Abstract
Objective
To investigate changes in the immature teeth of Sprague–Dawley rats during orthodontic treatment and to explore the changes in the peri-radicular alveolar bone through micro-computed tomography (CT).
Methods
Twenty-five 26-day-old male Sprague–Dawley rats were included. The maxillary left first molar was moved mesially under a continuous force of 30 cN, and the right first molar served as the control. After orthodontic treatment for 7, 14, 21, 28, and 42 days, the root length, tooth volume, and alveolar bone mineral density (BMD) around the mesial root were measured through micro-CT.
Results
The immature teeth continued to elongate after application of orthodontic force. The root length on the force side was significantly smaller than that on the control side, whereas the differences in the volume change between both sides were not statistically significant. Alveolar bone in the coronal part of the compression and tension sides showed no difference in BMD between the experimental and control groups. The BMD of the experimental group decreased from day 14 to day 42 in the apical part of the compression side and increased from day 7 to day 42 in the apical part of the tension side. The BMD of the experimental group decreased in the root apex part on day 7.
Early orthodontic treatment is strongly recommended for some malocclusions, such as crossbite and severe deep overjet, owing to its positive influences on craniofacial development, occlusal improvement, and psychological health.1,2 Generally, orthodontic treatment for such kind of malocclusions usually initiated in the late mixed or early permanent dentition stage,3 when many permanent teeth are still in the developmental stage. However, the effects of orthodontic force on root development have not yet been clarified, and the outcomes of the existing research are controversial.
Dalaie et al.4 found that applying orthodontic force to mandibular second premolars with immature apices may not have a damaging effect on the root and even accelerate root formation within a short period. Bauss et al.5 found that the root length of developing third molars treated orthodontically after transplantation could increase to the normal length. Nevertheless, according to Harris and Baker6 and Hendrix et al.,7 the roots of young permanent teeth undergoing treatment were shortened and did not reach normal length. The degree of root resorption was similar to that observed in adult patients.6,7 Animal experimental studies indicate that orthodontic forces may negatively impact root development in rats. Ren et al.8 reported that the incidence of root resorption showed no differences between young and adult rats. Zhao et al.9 observed a decrease in root length and morphological changes at the root apex on application of orthodontic forces in young rats. Owing to the contradictory findings of these studies, further research is required to confirm the effects of orthodontic forces on immature teeth.
Bone mineral density (BMD) has often been evaluated in studies on bone remodeling.10 Micro-computed tomography (micro-CT), which is non-invasive and provides high-resolution images, can contribute to rapid acquisition of information on hard tissue mineral content.11
Therefore, to further elucidate the effects of orthodontic force on root development, we investigated the three-dimensional (3D) changes in immature teeth and changes in the peri-radicular alveolar bone of Sprague–Dawley rats during orthodontic tooth movement using micro-CT.
All animal experiments were approved by the Animal Experimentation Ethics Committee of School of Stomatology, Capital Medical University, Beijing, China (approval no. KQYY-2013082601). Twenty-five 23-day-old male Sprague–Dawley rats (Vital River Laboratory, Beijing, China) were purchased. The rats were acclimatized for 3 days before the experiment, and the experiment was started when the rats were 26 days old. During the experiment, all rats were placed under constant temperature, with a standard 12-hour light/dark cycle, powder diet, and ad libitum water.
Twenty-five young rats were randomly divided into five groups, in which orthodontic force was applied for 7, 14, 21, 28, and 42 days (d7, d14, d21, d28, and d42, respectively; n = 5 in each group). The rats were anesthetized using intraperitoneal injection of 10% chloral hydrate solution (4 mL/kg body weight). A cervical groove of approximately 0.2 mm depth was prepared on the incisors with a dental low-speed handpiece. To induce mesial tooth movement, the maxillary first molar was connected to the maxillary incisors with a nickel-titanium closed-coil spring (Smart, Beijing, China) of 0.012-inch diameter using ligature wires of 0.2-mm diameter. In the molar region, the ligature passed through the contact point of the first and second molars. In the incisor region, the ligature passed through the cervical grooves. The spring was activated to supply a continuous force of 30 cN calibrated by a tension gauge.12 Then, the ligature wire was bonded to the incisors using an adhesive resin (3M Unitek, Monrovia, CA, USA). The maxillary right first molar without an orthodontic appliance served as the control (Figure 1A). The spring apparatus was checked daily and repaired if the appliance was loose or damaged. As the incisors continued to grow, we adjusted the orthodontic appliance once a week to maintain the level and direction of the force.13
The rats were euthanized by cervical dislocation under intraperitoneal anesthesia with 10% chloral hydrate solution (4 mL/kg body weight) at 7, 14, 21, 28, and 42 days after initiating orthodontic forces. The maxillae were dissected and fixed in 4% paraformaldehyde for 24 hours. Then, the maxillary specimens underwent micro-CT (Inveon; Siemens, München, Germany) under the following settings: 80 kV; 500 µA; exposure time, 2,000 msec; rotation, 360°; slice thickness, 18 µm. Images were reconstructed using the in-house micro-CT software, and approximately 704 cross-sectional images were generated from each sample. These images were exported to a Digital Imaging and Communications in Medicine (DICOM) format.
The mesial root length of the maxillary first molar was measured using the RadiAnt DICOM Viewer (Medixant, Poznan, Poland). The tomographic images were first rotated to standardize the measurement plane as that passing through the center of the mesial and distobuccal roots in the horizontal plane and coinciding with the long axis of the mesial root in the coronal plane. In sagittal views, the mesial root length was measured along the long axis of the root from the center of the root apical foramen to the line connecting the mesial and distal cemento-enamel junction (CEJ) of the maxillary first molar (Figure 2). The change in the root length was calculated as the difference in the root length at days 14 and 7, days 21 and 14, days 28 and 21, days 42 and 28, and days 42 and 7 of the experiment.
The tooth volume was evaluated using 3D processing software Mimics 20.0 (Materialise, Leuven, Belgium). First, the thresholding and crop mask tools were used to extract the maxillary first molar from its surrounding alveolar bone. Subsequently, the masks were edited in multi-planner reformation views to separate the maxillary first molars slice by slice. 3D reconstruction was performed after applying the region growing and smoothing mask tools, and the tooth volume was automatically calculated.
The movement of the maxillary first molar was assessed based on the crown distance, neck distance, apical foramen distance, and root inclination using RadiAnt DICOM Viewer (Medixant). Crown distance was the distance between the marginal ridge of the maxillary first and second molars. Neck distance was the distance between the CEJ of the maxillary first molar and second molars. Apical foramen distance was the distance between the mesial root apex of the maxillary first molar and mesiobuccal root apex of the maxillary second molar. Root inclination was the angle between the long axis of mesial root of the maxillary first molar and the horizontal plane (Figure 1B–D). The horizontal plane was defined as that passing through the mesial and distal CEJ of the maxillary second molar. The sagittal measurement plane was standardized as that passing through the center of the mesial root of the maxillary first molar and the mesiobuccal root of the maxillary second molar in the horizontal plane and coinciding with the long axis of the mesial root of the first molar in the coronal plane. Tooth movement and inclination were measured in sagittal views.
BMD was measured using micro-CT supporting software Inveon Research Workplace (Siemens) to evaluate alveolar bone remodeling. It has been proposed that biological responses differ in the coronal and apical parts of the mesial and distal sides of the roots.14 Therefore, classifying the peri-radicular alveolar bone into the coronal part of the compression side, coronal part of the tension side, apical part of the compression side, apical part of the tension side, and part with the root apex could help us better understand the changes in the alveolar bone at different locations during orthodontic treatment. In the coronal and apical regions, four 210 × 210 × 210 µm-cubes mesial and distal to the mesial root were selected as the region of interest (ROI).10 The distance between the cubes and the root was 200 µm. The ROI of the root apex part was defined as a cube of 210 × 210 × 100 µm below the apical foramen (Figure 3). Finally, the BMD was calculated based on the obtained Hounsfiled unit values.
All measurements were performed by researchers blinded to the allocation of the experimental and control groups.
All data were analyzed using the statistical software SPSS 20.0 (IBM Corp., Armonk, NY, USA). The normality of data distribution was determined with the Shapiro-Wilk test. For normally distributed data, a paired t-test was used to compare the experimental and control groups. Intergroup comparisons were performed using independent t-test. For non-normally distributed data, the Wilcoxon signed-rank test was used. p < 0.05 was set to be the level of significant difference. Normally distributed data are expressed as means and standard deviations, and non-normally distributed data are expressed as medians and interquartile ranges.
The body weight of the rats increased with time, and there was no significant difference in body weight between the experimental and control rats of the same age. The intraclass correlation coefficient evaluated by measured values was > 0.90 for all measurements.
The mesial root of the first maxillary molar was selected as the representative root to measure root length. In the control group, the root length increased considerably from day 7 to day 42 after initiating tooth movement (Table 1). There was also an obvious increase in tooth volume throughout the observation period (Table 2). The root development of the maxillary first molar of Sprague–Dawley rats was almost completed at day 42 of the experiment, i.e., at postnatal day (PN) 68.
After application of orthodontic force, the root length showed continuous growth up to day 42. The growth rate was faster in the earlier stages, with significant root elongation between days 7 and 21; the growth was slower in the later stages, with no obvious growth between days 21 and 42 (Table 1). In addition, the root length in the experimental group was shorter than that in the control group at each time point (Table 1; Figure 4A). We compared the changes in root length to further analyze the effect of orthodontic force on root development. The growth of the root between days 7 and 14 and days 28 and 42 after application of orthodontic force was significantly lower than that in the control group, and the change in the root length was considerably less in the experimental group than in the control group over the entire experimental duration (Table 1). Changes in the root apex shape were observed on days 21, 28, and 42 in the force group (Figure 2H–J).
The tooth volume increased from day 7 to day 42 after applying orthodontic force as did the volume on the control side. There was no significant difference in tooth volume between the experimental and the control groups, except on day 28, suggesting that orthodontic force did not affect the tooth volume (Table 2; Figure 4B).
Under orthodontic forces, the movement of the crown, neck, and apical foramen, and root inclination increased with time (Table 3; Figure 4C–F).
When comparing the BMD of alveolar bone on the compression and tension sides of the mesial root of the maxillary first molar, different responses of alveolar bone to orthodontic force were found. At the apical part of the compression side, the BMD of the experimental group was significantly lower than that of the control group at days 14, 21, 28, and 42. Meanwhile, at the apical part of the tension side, the BMD of the experimental group was significantly greater than the control group BMD at days 7, 21, 28, and 42. The BMD of the root apex part was lower in the experimental group than in the control group, with a statistically significant difference only at day 7 (Table 4; Figure 5).
Based on the present micro-CT study, we found that the immature roots of rats can continue to develop under orthodontic forces and the peri-radicular alveolar bone undergoes remodeling during tooth movement.
In our study, the root length and root volume on the control side increased until day 42 (PN 68). Zhao et al.9 found that root elongation was almost completed at PN 35, which was the longest observation time point of their study. The differences in the findings of the two studies may be attributed to the differences in the longest observation time points. Our findings are consistent with those of a study on the volume of immature roots in Wistar rats, which revealed complete root development at approximately 10 weeks.15 Therefore, when studying maxillary first molars in Sprague–Dawley rats, 10-week-old Sprague–Dawley rats should be selected to exclude root developmental interferences.
In this study, the immature root continued to significantly elongate under force, although the root length in the experimental group was significantly less than that in the control group. According to the literature, the root length differences might be related to the odontogenic capacity of the apical tissue of the immature root under force.16,17 It has been reported that occlusal function may affect root length by regulating root apical cell proliferation.18,19 We speculate that orthodontic forces in addition to occlusal forces in the experimental group may have affected root elongation more than the occlusal forces in the control group. Further histological and cytological studies are needed to explore the specific mechanisms of this phenomenon.
Zhao et al.9 observed alteration in the shape of the developing root apex during tooth movement, which is consistent with the present findings. Disturbances in the odontoblast arrangement and dentine formation due to mechanical forces may lead to bending of the root apex during development and consequent decrease in the root length.9 The external environment may therefore impact the shape of an immature root, which is also supported by Cao et al.,20 who observed that trauma to the primary predecessor tooth and interference between adjacent teeth may cause dilacerated teeth. Pavlidis et al.21 and Walia et al.22 reported that if impacted dilacerated teeth undergo timely orthodontic traction, the direction of the roots may alter and develop a proper spatial relationship with the crown.
In the present study, the tooth volume increased over time and showed no obvious differences between the control and experimental groups. This may be because the root is conical, and the loss of the apical portion does not affect the total volume. The experimental group showed lesser root length development with statistically significant differences compared to the control group; however, these differences did not affect the total volume of the tooth. The root tissues of immature teeth were also in development, e.g., uncalcified predentin may have protected the roots from the effects of resorbing cells.23,24 Deposition of cellular cementum can increase root volume and delay root resorption.24,25 However, Li et al.15 found that the final mesial root volume in the immature experimental group was significantly less than that in the control group. Considering the differences in the experimental design, such as the control setting, observation time points, and the volume definition during measurement, the comparability between the two studies is low.
In our previous clinical study, immature teeth with two-third root formation developed normally in orthodontic patients, and there was no significant difference in root length and tooth volume between the posttreatment roots of incomplete teeth and pretreatment roots of fully developed permanent teeth.26 These findings are not fully consistent with those of the present study. This discrepancy may be owing to different research designs and different types of tooth movements. The patients with mild crowding in our clinical study received only alignment treatment, whereas in the present study, we used young Sprague–Dawley rats as the experimental subjects to simulate the mesial and long-distance tooth movement. Moreover, in our clinical research, we could not introduce a control side in the same patients because of the ethical considerations, which may have also led to the differences in the findings of both studies.
We found obvious displacement of teeth in the experimental group; the higher root inclination angle compared with that in the control group indicates that the molars in the experimental group underwent mesial movement with a predominant tipping movement. Therefore, in our experiment, the mesial side of the mesial root of the upper first molar under orthodontic force was regarded as the pressure side and the distal side as the tension side, which is consistent with the findings of Li et al.15 and Mao et al.27
In the present study, the BMD at the apical part of the compression side was significantly less than that in the control group between days 14 and 42, which implies that greater bone resorption may occur on the compression side. Previous research has indicated that bone resorption begins at day 7 on the compression side under a continuous force.28 Another tooth movement experiment showed that the expression of osteoclast differentiation factor RANKL and the number of TRAP-positive osteoclasts on the compression side increased significantly at day 7 in young rats.29 Considering that histological reactions precede mineral changes, it is reasonable that our micro-CT findings indicate that significant resorption occurred day 14 onward.
The BMD in the apical part of the tension side was significantly increased at days 7, 21, 28, and 42 days of applying orthodontic forces, indicating that the orthodontic force promoted osteogenesis on the tension side. Further, Mao et al.27 observed that the BMD in the apical part of the tension side increased at day 7. However, Yoshida et al.30 measured the BMD on the distal side of the distobuccal root and found a decrease. The discrepancy in the findings might be related with the selection of different observation areas. Additionally, other in vivo and in vitro studies have demonstrated that a tension force could induce osteoclast apoptosis, osteogenic gene expression, and greater calcium deposition.31-33 These previous findings provide evidence for osteogenesis on the tension side.
Compared with the control group, the BMD on the coronal parts of both tension and compression sides showed no significant change under force. We hypothesized that since the alveolar bone was undergoing active growth and development in terms of not only the volume but also the mineral content, it may show few changes in bone mineral content due to a balance between bone deposition and resorption in these areas.
The BMD in the root apex part decreased significantly at day 7 and then returned to the level in the control group. A 3D finite element model showed that tipping could result in stress at the root apex,34 which may induce bone resorption and reduce BMD, resulting in tooth movement at the initial stage. As the turnover rate of the alveolar bone in developing rats is high,35 the deposition and resorption of the bone at the root apex part may be balanced even under combined occlusal, orthodontic, and growth and development forces.
According to the literature, the choice of force magnitude varies among orthodontic tooth movement experiments in rats. Some studies have shown that a 10-cN force on rat molars could produce more tooth movement and less root resorption than heavier forces.36,37 However, some researchers consider a 25-30-cN force as appropriate to induce orthodontic tooth movement in rats.12,38 Considering the reduction of spring force value along with tooth movement, a 30-cN force was applied in this study. Since different force magnitudes result in different degrees of root resorption,39 further studies could focus on root development under different force magnitudes.
However, this study has limitations. Occlusal interference is inevitable when teeth are moved in an oblique manner under orthodontic force; this occlusal interference was not excluded in our study. In future studies, the opposing teeth may be extracted to focus purely on the effect of orthodontic force on tooth roots.40 In addition, this study does not include the recovery process of tooth roots after orthodontic force termination, as well as the possibility of root restoring to normal shape and length. These aspects should therefore be evaluated in future.
The length and volume of the immature roots of the Sprague–Dawley rats continued to increase under a continuous force of 30 cN. During tooth movement in young rats, there was alveolar bone resorption on the compression side, bone formation on the tension side, and significant alveolar bone resorption in the root apex part only at the initial stage. The findings of this study suggest that appropriate early orthodontic treatment won’t cause adverse effects on the development of immature tooth roots. Meanwhile, orthodontists should pay attention to the developmental stage of young permanent teeth, the magnitude of orthodontic force and the duration of orthodontic treatment to prevent the potential adverse effects on immature teeth.
Notes
REFERENCES
1. Brierley CA, DiBiase A, Sandler PJ. 2017; Early Class II treatment. Aust Dent J. 62 Suppl 1:4–10. https://doi.org/10.1111/adj.12478. DOI: 10.1111/adj.12478. PMID: 28297093.

2. Ngan P, Moon W. 2015; Evolution of Class III treatment in orthodontics. Am J Orthod Dentofacial Orthop. 148:22–36. https://doi.org/10.1016/j.ajodo.2015.04.012. DOI: 10.1016/j.ajodo.2015.04.012. PMID: 26124025.

3. Fleming PS. 2017; Timing orthodontic treatment: early or late? Aust Dent J. 62 Suppl 1:11–9. https://doi.org/10.1111/adj.12474. DOI: 10.1111/adj.12474. PMID: 28297091.

4. Dalaie K, Badiee M, Behnaz M, Kavousinejad S. 2021; Effect of orthodontic forces on root length of immature mandibular second premolars: a split-mouth randomized clinical trial. Dental Press J Orthod. 26:e2119355. https://doi.org/10.1590/2177-6709.26.5.e2119355.oar. DOI: 10.1590/2177-6709.26.5.e2119355.oar. PMID: 35640080. PMCID: PMC8576854. PMID: 24c4b858170847b2abd2a5940cbcd279.

5. Bauss O, Sadat-Khonsari R, Engelke W, Kahl-Nieke B. 2003; Results of transplanting developing third molars as part of orthodontics space management. Part 2: results following the orthodontic treatment of transplanted developing third molars in cases of aplasia and premature loss of teeth with atrophy of the alveolar process. J Orofac Orthop. 64:40–7. https://doi.org/10.1007/s00056-003-0132-y. DOI: 10.1007/s00056-003-0132-y. PMID: 12557106.

6. Harris EF, Baker WC. 1990; Loss of root length and crestal bone height before and during treatment in adolescent and adult orthodontic patients. Am J Orthod Dentofacial Orthop. 98:463–9. https://doi.org/10.1016/s0889-5406(05)81656-7. DOI: 10.1016/S0889-5406(05)81656-7. PMID: 2239846.

7. Hendrix I, Carels C, Kuijpers-Jagtman AM, Van 'T Hof M. 1994; A radiographic study of posterior apical root resorption in orthodontic patients. Am J Orthod Dentofacial Orthop. 105:345–9. DOI: 10.1016/S0889-5406(94)70128-8. PMID: 8154459.

8. Ren Y, Maltha JC, Liem RS, Stokroos I, Kuijpers-Jagtman AM. 2008; Age-dependent external root resorption during tooth movement in rats. Acta Odontol Scand. 66:93–8. https://doi.org/10.1080/00016350801982522. DOI: 10.1080/00016350801982522. PMID: 18446550.

9. Zhao L, Matsumoto Y, Ono T, Iseki S. 2019; Effects of mechanical force application on the developing root apex in rat maxillary molars. Arch Oral Biol. 101:64–76. https://doi.org/10.1016/j.archoralbio.2019.03.010. DOI: 10.1016/j.archoralbio.2019.03.010. PMID: 30903951.

10. An J, Li Y, Liu Z, Wang R, Zhang B. 2017; A micro-CT study of microstructure change of alveolar bone during orthodontic tooth movement under different force magnitudes in rats. Exp Ther Med. 13:1793–8. https://doi.org/10.3892/etm.2017.4186. DOI: 10.3892/etm.2017.4186. PMID: 28565769. PMCID: PMC5443305.

11. Swain MV, Xue J. 2009; State of the art of Micro-CT applications in dental research. Int J Oral Sci. 1:177–88. https://doi.org/10.4248/ijos09031. DOI: 10.4248/IJOS09031. PMID: 20690421. PMCID: PMC3470105.

12. Shintcovsk RL, Knop L, Tanaka OM, Maruo H. 2014; Nicotine effect on bone remodeling during orthodontic tooth movement: histological study in rats. Dental Press J Orthod. 19:96–107. https://doi.org/10.1590/2176-9451.19.2.096-107.oar. DOI: 10.1590/2176-9451.19.2.096-107.oar. PMID: 24945520. PMCID: PMC4296601. PMID: 6c1142eabdb14afa86cec9253264099b.

13. Kong X, Cao M, Ye R, Ding Y. 2010; Orthodontic force accelerates dentine mineralization during tooth development in juvenile rats. Tohoku J Exp Med. 221:265–70. https://doi.org/10.1620/tjem.221.265. DOI: 10.1620/tjem.221.265. PMID: 20625241.

14. Leong NL, Hurng JM, Djomehri SI, Gansky SA, Ryder MI, Ho SP. 2012; Age-related adaptation of bone-PDL-tooth complex: Rattus-Norvegicus as a model system. PLoS One. 7:e35980. https://doi.org/10.1371/journal.pone.0035980. DOI: 10.1371/journal.pone.0035980. PMID: 22558292. PMCID: PMC3340399. PMID: 39413ee9480d4cb6ad409b68b02f9c1f.

15. Li X, Xu J, Yin Y, Liu T, Chang L, He S, et al. 2021; Notch signaling inhibition protects against root resorption in experimental immature tooth movement in rats. Am J Orthod Dentofacial Orthop. 159:426–34.e5. https://doi.org/10.1016/j.ajodo.2020.05.012. DOI: 10.1016/j.ajodo.2020.05.012. PMID: 33568273.

16. Owens PD. 1978; Ultrastructure of Hertwig's epithelial root sheath during early root development in premolar teeth in dogs. Arch Oral Biol. 23:91–104. https://doi.org/10.1016/0003-9969(78)90145-0. DOI: 10.1016/0003-9969(78)90145-0. PMID: 274924.

17. Xu L, Tang L, Jin F, Liu XH, Yu JH, Wu JJ, et al. 2009; The apical region of developing tooth root constitutes a complex and maintains the ability to generate root and periodontium-like tissues. J Periodontal Res. 44:275–82. https://doi.org/10.1111/j.1600-0765.2008.01129.x. DOI: 10.1111/j.1600-0765.2008.01129.x. PMID: 19261077.

18. Nakasone N, Yoshie H. 2011; Occlusion regulates tooth-root elongation during root development in rat molars. Eur J Oral Sci. 119:418–26. https://doi.org/10.1111/j.1600-0722.2011.00856.x. DOI: 10.1111/j.1600-0722.2011.00856.x. PMID: 22112026.

19. Motokawa M, Terao A, Karadeniz EI, Kaku M, Kawata T, Matsuda Y, et al. 2013; Effects of long-term occlusal hypofunction and its recovery on the morphogenesis of molar roots and the periodontium in rats. Angle Orthod. 83:597–604. https://doi.org/10.2319/081812-661.1. DOI: 10.2319/081812-661.1. PMID: 23148606. PMCID: PMC8754027.

20. Cao D, Shao B, Izadikhah I, Xie L, Wu B, Li H, et al. 2021; Root dilaceration in maxillary impacted canines and adjacent teeth: a retrospective analysis of the difference between buccal and palatal impaction. Am J Orthod Dentofacial Orthop. 159:167–74. https://doi.org/10.1016/j.ajodo.2019.12.019. DOI: 10.1016/j.ajodo.2019.12.019. PMID: 33342674.

21. Pavlidis D, Daratsianos N, Jäger A. 2011; Treatment of an impacted dilacerated maxillary central incisor. Am J Orthod Dentofacial Orthop. 139:378–87. https://doi.org/10.1016/j.ajodo.2009.10.040. DOI: 10.1016/j.ajodo.2009.10.040. PMID: 21392694.

22. Walia PS, Rohilla AK, Choudhary S, Kaur R. 2016; Review of dilaceration of maxillary central incisor: a mutidisciplinary challenge. Int J Clin Pediatr Dent. 9:90–8. https://doi.org/10.5005/jp-journals-10005-1341. DOI: 10.5005/jp-journals-10005-1341. PMID: 27274164. PMCID: PMC4890071.

23. Winter BU, Stenvik A, Vandevska-Radunovic V. 2009; Dynamics of orthodontic root resorption and repair in human premolars: a light microscopy study. Eur J Orthod. 31:346–51. https://doi.org/10.1093/ejo/cjp020. DOI: 10.1093/ejo/cjp020. PMID: 19465737.

24. Reitan K. 1974; Initial tissue behavior during apical root resorption. Angle Orthod. 44:68–82. https://doi.org/10.1043/0003-3219(1974)044%3C0068:itbdar%3E2.0.co;2. DOI: 10.1043/0003-3219(1974)044<0068:ITBDAR>2.0.CO;2. PMID: 4520953.

25. Star H, Chandrasekaran D, Miletich I, Tucker AS. 2017; Impact of hypofunctional occlusion on upper and lower molars after cessation of root development in adult mice. Eur J Orthod. 39:243–50. https://doi.org/10.1093/ejo/cjw051. DOI: 10.1093/ejo/cjw051. PMID: 27567636.

26. Wan J, Zhou S, Wang J, Zhang R. 2023; Three-dimensional analysis of root changes after orthodontic treatment for patients at different stages of root development. Am J Orthod Dentofacial Orthop. 163:60–7. https://doi.org/10.1016/j.ajodo.2021.08.025. DOI: 10.1016/j.ajodo.2021.08.025. PMID: 36195543.

27. Mao Y, Wang L, Zhu Y, Liu Y, Dai H, Zhou J, et al. 2018; Tension force-induced bone formation in orthodontic tooth movement via modulation of the GSK-3β/β-catenin signaling pathway. J Mol Histol. 49:75–84. https://doi.org/10.1007/s10735-017-9748-x. DOI: 10.1007/s10735-017-9748-x. PMID: 29224185. PMCID: PMC5750339.

28. Ru N, Liu SS, Zhuang L, Li S, Bai Y. 2013; In vivo microcomputed tomography evaluation of rat alveolar bone and root resorption during orthodontic tooth movement. Angle Orthod. 83:402–9. https://doi.org/10.2319/031312-219.1. DOI: 10.2319/031312-219.1. PMID: 23030553. PMCID: PMC8763084.

29. Li X, Li M, Lu J, Hu Y, Cui L, Zhang D, et al. 2016; Age-related effects on osteoclastic activities after orthodontic tooth movement. Bone Joint Res. 5:492–9. https://doi.org/10.1302/2046-3758.510.bjr-2016-0004.r2. DOI: 10.1302/2046-3758.510.BJR-2016-0004.R2. PMID: 27769958. PMCID: PMC5108356.

30. Yoshida T, Yamaguchi M, Utsunomiya T, Kato M, Arai Y, Kaneda T, et al. 2009; Low-energy laser irradiation accelerates the velocity of tooth movement via stimulation of the alveolar bone remodeling. Orthod Craniofac Res. 12:289–98. https://doi.org/10.1111/j.1601-6343.2009.01464.x. DOI: 10.1111/j.1601-6343.2009.01464.x. PMID: 19840281.

31. Kobayashi Y, Hashimoto F, Miyamoto H, Kanaoka K, Miyazaki-Kawashita Y, Nakashima T, et al. 2000; Force-induced osteoclast apoptosis in vivo is accompanied by elevation in transforming growth factor beta and osteoprotegerin expression. J Bone Miner Res. 15:1924–34. https://doi.org/10.1359/jbmr.2000.15.10.1924. DOI: 10.1359/jbmr.2000.15.10.1924. PMID: 11028444.

32. Qin J, Hua Y. 2016; Effects of hydrogen sulfide on the expression of alkaline phosphatase, osteocalcin and collagen type I in human periodontal ligament cells induced by tension force stimulation. Mol Med Rep. 14:3871–7. https://doi.org/10.3892/mmr.2016.5680. DOI: 10.3892/mmr.2016.5680. PMID: 27573279.

33. Zhang R, Wan J, Wang H. 2019; Mechanical strain triggers differentiation of dental mesenchymal stem cells by activating osteogenesis-specific biomarkers expression. Am J Transl Res. 11:233–44. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6357315/. PMID: 30787982. PMCID: PMC6357315.
34. Bicǎ C, Brezeanu L, Bicǎ D. 2013; A finite element model of force distribution from tooth movement. Metal Int. 18:12–5. https://www.proquest.com/openview/6e242fcd5829fba2e89912e9b225893d/1?pq-origsite=gscholar&cbl=886383.
35. Misawa Y, Kageyama T, Moriyama K, Kurihara S, Yagasaki H, Deguchi T, et al. 2007; Effect of age on alveolar bone turnover adjacent to maxillary molar roots in male rats: a histomorphometric study. Arch Oral Biol. 52:44–50. https://doi.org/10.1016/j.archoralbio.2006.06.012. DOI: 10.1016/j.archoralbio.2006.06.012. PMID: 17125731.
36. Gonzales C, Hotokezaka H, Yoshimatsu M, Yozgatian JH, Darendeliler MA, Yoshida N. 2008; Force magnitude and duration effects on amount of tooth movement and root resorption in the rat molar. Angle Orthod. 78:502–9. https://doi.org/10.2319/052007-240.1. DOI: 10.2319/052007-240.1. PMID: 18416627.
37. Kraiwattanapong K, Samruajbenjakun B. 2019; Tissue response resulting from different force magnitudes combined with corticotomy in rats. Angle Orthod. 89:797–803. https://doi.org/10.2319/090418-645.1. DOI: 10.2319/090418-645.1. PMID: 30896251. PMCID: PMC8111850.
38. Li C, Chung CJ, Hwang CJ, Lee KJ. 2019; Local injection of RANKL facilitates tooth movement and alveolar bone remodelling. Oral Dis. 25:550–60. https://doi.org/10.1111/odi.13013. DOI: 10.1111/odi.13013. PMID: 30536847.
39. Nakano T, Hotokezaka H, Hashimoto M, Sirisoontorn I, Arita K, Kurohama T, et al. 2014; Effects of different types of tooth movement and force magnitudes on the amount of tooth movement and root resorption in rats. Angle Orthod. 84:1079–85. https://doi.org/10.2319/121913-929.1. DOI: 10.2319/121913-929.1. PMID: 24754797. PMCID: PMC8638510.
40. Marcantonio CC, Nogueira AVB, Leguizamón NDP, de Molon RS, Lopes MES, Silva RCL, et al. 2021; Effects of obesity on periodontal tissue remodeling during orthodontic movement. Am J Orthod Dentofacial Orthop. 159:480–90. https://doi.org/10.1016/j.ajodo.2019.12.025. DOI: 10.1016/j.ajodo.2019.12.025. PMID: 33563505.

Figure 1
A, Rat model of orthodontic tooth movement; B, sagittal view. The yellow line represents the horizontal plane passing through the mesial and distal cemento-enamel junction of the maxillary second molar; C, horizontal view. The red line represents the sagittal plane passing through the center of the mesial root of the maxillary first molar and the mesiobuccal root of the maxillary second molar; the green dotted line indicates the division between the upper first and second molars, and the upper second and third molars. D, Measurement of movement at the crown (a), neck (b), apical foramen (c), and inclination of the root (d). m1, represents the maxillary first molar; m2, represents the maxillary second molar; m3, represents the maxillary third molar.
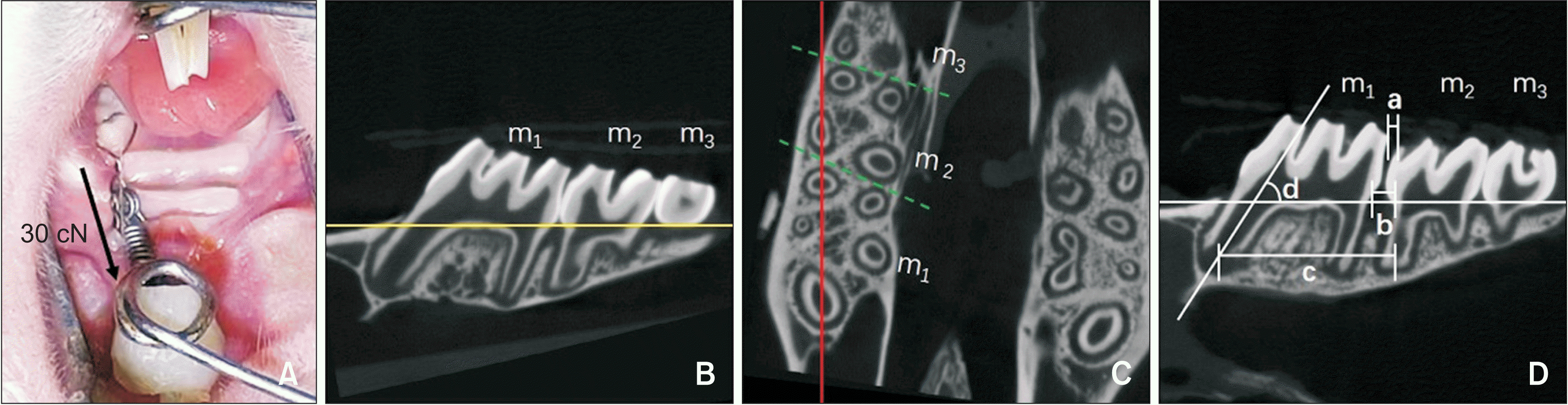
Figure 2
The mesial root length of the maxillary first molar. A–E, Micro-computed tomography (CT) reconstruction of the maxillary first molars in the control group at 7, 14, 21, 28, and 42 days after applying orthodontic force. F–J, Micro-CT reconstruction of the maxillary first molars of the orthodontic force group at 7, 14, 21, 28, and 42 days after applying orthodontic force. The blue and green arrows represent the cemento-enamel junction and mesial root length, respectively. H–J, The root apex shape was altered at 21, 28, and 42 days after application of orthodontic force.
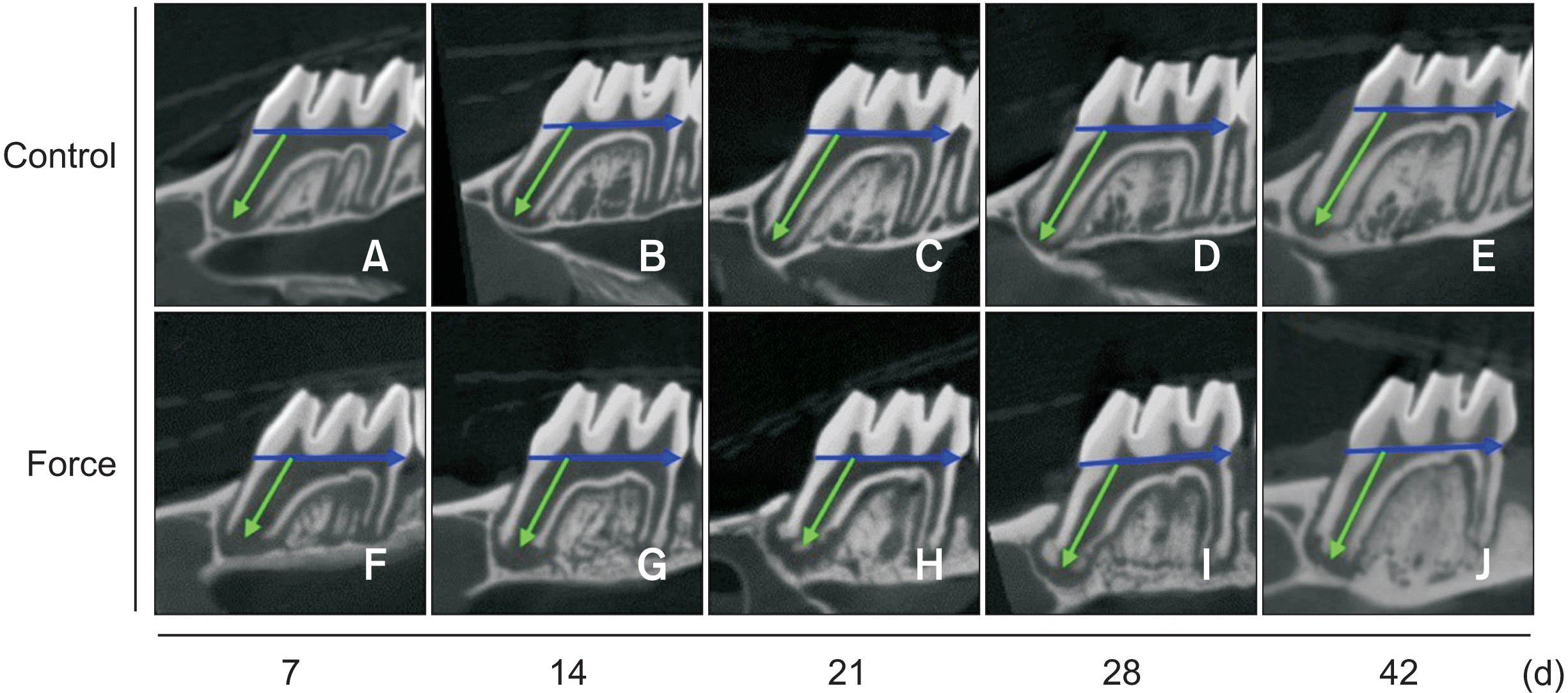
Figure 3
Selection of regions of interest (ROI). 210 × 210 × 210-µm cubes, 200 µm mesial and distal to the mesial root. In the apical region, the ROI was defined as a 210 × 210 × 100-µm cube below the apical foramen. A, Sagittal view; B, horizontal view; C, coronal view. Coronal part of the compression side (M1), coronal part of the tension side (D1), apical part of the compression side (M2), apical part of the tension side (D2), and root apex part (A).
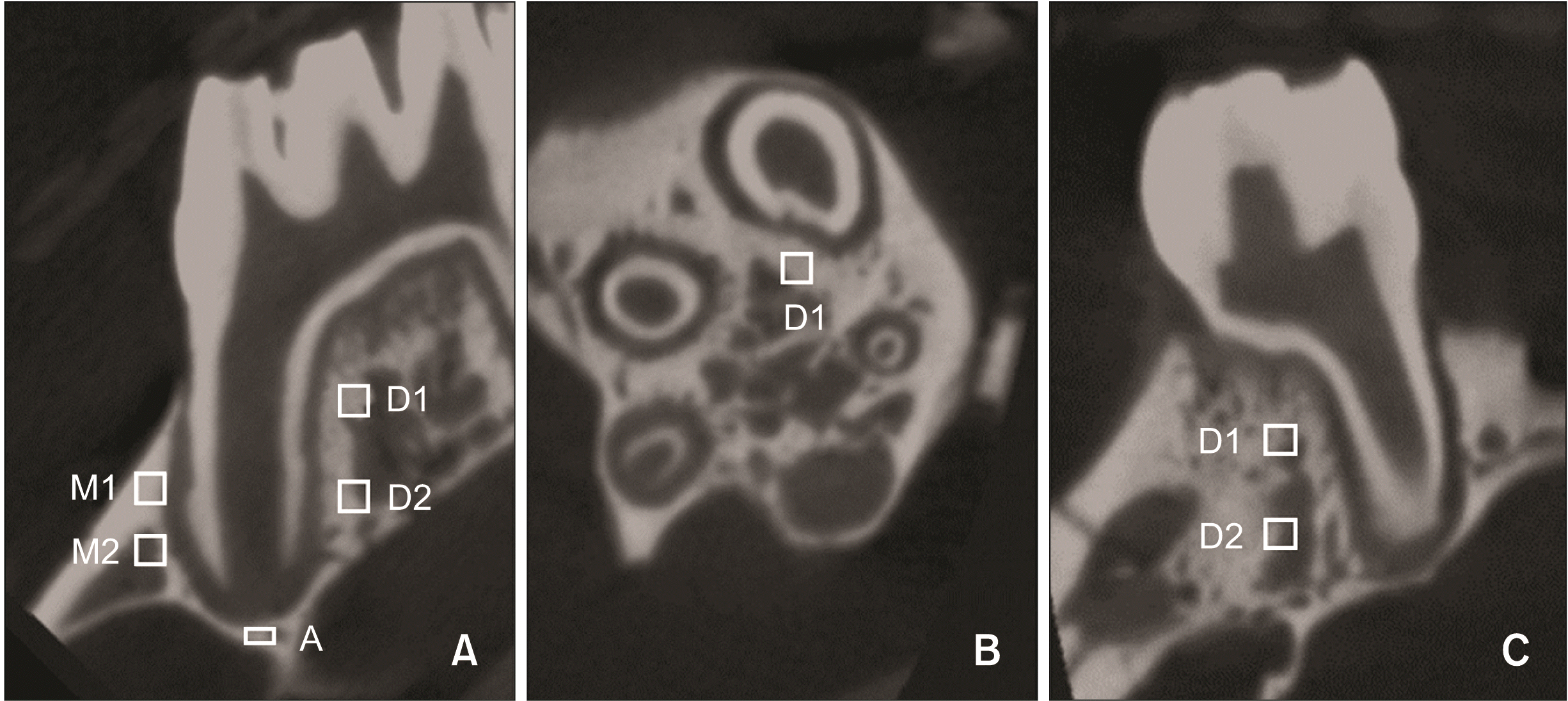
Figure 4
Graphical representation of the root length (mm), tooth volume (mm3), and tooth movement at different time points. A, Root length; B, tooth volume; C, crown distance; D, neck distance; E, apical foramen distance; F, root inclination. *p < 0.05.
Figure 5
Changes in the bone mineral density (BMD) of the alveolar bone at different time points. A, Coronal part of the compression side (M1). B, Apical part of the compression side (M2). C, Coronal part of the tension side (D1). D, Apical part of the tension side (D2). E, Root apex part (A). Box edges represent the upper and lower quantiles, the middle lines in the boxes represent the medians, and the whiskers represent the maxima and minima. *p < 0.05.
Table 1
Descriptive statistics of the mesial root length
| Group/variable | 7 d | 14 d | 21 d | 28 d | 42 d |
|---|---|---|---|---|---|
| Root length (mm) | |||||
| Force | 1.939 ± 0.063 | 2.069 ± 0.116 | 2.303 ± 0.142 | 2.361 ± 0.040 | 2.411 ± 0.090 |
| Control | 2.094 ± 0.019 | 2.334 ± 0.049 | 2.655 ± 0.088 | 2.595 ± 0.074 | 2.828 ± 0.088 |
| p-value† | 0.003* | 0.002* | 0.004* | 0.001* | < 0.001* |
| 7/14 d | 7/21 d | 7/28 d | 7/42 d | 14/21 d | 14/28 d | 14/42 d | 21/28 d | 21/42 d | 28/42 d | |
|---|---|---|---|---|---|---|---|---|---|---|
| Pairwise comparisons of root length (p-values‡) | ||||||||||
| Force | 0.059 | 0.001* | < 0.001* | < 0.001* | 0.021* | 0.001* | 0.001* | 0.408 | 0.189 | 0.287 |
| Control | < 0.001* | < 0.001* | < 0.001* | < 0.001* | < 0.001* | < 0.001* | < 0.001* | 0.277 | 0.015* | 0.002* |
| ΔL14–7 d | ΔL21–14 d | ΔL28–21 d | ΔL42–28 d | ΔL42–7 d | |
|---|---|---|---|---|---|
| Root length change (mm) | |||||
| Force | 0.129 ± 0.082 | 0.235 ± 0.182 | 0.058 ± 0.151 | 0.050 ± 0.089 | 0.472 ± 0.091 |
| Control | 0.240 ± 0.046 | 0.321 ± 0.100 | –0.060 ± 0.151 | 0.233 ± 0.136 | 0.740 ± 0.090 |
| p-value† | 0.031* | 0.298 | 0.251 | 0.024* | 0.001* |
Table 2
Descriptive statistics of the tooth volume
| Group/variable | 7 d | 14 d | 21 d | 28 d | 42 d |
|---|---|---|---|---|---|
| Tooth volume (mm3) | |||||
| Force | 8.313 ± 0.332 | 9.209 ± 0.318 | 9.252 ± 0.352 | 9.876 ± 0.687 | 10.218 ± 0.405 |
| Control | 8.289 ± 0.219 | 9.309 ± 0.381 | 9.402 ± 0.225 | 10.053 ± 0.644 | 10.386 ± 0.467 |
| p-value† | 0.909 | 0.368 | 0.079 | 0.016* | 0.410 |
| 7/14 d | 7/21 d | 7/28 d | 7/42 d | 14/21 d | 14/28 d | 14/42 d | 21/28 d | 21/42 d | 28/42 d | |
|---|---|---|---|---|---|---|---|---|---|---|
| Pairwise comparisons (p-values‡) | ||||||||||
| Force | 0.004* | 0.002* | 0.002* | < 0.001* | 0.857 | 0.094 | 0.004* | 0.108 | 0.004* | 0.364 |
| Control | 0.001* | < 0.001* | < 0.001* | < 0.001* | 0.653 | 0.057 | 0.004* | 0.065 | 0.003* | 0.376 |
Table 3
Descriptive statistics of the tooth movement distance and root inclination
| Crown distance (mm) | Neck distance (mm) | Apical foramen distance (mm) | Root inclination (°) | |
|---|---|---|---|---|
| 7 d | ||||
| Force | 0.15 ± 0.02 | 0.39 ± 0.01 | 4.03 ± 0.07 | 47.56 ± 7.10 |
| Control | 0.10 ± 0.02 | 0.31 ± 0.04 | 4.00 ± 0.07 | 42.38 ± 9.04 |
| p-value† | 0.006* | 0.010* | 0.230 | 0.011* |
| 14 d | ||||
| Force | 0.35 ± 0.10 | 0.45 ± 0.09 | 4.12 ± 0.03 | 49.52 ± 0.41 |
| Control | 0.13 ± 0.02 | 0.29 ± 0.10 | 4.07 ± 0.09 | 44.18 ± 2.69 |
| p-value† | 0.005* | 0.022* | 0.329 | 0.008* |
| 21 d | ||||
| Force | 0.41 ± 0.03 | 0.54 ± 0.02 | 4.14 ± 0.04 | 52.38 ± 0.77 |
| Control | 0.14 ± 0.02 | 0.24 ± 0.03 | 4.08 ± 0.07 | 44.50 ± 1.77 |
| p-value† | < 0.001* | < 0.001* | 0.063 | 0.002* |
| 28 d | ||||
| Force | 0.68 ± 0.04 | 0.61 ± 0.04 | 4.39 ± 0.09 | 57.62 ± 1.40 |
| Control | 0.14 ± 0.02 | 0.23 ± 0.04 | 4.34 ± 0.13 | 44.28 ± 1.07 |
| p-value† | < 0.001* | < 0.001* | 0.176 | < 0.001* |
| 42 d | ||||
| Force | 1.06 ± 0.05 | 0.95 ± 0.04 | 4.44 ± 0.06 | 65.68 ± 3.08 |
| Control | 0.13 ± 0.03 | 0.28 ± 0.07 | 4.35 ± 0.11 | 43.94 ± 2.16 |
| p-value† | < 0.001* | < 0.001* | 0.167 | < 0.001* |
Table 4
Descriptive statistics of alveolar bone density in regions of interest
| Group | M1 (mg/mL) | M2 (mg/mL) | D1 (mg/mL) | D2 (mg/mL) | A (mg/mL) |
|---|---|---|---|---|---|
| 7 d | |||||
| Force | 2,326.74 (2,229.86–2,336.68) | 1,810.70 (1,402.58–1,991.52) | 1,975.46 (1,836.70–2,068.16) | 2,000.46 (1,975.79–2,034.66) | 1,613.64 (1,551.41–1,810.86) |
| Control | 2,374.98 (2,343.17–2,405.02) | 1,886.34 (1,879.80–2,064.73) | 1,911.12 (1,834.68–2,132.19) | 1,605.38 (1,477.83–1,732.88) | 1,902.28 (1,730.93–1,941.27) |
| p-value† | 0.080 | 0.225 | 0.893 | 0.043* | 0.043* |
| 14 d | |||||
| Force | 2,371.09 (2,002.75–2,380.12) | 1,730.75 (1,654.17–1,815.73) | 1,998.26 (1,773.18–2,202.85) | 1,986.75 (1,781.84–2,073.07) | 1,893.08 (1,813.57–2,047.42) |
| Control | 2,429.62 (1,365.57–2,457.00) | 2,003.23 (1,887.32–2,296.26) | 1,999.83 (1,929.88–2,039.86) | 1,842.86 (1,800.87–1,882.26) | 1,930.31 (1,890.77–2,107.50) |
| p-value† | 0.080 | 0.043* | 0.893 | 0.138* | 0.500 |
| 21 d | |||||
| Force | 2,339.22 (1,781.40– 2,380.44) | 1,892.40 (1,819.98–2,007.95) | 2,116.51 (2,096.31–2,140.90) | 2,132.78 (2,041.36–2,232.84) | 1,878.67 (1,785.68–1,917.10) |
| Control | 2,353.33 (2,297.80– 2,388.88) | 2,213.88 (2,100.57–2,283.65) | 1,954.10 (1,883.17–2,161.84) | 1,796.48 (1,711.85–1,897.83) | 1,939.10 (1,886.00–2,057.64) |
| p-value† | 0.225 | 0.043* | 0.225 | 0.043* | 0.225 |
| 28 d | |||||
| Force | 2,357.36 (2,132.14– 2,394.06) | 2,011.83 (1,912.68–2,190.39) | 2,235.81 (2,083.64–2,303.32) | 2,071.96 (2,062.42–2,174.35) | 1,871.56 (1,621.47–1,953.30) |
| Control | 2,400.00 (2,266.11– 2,420.48) | 2,304.89 (2,094.10–2,339.49) | 2,001.29 (1,935.23–2,364.88) | 1,736.05 (1,719.26–1,770.36) | 1,977.21 (1,943.43–2,110.66) |
| p-value† | 0.893 | 0.043* | 0.500 | 0.043* | 0.138 |
| 42 d | |||||
| Force | 2,276.99 (2,176.86– 2,354.28) | 2,039.78 (1,865.21–2,077.60) | 2,169.27 (2,122.65–2,257.98) | 2,149.14 (2,047.29–2,205.98) | 1,928.06 (1,797.17–2,007.39) |
| Control | 2,359.06 (2,308.82– 2,463.22) | 2,380.55 (2,292.00–2,475.90) | 2,136.80 (2,034.70–2,279.57) | 1,698.62 (1,570.96–1,758.74) | 1,943.49 (1,874.80–2,286.51) |
| p-value† | 0.080 | 0.043* | 0.686 | 0.043* | 0.138 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download