INTRODUCTION
Monolithic zirconia restorations are superior to porcelain-veneered zirconia restorations in regards to fracture and chipping,
1-3 however, their main drawback is low translucency.
1 One suggested strategy to increase translucency is to increase the yttrium oxide content (Y
2O
3) and subsequently the cubic phase.
4 Partially stabilized zirconia is obtained by addition of 4–6 mol% Y
2O
3 while full cubic stabilized zirconia (FSZ) is obtained by addition of over 8 mol% Y
2O
3.
5,6 FSZ has a lower alumina content, relatively fine particles, and optically isotropic cubic phase. Thus, it has lower grain boundary and light scattering, and higher translucency.
7-9
Correction of crowding and tooth alignment not only provides optimal esthetics, but also improves the periodontal status and enhances oral hygiene.
10 Thus, with an increase in older individuals seeking orthodontic treatment,
11 orthodontists encounter patients with various types of fixed dental restorations including zirconia. However, information regarding the effect of orthodontic treatment on different properties of zirconia is scarce.
Color parameters such as color stability and translucency are among the important clinical success criteria for any restoration.
1,12,13 Thus, it is imperative to assess the effect of different processes on these parameters. In orthodontic bracket bonding to zirconia, it is essential to create a reliable bond strength to withstand orthodontic and masticatory forces.
14 Nonetheless, orthodontic bracket bonding on ceramic restorations may increase the surface roughness (due to surface treatment or residual resin removal), change the esthetic appearance of restoration, decrease its longevity, enhance plaque retention, leading to periodontal problems and restoration discoloration.
15 Since zirconia is devoid of glass phase, hydrofluoric acid cannot be used to enhance its bond strength.
16 The suggested strategies for surface treatment of zirconia to enhance its bond strength include airborne particle abrasion (APA), silica-coating, and laser irradiation.
16 The effect of different surface treatments in the process of orthodontic bonding, and orthodontic treatment in general, on color parameters and color stability of enamel,
17-24 and feldspathic porcelain
15,25-27 has been previously studied. To the best of the authors’ knowledge, no previous study has addressed the effect of orthodontic treatment on color parameters of zirconia restorations. Thus, this study aimed to assess the color properties, color stability, and translucency of FSZ following orthodontic bonding with different surface treatments and coffee thermocycling (CTC). The null hypothesis was that the color properties, color stability, and translucency of FSZ would not be influenced by orthodontic bonding with different surface treatments and CTC.
MATERIALS AND METHODS
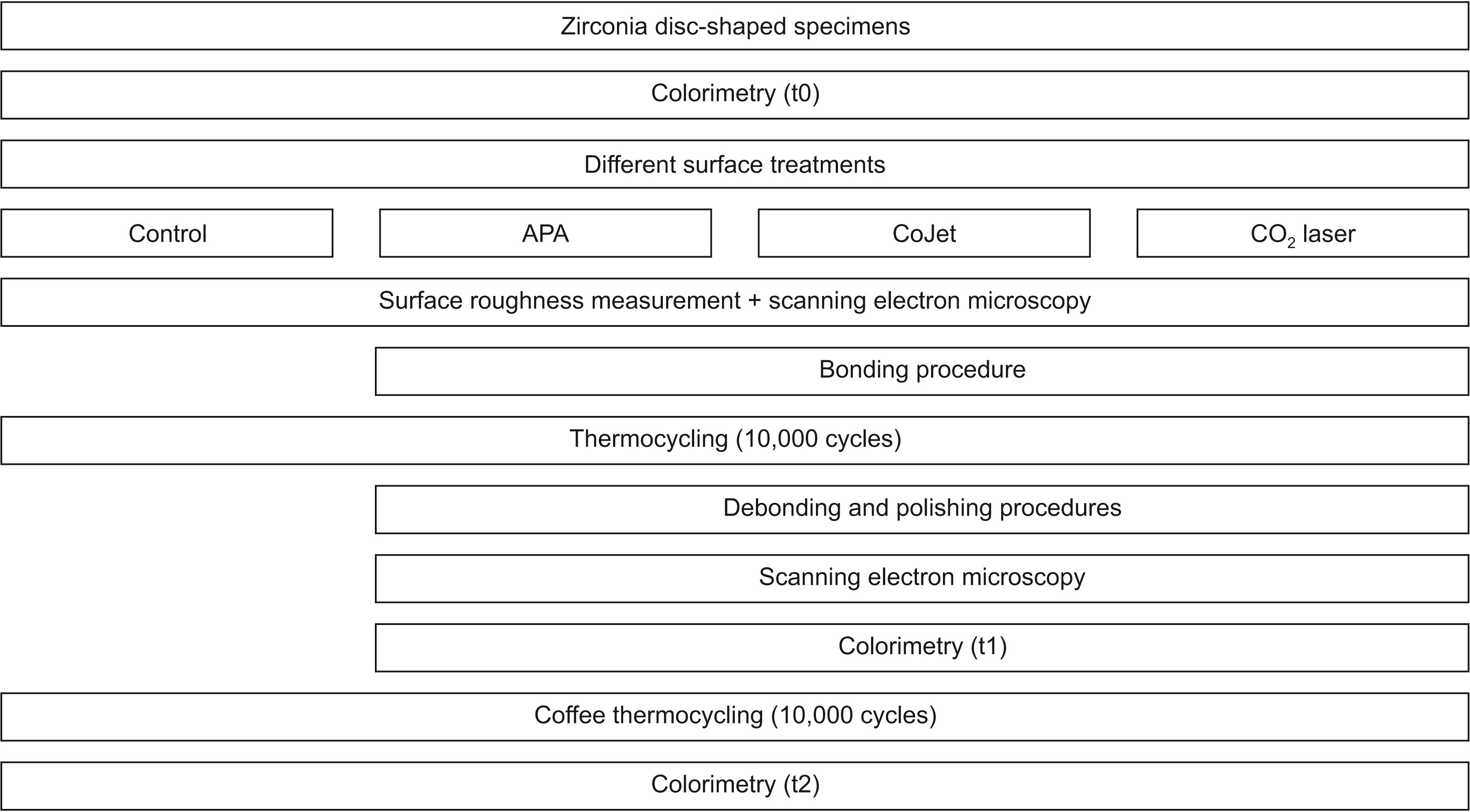
A total of 120 disc-shaped specimens with 10 mm diameter and 1 mm thickness were fabricated from pre-sintered zirconia blocks (Ceramill Zolid FX Multilayer; Amann Girrbach, Koblach, Austria) using a computer-aided design and computer-aided manufacturing (CAD-CAM) milling machine (Ceramill motion 2; Amann Girrbach), and sintered according to the manufacturer’s instructions. The bonding surface of specimens was wet-polished using 600- to 1,200-grit abrasive papers with a grinding/polishing machine (300 rpm, 15 seconds, 10-N load). The final thickness of specimens was measured by a digital caliper (Mitutoyo ABSOLUTE 500-197-20; Mitutoyo Corp., Kanagawa, Japan). The fabricated specimens were then assigned to the following four groups (n = 40) for different surface treatments: 1) Control group with no surface treatment and completely intact specimens that did not undergo orthodontic bonding; 2) APA; 3) Silica-coating with CoJet; and 4) carbon dioxide (CO2) laser.
Prior to surface treatments, the specimens were cleaned in an ultrasonic bath containing 96% isopropanol at room temperature for 3 minutes, and were then dried. In the APA group, the bonding surface of specimens was airborne-particle abraded by an intraoral sandblaster (Microsandblaster; Dento-Prep Ronvig, Daugård, Denmark) with 25-µm alumina particles from 10 mm distance with 0.25 MPa pressure, and 90-degree angle for 20 seconds.
28 In the CoJet group, 30-µm silica-coated alumina particles (CoJet sand; 3M ESPE, Seefeld, Germany) were used by an intraoral sandblaster (Microsandblaster; Dento-Prep Ronvig) on the surface from 10 mm distance with 0.25 MPa pressure, and 90-degree angle for 20 seconds.
16,28 In the laser group, the surface of specimens was covered with graphite powder (HB pencil) and subjected to CO
2 laser (DS 10UD; Daeshin, Yongin, Korea) under coolant. The laser parameters included 10,600 nm wavelength, 4 W power, 159.22 mJ energy density, 50 seconds irradiation time, 4 mm focal spot, and continuous-wave mode.
29,30
Following the surface treatments and prior to quantitative assessment of the surface, the specimens were completely rinsed, and allowed 24 hours to dry. The mean surface roughness (Ra) of specimens was measured by a profilometer (TR200; Time Group Inc., Beijing, China).
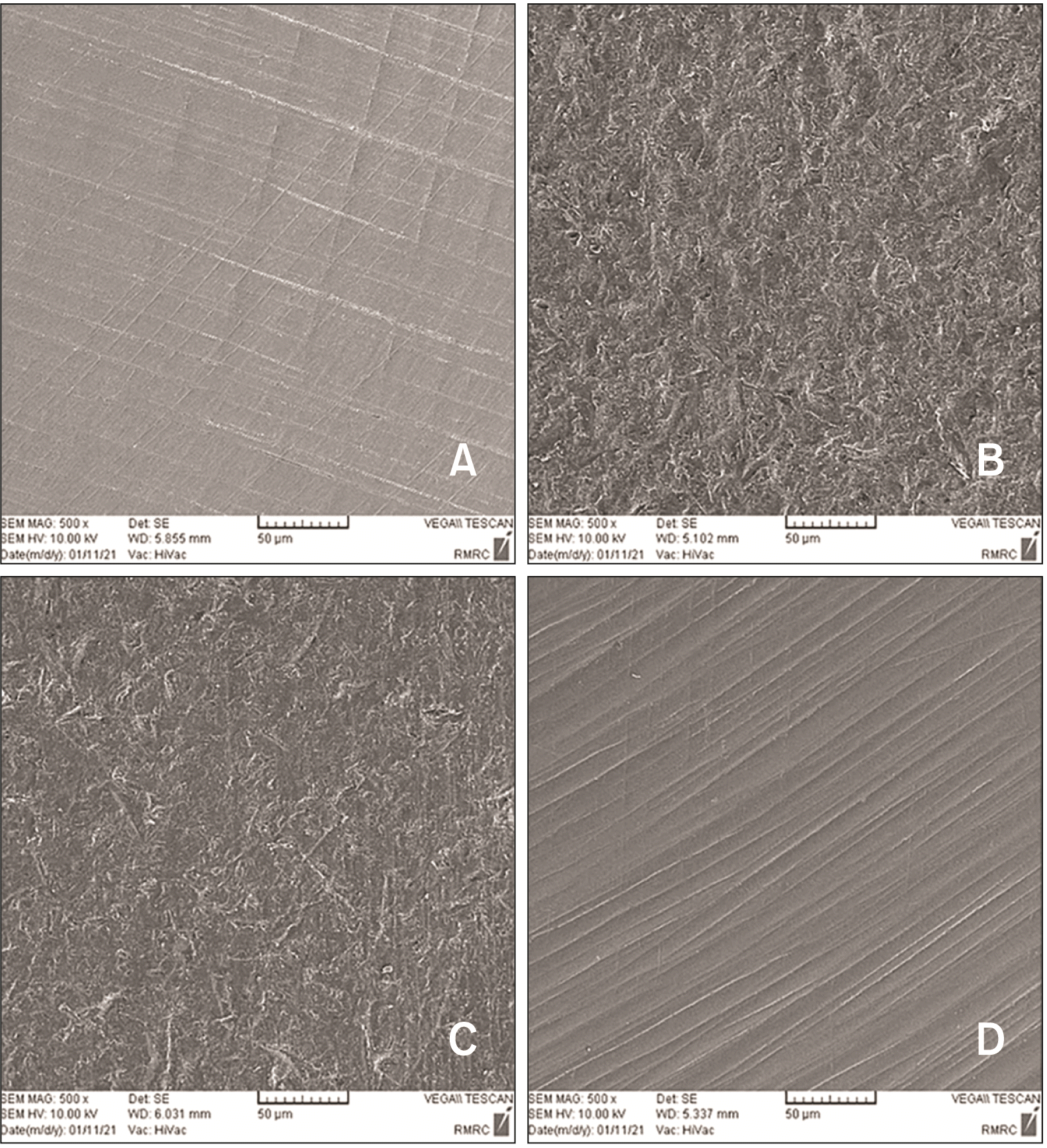
One additional specimen was fabricated in each study group for qualitative assessment of the morphological surface changes caused by each surface treatment using scanning electron microscopy (SEM, Vega; Tescan, Brno, Czech Republic). Micrographs were captured at ×500 magnification (
Figure 1).
Prior to bonding, the specimens were cleaned again in an ultrasonic bath and air-dried. The bonding process was conducted according to the manufacturer’s instructions. First, silane primer containing MDP (Clearfil Ceramic primer; Kuraray Medical, Tokyo, Japan) was applied on the bonding surface. Next, mandibular central incisor metal brackets (American orthodontics, Sheboygan, WI, USA) were bonded to the treated surface with adhesive (GC Ortho Connect; GC Orthodontics, Breckerfeld, Germany). After removal of excess adhesive, the mesial and distal surfaces of metal brackets were light-cured for 20 seconds using a curing unit (Elipar Freelight 2; 3M ESPE, St Paul, MN, USA) with a light intensity of 650 mW/cm2.
All specimens (in control and bonded groups) were stored in distilled water at 37°C for 24 hours. Next, they underwent 10,000 thermal cycles between 5–55°C with a dwell time of 30 seconds and transfer time of 10 seconds.
31 In all bonded specimens, brackets were removed by gentle force using a plier. To remove the residual adhesive, a 30-flute tungsten carbide bur (0197; Drendel and Zweiling Diamant GmbH, Lemgo, Germany) was used at low-speed (20,000 rpm)
32 under copious water irrigation.
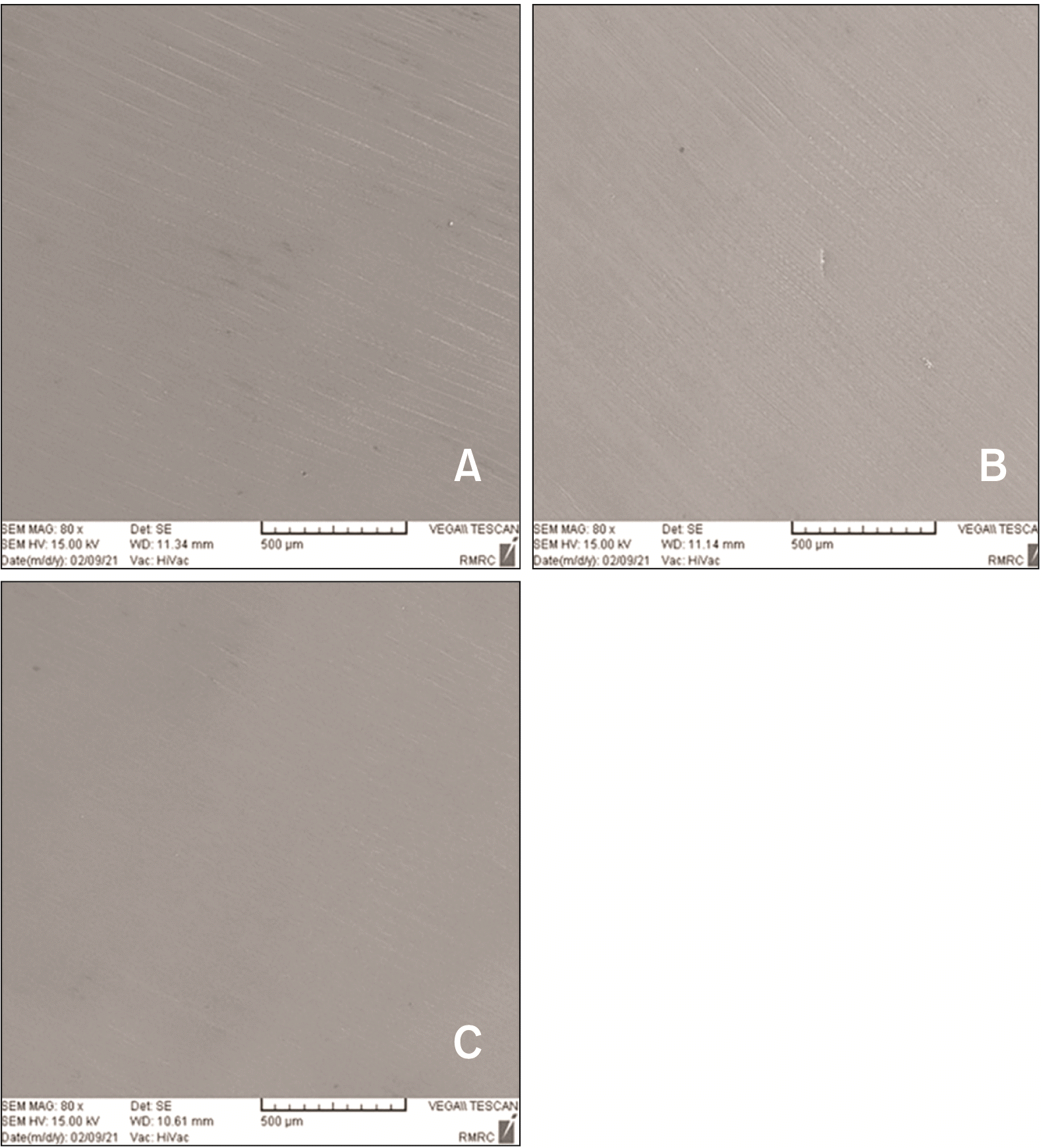
After ensuring the absence of residual adhesive on the surface by inspection under loupe magnification, polishing was performed by using a 3-step ceramic polishing system (Illume 3 Step Zirconia RA; Prima Dental Manufacturing Ltd, Gloucester, UK) with a low-speed hand-piece (10,000 rpm) with gentle pressure under water coolant. A new polishing kit was used for every 5 specimens. The specimens were polished with brushing movement in one direction for 30 seconds and in another direction with 90-degree angle relative to the previous direction for another 30 seconds. The polishing process was continued until the zirconia surface appeared smooth to the naked eye. Moreover, to ensure optimal quality of polishing, the surface of two additional specimens in each group underwent SEM assessment at ×80 magnification after polishing (
Figure 2). All procedures were performed by the same operator.
For CTC, all groups underwent 10,000 thermal cycles between 5–55°C with a dwell time of 30 seconds and a transfer time of 10 seconds. Coffee solution was prepared by adding a rounded tablespoon coffee to 177 mL of water according to previous studies.
1,13 The coffee solution was refreshed every 12 hours. After CTC, the specimens were brushed circumferentially with toothpaste under running water 12 times and dried.
1,13
A spectrophotometer was used for colorimetry (I1 Pro2 Spectrophotometer; Xrite, Grand Rapids, MI, USA). The aperture size of the instrument was 4 mm in diameter. The instrument spectral range was between 380 to 730 nm with 10 nm interval with the circumferential 0°/45° viewing geometry. Colorimetry was repeated three times for the test groups that underwent orthodontic treatment: before surface treatment (baseline or t0), after debonding and polishing (t1), and after CTC (t2), and twice for the control group: before surface treatment (baseline or t0) and after CTC (t2) (
Figure 3). Prior to the measurements, the specimens were cleaned in an ultrasonic bath and dried. A scalpel was used to create an indentation in the specimen margin at one side to mark the measurement surface and enable reproducible placement of specimens in the spectrophotometer. For colorimetry, standard black and white backgrounds (Sealed Paper Charts; Leneta, Bergen, NJ, USA) were used. The specimens were placed at the center, and measurements were repeated three times for each specimen. The L*, a*, b*, C*, and h° parameters were recorded. L* indicates lightness, a* indicates redness (positive)-greenness (negative), b* indicates yellowness (positive)-blueness (negative), C* indicates chroma, and h° indicates the hue angle. To calculate the color difference (∆E), the values measured against the white background were used with the CIEDE2000 (∆E
00) formula:
33
In this study, CIEDE2000 50% perceptibility threshold (PT) equal to 0.8 units and 50% acceptability threshold (AT) equal to 1.8 units were considered acceptable according to Paravina et al.
34
The translucency parameter (TP) was calculated according to the following formula based on the difference in L*, a*, and b* parameters measured against the standard white and black backgrounds:
Also, PT of translucency was considered to be 0.62 and AT of translucency was considered to be 2.62.
35 The TP was calculated at t0, t1, and t2 for the test groups, and t0 and t2 for the control group. The TP of the control group was used as the baseline TP for all groups.
The Shapiro–Wilk test was applied to analyze the normality of data distribution. The homogeneity of variances was evaluated by the Levene’s test. Repeated measures analysis of variance (ANOVA) was applied to compare color parameters, TP, and ∆E00 within each test group at different time points, followed by pairwise comparisons by the Bonferroni test. Paired t-test was used to compare color parameters and TP in the control group between t0 and t2. Other comparisons were made by ANOVA. Pairwise comparisons were performed by the Tukey’s test in case of homogeneity of variances, and Games–Howell test in case of non-homogeneity of variances. Accordingly, ANOVA followed by Tukey’s test (for pairwise comparisons) were applied to compare TP and ∆E00 among different groups at each time point; whereas, ANOVA followed by Games–Howell test (for pairwise comparisons) were applied to compare ∆E00t0t2, TP at t2, and ∆TP (changes in TP) based on different surface treatments. All statistical analyses were carried out using SPSS version 26 (IBM Co., Armonk, NY, USA) at 0.05 level of significance.
RESULTS
The mean and standard deviation of surface roughness were 0.13 ± 0.02 µm in the control group, 0.37 ± 0.05 µm in the APA group, 0.33 ± 0.05 µm in the CoJet group, and 0.18 ± 0.03 µm in the laser group (
Figure 1). ANOVA revealed a significant difference in this regard among the groups (
p < 0.001, F = 329.80). Pairwise comparisons showed that the surface roughness was highest in APA group, and then CoJet, laser and control in decreasing order with significant differences between the groups (
p < 0.05).
Repeated measures ANOVA indicated that the changes in L*, a*, and h° at different time points were significant within each test group (
p < 0.001). All pairwise comparisons of different time points revealed significant differences (
p < 0.001). Regarding the ascending trend of change in b* parameter from t0 to t2, significant differences between different time points were only noted in the CoJet group (
p < 0.001). This trend was not significant from t0 to t1 (
p = 0.197), but the changes from t1 to t2, and t0 to t2 were significant (
p < 0.001). Regarding the descending trend of change in C*, this change was only significant in the CoJet group such that all pairwise comparisons yielded significant differences (
p < 0.001). In the control group, all color parameters experienced a significant change between t0 to t2 according to paired
t-test (
p < 0.001). At t2, the L*, a*, C* and h° parameters significantly decreased while the b* parameter significantly increased (
Table 1).
Table 2 shows the comparison of the ∆E
00 between t0t1, t1t2, and t0t2 for different surface treatments. Results showed a significant difference in ∆E
00 between the test groups at the three time points (
p < 0.001). Pairwise comparisons of the groups showed that the ∆E
00t0t1 in the laser group was significantly lower than that in the APA (
p < 0.001) and CoJet (
p < 0.001) groups, and the difference between CoJet and APA was not significant (
p = 0.095). Regarding ∆E
00t1t2, the APA group showed a significantly higher mean value than the laser (
p < 0.001) and CoJet (
p = 0.003) groups. Also, the mean value in the CoJet group was significantly higher than that in the laser group (
p < 0.001). Comparison of ∆E
00t0t2 between the control (0.19 ± 0.02) and other groups by ANOVA revealed a significant difference among the four groups (
p < 0.001). Pairwise comparisons of the groups revealed significant differences between all groups (
p < 0.001) except for the difference between the laser and CoJet groups (
p = 0.511).
Comparison of ∆E00 within each group showed that in the APA and laser groups, the mean value during t0t1 was significantly lower than that during t1t2 (p < 0.001) and t0t2 (p < 0.001). Also, the mean value during t1t2 was significantly lower than that during t0t2 (p < 0.001). Nonetheless, in the CoJet group, only ∆E00t0t1 was significantly lower than ∆E00t1t2 (p = 0.011). Other comparisons revealed no significant difference (p > 0.05).
Regarding the TP, a significant differences were found among the three surface treatments at three assessment time points (
Table 3). At t1, TP in the laser group was significantly lower than that in the APA (
p < 0.001) and CoJet (
p < 0.001) groups. However, the difference between the APA and CoJet groups was not significant in this regard (
p > 0.05). Comparison of TP at t2 between the control and other groups by ANOVA showed no significant difference between the CoJet group and APA (
p = 0.082) and laser (
p = 0.717) groups. Nonetheless, the pairwise comparisons revealed significant differences (
p < 0.001). Comparison of time points showed that in all groups, TP significantly increased from t0 to t2 (
p < 0.001).
Table 4 compares the changes in TP (∆TP) at different time points. Significant differences were noted among different groups in ∆TPt0t1 and ∆TPt0t2 (
p < 0.001); while, this difference was not significant for ∆TPt1t2 (
p = 0.058). Pairwise comparisons showed no significant difference between the APA and CoJet groups regarding ∆TPt0t1 and ∆TPt0t2 (
p = 0.762 and
p = 0.818, respectively); however, these values were significantly lower in the laser group (
p < 0.001).
DISCUSSION
The present results showed significant effect of orthodontic bonding with different surface treatments and CTC on color parameters, color stability, and TP of FSZ. Thus, the null hypothesis of the study was rejected. In general, there is no similar study on zirconia to compare our results with. Nonetheless, some relevant studies have been conducted on enamel and feldspathic porcelain, reporting controversial results. Some studies reported that the color change caused by orthodontic treatment exceeded the clinically acceptable threshold, and even polishing could not correct it.
19,21,26 However, other studies reported that the color change was insignificant and reversible.
17,18,23,27,36
In the present study, since the amount of residual adhesive remaining on the surface of specimens after debonding was variable, it was not possible to standardize the specimens regarding the polishing time. Therefore, according to previous studies,
32,37 achieving a visually smooth surface was considered as the criterion for completion and termination of polishing. Moreover, to ensure optimal quality of polishing, two additional specimens from each group underwent SEM assessment after polishing. Nonetheless, future studies are recommended to assess the surface roughness of specimens after polishing to ensure no significant difference among the specimens in this regard.
To better simulate the clinical setting in the present study, a plier was used for debonding instead of a universal testing machine, similar to previous studies.
27,32,37 However, one previous study
26 used a universal testing machine to standardize the debonding process of specimens. It appears that using a universal testing machine decreases the generalizability of the results. For instance, the residual adhesive on the specimen surface may be much lower or higher than what it seems. Thus, although using a plier may cause higher variability after debonding in specimens, we tried to compensate for it by increasing the sample size.
The current results revealed that in comparison of different surface treatments applied, the APA group showed minimum and the laser and CoJet groups showed maximum color stability after orthodontic bonding and CTC. Minimum color change was noted when comparing before and after orthodontic treatment, and CTC had a higher contribution in discoloration of bonded specimens. The discoloration caused in control specimens following CTC was insignificant, and bonded groups showed much lower color stability than intact specimens after CTC. Nonetheless, the calculated ∆E
00 did not exceed AT or even PT in any group at any time point. Perceptibility refers to judgment regarding presence/absence of precise color match between the tooth and restoration, and lower perceptibility values indicate a color match close to perfect.
9 Acceptability refers to acceptable color change of restoration, and is within the range of PT and unacceptable color mismatch.
9 Regarding TP, the results showed that orthodontic treatment changed the TP after CTC irrespective of the type of surface treatment. TP significantly increased from t0 to t2, and the share of CTC after orthodontic treatment in this increase was lower than the share of orthodontic treatment alone. Lower share of CTC in alterations of TP was in line with the findings of a review study by Papageorgiou-Kyrana et al.,
4 who showed that irrespective of the type of zirconia, the effect of aging was insignificant and below the AT and PT in thicknesses over 0.5 mm. A significant increase in TP was also noted in the control specimens after CTC in the present study, although the ∆TP in the control group was significantly lower than that in the test groups. The ∆TP in different surface treatment groups before and after CTC (T0 to T2) was lower than AT and higher than PT; while, ∆TP was lower than PT in the control group (0.1 ± 0.02). This finding highlighted the significant effect of orthodontic treatment on susceptibility of zirconia to change in translucency following aging. The increase in TP was in line with the reduction in L* parameter. According to the Lambert’s law, decreasing the thickness decreases light absorption and subsequently increases light transmission.
33
Similar to the present study, several other studies reported that the discoloration of zirconia due to CTC or UV aging alone or CTC following clinical adjustment was lower than AT,
1,9,12,13,37 and showed that translucency increased with aging and also with increased frequency of thermal cycles.
7,8,12,31 Conversely, Turgut
9 reported that the translucency of monolithic zirconia decreased by 1.8 to 6.0 units due to UV aging. Al-Zordk and Saker
37 reported a reduction in translucency between 0.42 to 1.51 units due to clinical adjustment and subsequent CTC. Variations in the results can be due to the use of different types of zirconia and different aging protocols.
The present results indicated that all test groups under orthodontic treatment had higher translucency than the control group after CTC. This finding may be due to two reasons. The first reason may be the higher surface roughness of specimens that underwent orthodontic treatment. It has been demonstrated that smoother surfaces have higher light reflection, less passage of light, and lower translucency.
38,39 Surface roughness can affect the translucency of monolithic CAD/CAM restorations such as zirconia.
40 Moreover, it has been demonstrated that despite polishing after orthodontic treatment, the surface roughness of the underlying substrate may not reach the baseline value.
26,41 Thus, specimens that underwent surface treatments exhibited increase in surface roughness leading to greater increase in translucency compared with the control group. The second reason may be the thickness of material, because lower thickness is associated with higher translucency.
39 The surface treatments and debonding processes followed by the final finishing and polishing might have decreased the specimen thickness and increased the translucency.
In the present study, only one type of polishing kit and method on a limited thickness of zirconia was evaluated. Moreover, this study had an inherent limitations of in vitro study design which could not take intraoral factors such as oral hygiene, saliva, pH, and temperature into account. Also, both surfaces of zirconia were stained in our study whereas in a clinical environment, only one surface would be stained as the other surface would be bonded to a tooth. Thus, an in vivo study is warranted to further evaluate the color properties using different polishing techniques on various thicknesses of zirconia.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download