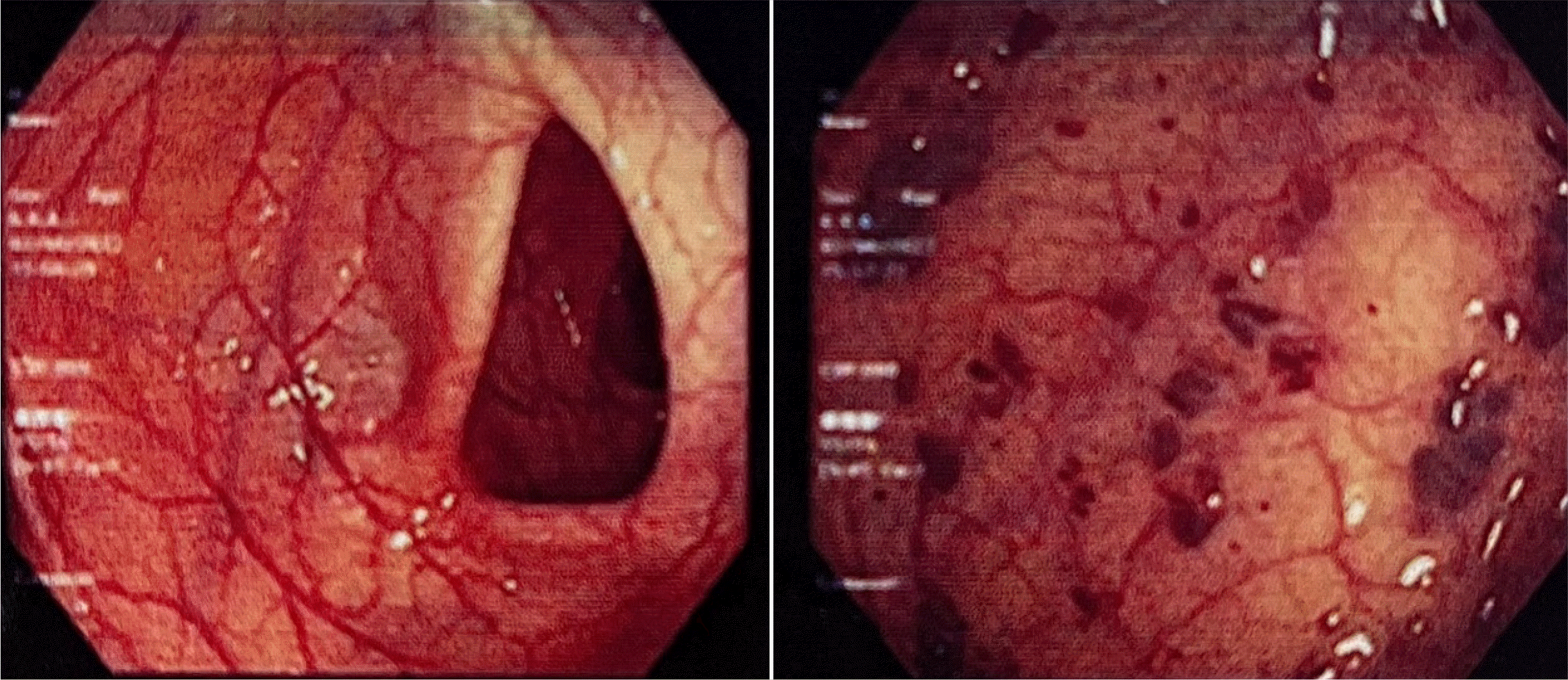
Fig. 2
Intraoperative findings revealed several extensively tangled vascular lesions that covered the entire sigmoid colon and rectum from the intestinal wall to the corresponding mesentery.
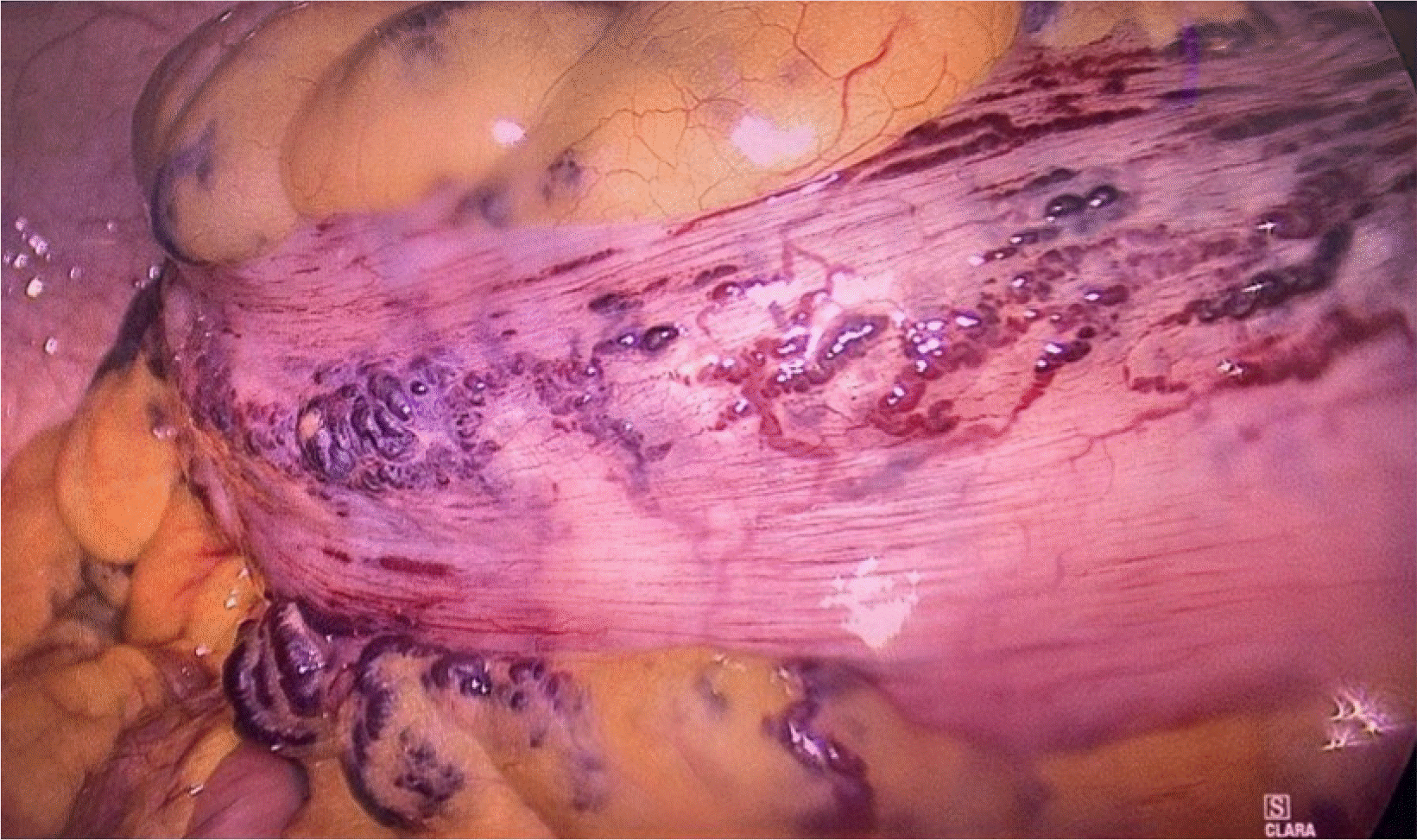
Fig. 3
Gross specimen showed the corresponding extensive vascular lesions on the sigmoid-rectal wall.
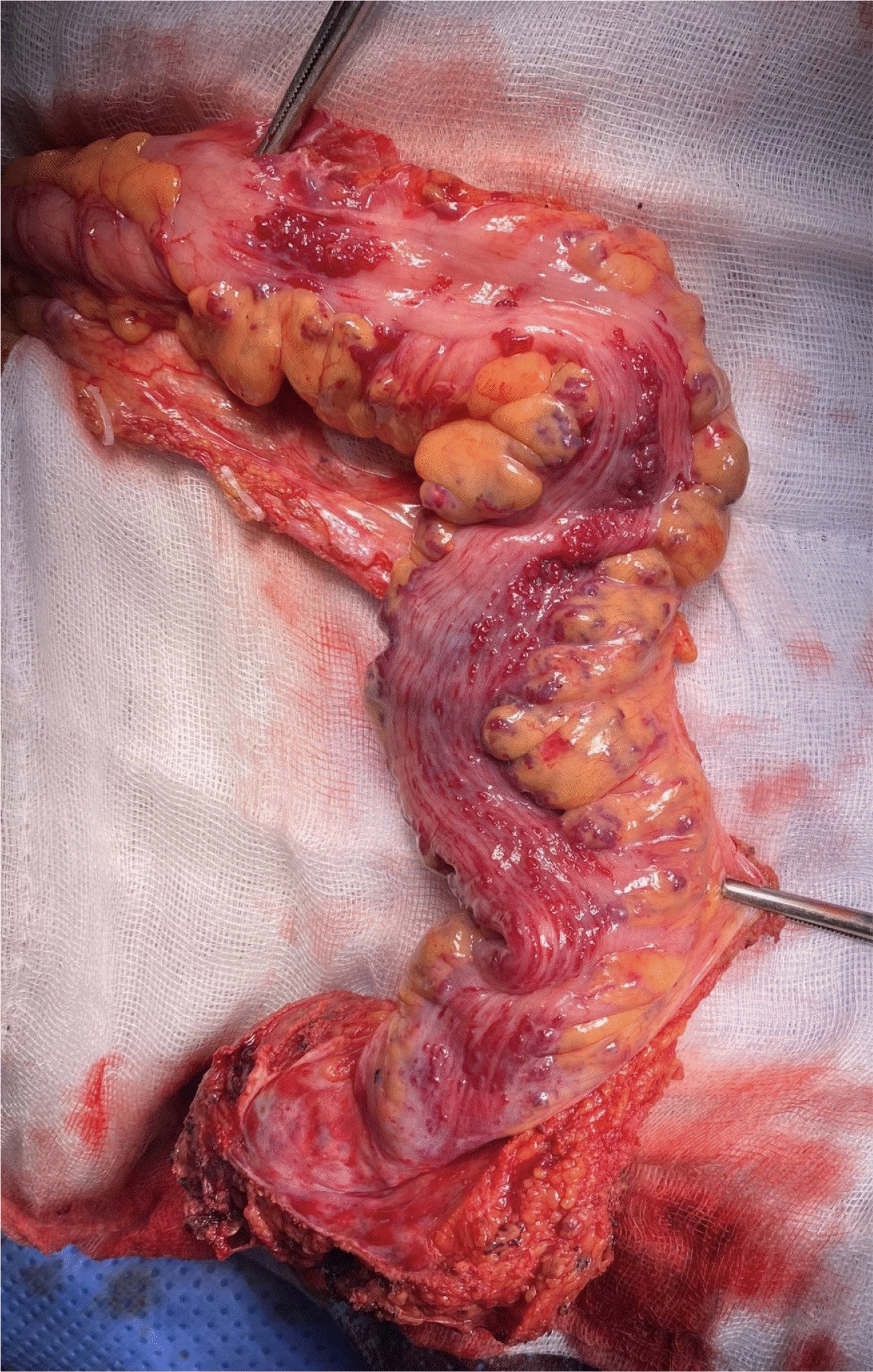
Fig. 4
Histopathology report demonstrated a colonic arteriovenous malformation consisting of (A) blood-filled vessels in the submucosal layer (H&E, ×40) and (B) the muscular layer (H&E, ×40). On the high power field, (C) large vessels with dilated lumens and some abnormal thick walls or transmural involvement (H&E, ×100); and (D) calcifying deposit (H&E, ×100).




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download