Abstract
Backgrounds/Aims
Postoperative pancreatic fistula (POPF) after pancreaticoduodenectomy (PD) is a source of major morbidity and mortality. Early diagnosis and treatment of POPF is mandatory to improve patient outcomes and clinical risk scores may be ombined with postoperative drain fluid amylase (DFA) values to stratify patients. The aim of this pilot study was to etermine if intraoperative fluid amylase (IFA) values correlate with DFA1 and POPF.
Methods
In patients undergoing PD from February to November 2020, intraoperative samples of intra-abdominal fluid adjacent to the pancreatic anastomosis were taken and sent for fluid amylase measurement prior to abdominal closure. Data regarding patient demographics, postoperative DFA values, complications, and mortality were prospectively collected.
Results
Data were obtained for 52 patients with a median alternative Fistula Risk Score (aFRS) of 9.9. Postoperative complications occurred in 20 (38.5%) patients (five Clavien grade ≥ 3). There were eight POPFs and two patients died (pneumonia/sepsis). There was a significant correlation between IFA and DFA1 (R2 = 0.713; p < 0.001) and DFA3 (p < 0.001), and the median IFA was higher in patients with POPF than patients without (1,232.5 vs. 122; p = 0.0003). IFA > 260 U/L predicted POPF with sensitivity, specificity, positive and negative predictive values of 88.0%, 75.0%, 39.0%, and 97.0%, respectively. The incidence of POPF was 43.0% in high-risk (high aFRS/IFA) and 0% in lowrisk patients (low aFRS/IFA).
Pancreaticoduodenectomy (PD) is a potentially curative treatment for tumors in the pancreatic head and periampullary region [1,2], but is associated with perioperative morbidity rates of 40%–58% even in high volume centers [3]. Postoperative pancreatic fistula (POPF) remains the most important surgical complication after PD and may be associated with organ failure, prolonged hospitalization, and mortality [4]. Several factors, including body mass index, pancreatic duct width, and texture affect the incidence of POPF and have been incorporated into various clinical risk scores [5]. A range of mitigation strategies, such as peri-anastomotic drainage, pancreatic duct stents (internal or external), and somatostatin analogues have been adopted to reduce the incidence and/or clinical impact of POPF in high-risk patients, although evidence to support these approaches is limited [6].
POPF has been defined as the presence of amylase-rich fluid on or after the third postoperative day [7], although it is clear that the first postoperative day drain fluid amylase (DFA) is an accurate predictor of POPF and may be used to guide early postoperative management [8]. Indeed, a randomized trial of early versus late drain removal in patients with a low DFA postoperative day 1 demonstrated significantly lower postoperative morbidity in the early drain removal group [9]. Routine drainage, after a range of abdominal operations including liver resection and colectomy [10], is unnecessary and potentially harmful. However, this does not seem to apply to patients undergoing PD, and a recent randomized trial was stopped early due to higher mortality in the no-drain group [11]. Since the vast majority of POPFs may be detected on the first postoperative day after PD, this may indicate that POPFs usually occur due to immediate anastomotic failure rather than ischemia. Hence, we hypothesize that it may be possible to detect pancreatic juice intraoperatively in the peri-anastomotic region after completion of the pancreatic anastomosis, and that the presence of peri-anastomotic pancreatic juice may correlate with POPF. Therefore, the aim of this study was to evaluate if intraoperative fluid amylase (IFA) levels correlated with postoperative DFA and/or POPFs.
Data were collected prospectively from consecutive patients who underwent PD at University Hospital Birmingham, UK, from February to November 2020 (including patients admitted to the Priory Hospital Birmingham during the COVID-19 pandemic). The study was registered as an audit with the Trust Clinical Audit Department (CARMS number 15827), and ethical approval was not required. The study was approved by our Institution Review Board and ethical procedure in accordance with guidelines set out by research at University Hospitals Birmingham and clinical audit management team with CARMS number 15827. Preoperative data was collected regarding patient demographics, comorbidity, indication for surgery, preoperative biliary stenting, neoadjuvant chemotherapy and body mass index. The alternate Fistula Risk Score (aFRS) was calculated for each patient. Patients underwent either a pylorus-preserving PD (PPPD) or classical Whipple (CW) according to surgeon preference. Pancreatic reconstruction was carried out using a pancreaticojejunal anastomosis (PJA) on a single jejunal loop in all cases, with either a duct-mucosa or dunking technique, according to individual surgeon preference [12]. After completion of the PJA, the upper abdominal cavity was thoroughly irrigated with a minimum of 500 mL sterile water and suctioned to remove any trace of pancreatic juice that had accumulated between transection of the pancreatic neck and completion of the PJA. Reconstruction was then completed with an end-side hepaticojejunostomy and either an antecolic end-side duodenojejunostomy (PPPD) or retrocolic gastrojejunostomy (CW). After completion of both biliary and gastric anastomoses and before final irrigation and suction, two 5 mL samples of abdominal fluid were taken immediately adjacent to the PJA (cranial and caudal to PJA) and sent for amylase analysis (IFA). Following irrigation and suction, one or two large bore passive drains were positioned adjacent to the PJA prior to abdominal wound closure. Postoperative management followed an Enhanced Recovery After Surgery pathway, which has been described previously [13], and included drain management that was dictated by drain fluid amylase values on the first (DFA1) and third (DFA3) postoperative days. Data regarding postoperative complications were collected prospectively and classified according to Clavien-Dindo [14]. POPF was defined and graded according to the 2016 update of the International Study Group of Pancreatic Fistula (ISGPF) [15].
Continuous data were reported as median and interquartile ranges (IQR). Pearson correlation was used to compare IFA with DFA1/DFA3. Receiver operating characteristic (ROC) analysis was used to detemine optimal cut-off values of IFA to predict or exclude POPF. Categorical and continuous variables were analyzed using Mann–Whitney U and Fisher’s exact test, respectively. All analyses were performed using IBM SPSS 25 (IBM Corp., Armonk, NY, USA), with p < 0.05 deemed to be indicative of statistical significance throughout. Patients with missing data were excluded on a per analysis basis.
A total of 52 consecutive patients underwent PD (49 PPPD, 3 CW) during the study period (mean age 63.5 years; 53.8% female). The indication for surgery was pancreatic adenocarcinoma (29), ampullary adenocarcinoma (14), duodenal adenocarcinoma (6), and intraductal papillary mucinous neoplasm (3). Table 1 represents demographic analysis based on IFA ≥ 260. The median aFRS score was 9.9 (4.9–19.65) and the PJA was fashioned by either duct-to-mucosa (38) or dunking anastomoses (14). Out of 52, 20 patients (38.5%) developed postoperative complications (Clavien-Dindo grades 1–2: 15; grade ≥ 3: 5), including eight POPF (15.4%; biochemical leak, five; clinically relevant [CR], three) and delayed gastric emptying in five patients. One patient required reoperation due to POPF and two patients (3.8%) died within 90 days (COVID-19 infection, one; hospital-acquired pneumonia/sepsis, one).
The median IFA values cranial and caudal to the pancreatic anastomosis were 61.75 U/L (IQR: 136.0–368.5 U/L) and 50 U/L (IQR: 107.5–408.5 U/L), respectively. The highest value of IFA (either cranial or caudal to the anastomosis) was incorporated into subsequent analysis. There was a significant positive correlation between IFA and both DFA1 (Pearson’s correlation: R2 = 0.713; p < 0.001) and DFA3 (p < 0.001) (Fig. 1). Median IFA was significantly higher in patients who developed POPF (1,232.5, IQR 695.3–1,889.0) compared to those without POPF (122, IQR 61.3–168.8; p = 0.0003). ROC analysis identified optimal cut-off values for IFA of 236 U/L and 260 U/L for predicting DFA1 > 350 and POPF, respectively (Fig. 2, 3). The sensitivity, specificity, positive and negative predictive values of IFA in predicting DFA1 > 350 were 82.0%, 83.0%, 70.0%, and 90.0%, respectively. The sensitivity, specificity, positive and negative predictive values of IFA in predicting POPF were 88.0%, 75.0%, 39.0%, and 97.0%, respectively.
The incidence of POPF in high-risk (aFRS ≥ 9.9) and low-risk patients (aFRS < 9.9) was 26.9% (7/26) and 3.8% (1/26), respectively. A combination of both aFRS and IFA ≥ 260 improved the clinical utility of aFRS, since the incidence of POPF was 43.0% in patients with both high aFRS and high IFA, while the incidence of POPF was 0% (0/22) in patients with both low aFRS and low IFA.
The main finding of this study is that IFA levels strongly correlated with early postoperative DFA levels (on the first postoperative day) and POPF. This finding suggests that, at least in a proportion of patients, POPF may be detected almost immediately after completion of the pancreatic anastomosis, indicating anastomotic failure rather than ischemia, and concurs with data from a previous study [16]. It is theoretically possible that the fluid amylase detected intraoperatively may occur due to pancreatic trauma and/or pancreatitis, rather than from an immediate anastomotic leak. There has been some interest in the concept of postoperative pancreatitis after PD, but this may be a biochemical phenomenon rather than a CR complication, since studies adopted a very low threshold value of serum amylase (>100 U/L) to define postoperative pancreatitis [17,18], and results do not appear to be reproducible [19]. A high IFA predicted POPF with a high sensitivity and specificity, while a low IFA (< 260 U/L) excluded a POPF with a negative predictive value of 97.0%. The relationship between IFA and POPF has potential clinical utility. POPF is a major source of morbidity after PD, and there are several available risk scores that stratify patients prior to surgery. The cornerstone of management of POPF is early detection and aggressive management. However, there are several intraoperative mitigation strategies that have been advocated which aim to prevent or reduce the clinical impact of POPF, including pancreatic duct stenting, peri-anastomotic drainage, and total pancreatectomy [6]. These interventions are not entirely without risk, and although routine drainage is advocated after PD, prolonged drainage is associated with significantly increased morbidity [9], which is presumably due to ascending infection along the drain. Early drain removal is therefore advisable in patients without a POPF and is usually based on postoperative DFA levels [9,20-23]. Measurement of IFA may provide additional information to established preoperative risk scores, and may even facilitate a no-drain strategy for low-risk patients with a low IFA. It would be necessary to compare such a strategy with early drain removal in a prospective randomized trial. By contrast, in high-risk patients, a high IFA may prompt mitigation strategies, such as an external pancreatic duct stent [6], multiple peri-anastomotic drains, or even a total pancreatectomy. A recent study by Marchegiani et al. (2022) [24], compared short and long-term outcomes between PD and total pancreatectomy and concluded that completion total pancreatectomy could be considered, but only in a few selected patients at very high risk of POPF. Additional prognostic information, provided by IFA measurement, may help to identify a subgroup of patients at very high risk of a CR-POPF, in whom a completion total pancreatectomy may be justified. To our knowledge, this is only the second study to evaluate the clinical utility of IFA, and our findings were comparable to those by de Reuver et al. [16]. A potential logistic issue with IFA analysis is that the sample is obtained near the end of the surgical procedure and may take 1–2 hours to be analyzed, potentially preventing implementation of intraoperative mitigation strategies. Thus, at present, the utility of IFA is limited to influencing early postoperative management (i.e. on the evening of surgery), such as early drain removal in low-risk patients.
There are two main limitations of this study. First, due to its small sample size, it was not possible to identify a cut-off value for IFA that predicted CR-POPF, and therefore larger cohort studies are warranted to investigate this. Nonetheless, despite the small sample size, IFA significantly correlated with both DFA1 and POPF in this study, suggesting that IFA is a valid tool and may have clinical utility. Second, a rapid bedside fluid amylase assay is not currently available, and therefore in this study the IFA result only became available postoperatively. If the clinical utility of IFA is confirmed by larger studies, then there would be a need to develop a rapid assay so that the result could be used to guide intraoperative mitigation strategies.
In conclusion, measurement of IFA after completion of the pancreatic anastomosis during PD may be a useful adjunct to clinical fistula risk scores, potentially allowing earlier risk stratification. High-risk patients may benefit from intraoperative mitigation strategies, while low-risk patients may be selected for a no-drain strategy. Large, prospective, multi-center studies would be required to further evaluate the clinical applicability of IFA measurement.
In conclusion, IFA values closely correlated with POPF and may be a useful adjunct to clinical risk scores to stratify patients during PD. Larger, prospective studies are needed to validate the findings of this study and to determine if IFA may have a role in guiding mitigation strategies during PD.
ACKNOWLEDGEMENTS
The Preliminary findings were presented at the 14th Congress of the European-African Hepato-Pancreato-Biliary Association (E-AHPBA); 15–17 September 2021, Bibalo, Spain. HPB 2021;23(Suppl 3):728-729.
REFERENCES
1. Tsai CY, Lai BR, Wang SY, Liao CH, Liu YY, Kang SC, et al. 2017; The impact of preoperative etiology on emergent pancreaticoduodenectomy for non-traumatic patients. World J Emerg Surg. 12:21. DOI: 10.1186/s13017-017-0133-6. PMID: 28469698. PMCID: PMC5414322.

2. Whipple AO, Parsons WB, Mullins CR. 1935; Treatment of carcinoma of the ampulla of vater. Ann Surg. 102:763–779. DOI: 10.1097/00000658-193510000-00023. PMID: 17856666. PMCID: PMC1391173.
3. Lubrano J, Bachelier P, Paye F, Le Treut YP, Chiche L, Sa-Cunha A, et al. 2018; Severe postoperative complications decrease overall and disease free survival in pancreatic ductal adenocarcinoma after pancreaticoduodenectomy. Eur J Surg Oncol. 44:1078–1082. DOI: 10.1016/j.ejso.2018.03.024. PMID: 29685757.

4. Khajanchee YS, Johnston WC, Cassera MA, Hansen PD, Hammill CW. 2017; Characterization of pancreaticojejunal anastomotic healing in a porcine survival model. Surg Innov. 24:15–22. DOI: 10.1177/1553350616674638. PMID: 27794116.

5. Callery MP, Pratt WB, Kent TS, Chaikof EL, Vollmer CM Jr. 2013; A prospectively validated clinical risk score accurately predicts pancreatic fistula after pancreatoduodenectomy. J Am Coll Surg. 216:1–14. DOI: 10.1016/j.jamcollsurg.2012.09.002. PMID: 23122535.

6. Ecker BL, McMillan MT, Asbun HJ, Ball CG, Bassi C, Beane JD, et al. 2018; Characterization and optimal management of high-risk pancreatic anastomoses during pancreatoduodenectomy. Ann Surg. 267:608–616. DOI: 10.1097/SLA.0000000000002327. PMID: 28594741.
7. Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, Izbicki J, et al. 2005; Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery. 138:8–13. DOI: 10.1016/j.surg.2005.05.001. PMID: 16003309.

8. Israel JS, Rettammel RJ, Leverson GE, Hanks LR, Cho CS, Winslow ER, et al. 2014; Does postoperative drain amylase predict pancreatic fistula after pancreatectomy? J Am Coll Surg. 218:978–987. DOI: 10.1016/j.jamcollsurg.2014.01.048. PMID: 24680573.

9. Bassi C, Molinari E, Malleo G, Crippa S, Butturini G, Salvia R, et al. 2010; Early versus late drain removal after standard pancreatic resections: results of a prospective randomized trial. Ann Surg. 252:207–214. DOI: 10.1097/SLA.0b013e3181e61e88. PMID: 20622661.
10. Petrowsky H, Demartines N, Rousson V, Clavien PA. 2004; Evidence-based value of prophylactic drainage in gastrointestinal surgery: a systematic review and meta-analyses. Ann Surg. 240:1074–1084. discussion 1084–1085. DOI: 10.1097/01.sla.0000146149.17411.c5. PMID: 15570212. PMCID: PMC1356522.
11. Van Buren G 2nd, Bloomston M, Hughes SJ, Winter J, Behrman SW, Zyromski NJ, et al. 2014; A randomized prospective multicenter trial of pancreaticoduodenectomy with and without routine intraperitoneal drainage. Ann Surg. 259:605–612. DOI: 10.1097/SLA.0000000000000460. PMID: 24374513.

12. Shukla PJ, Barreto SG, Fingerhut A, Bassi C, Büchler MW, Dervenis C, et al. 2010; Toward improving uniformity and standardization in the reporting of pancreatic anastomoses: a new classification system by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 147:144–153. DOI: 10.1016/j.surg.2009.09.003. PMID: 19879614.

13. Sutcliffe RP, Hamoui M, Isaac J, Marudanayagam R, Mirza DF, Muiesan P, et al. 2015; Implementation of an enhanced recovery pathway after pancreaticoduodenectomy in patients with low drain fluid amylase. World J Surg. 39:2023–2030. DOI: 10.1007/s00268-015-3051-3. PMID: 25809067.

14. Dindo D, Demartines N, Clavien PA. 2004; Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 240:205–213. DOI: 10.1097/01.sla.0000133083.54934.ae. PMID: 15273542. PMCID: PMC1360123.
15. Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, et al. 2017; The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery. 161:584–591. DOI: 10.1016/j.surg.2016.11.014. PMID: 28040257.

16. de Reuver PR, Gundara J, Hugh TJ, Samra JS, Mittal A. 2016; Intra-operative amylase in peri-pancreatic fluid independently predicts for pancreatic fistula post pancreaticoduodectomy. HPB (Oxford). 18:608–614. DOI: 10.1016/j.hpb.2016.05.007. PMID: 27346142. PMCID: PMC4925798.

17. Chen H, Wang W, Ying X, Deng X, Peng C, Cheng D, et al. 2020; Predictive factors for postoperative pancreatitis after pancreaticoduodenectomy: a single-center retrospective analysis of 1465 patients. Pancreatology. 20:211–216. DOI: 10.1016/j.pan.2019.11.014. PMID: 31831390.

18. Bannone E, Andrianello S, Marchegiani G, Masini G, Malleo G, Bassi C, et al. 2018; Postoperative acute pancreatitis following pancreaticoduodenectomy: a determinant of fistula potentially driven by the intraoperative fluid management. Ann Surg. 268:815–822. DOI: 10.1097/SLA.0000000000002900. PMID: 30004917.
19. Yoo D, Park SY, Hwang DW, Lee JH, Song KB, Lee W, et al. 2021; Lack of association between postoperative pancreatitis and other postoperative complications following pancreaticoduodenectomy. J Clin Med. 10:1179. DOI: 10.3390/jcm10061179. PMID: 33799863. PMCID: PMC8001526.

20. Liu Y, Li Y, Wang L, Peng CJ. 2018; Predictive value of drain pancreatic amylase concentration for postoperative pancreatic fistula on postoperative day 1 after pancreatic resection: an updated meta-analysis. Medicine (Baltimore). 97:e12487. DOI: 10.1097/MD.0000000000012487. PMID: 30235751. PMCID: PMC6160246.
21. Bertens KA, Crown A, Clanton J, Alemi F, Alseidi AA, Biehl T, et al. 2017; What is a better predictor of clinically relevant postoperative pancreatic fistula (CR-POPF) following pancreaticoduodenectomy (PD): postoperative day one drain amylase (POD1DA) or the fistula risk score (FRS)? HPB (Oxford). 19:75–81. DOI: 10.1016/j.hpb.2016.10.001. PMID: 27825541.

22. Sutcliffe RP, Battula N, Haque A, Ali A, inivasan P Sr, Atkinson SW, et al. 2012; Utility of drain fluid amylase measurement on the first postoperative day after pancreaticoduodenectomy. World J Surg. 36:879–883. DOI: 10.1007/s00268-012-1460-0. PMID: 22354484.

23. Conlon KC, Labow D, Leung D, Smith A, Jarnagin W, Coit DG, et al. 2001; Prospective randomized clinical trial of the value of intraperitoneal drainage after pancreatic resection. Ann Surg. 234:487–493. discussion 493–494. DOI: 10.1097/00000658-200110000-00008. PMID: 11573042. PMCID: PMC1422072.

24. Marchegiani G, Perri G, Burelli A, Zoccatelli F, Andrianello S, Luchini C, et al. 2022; High-risk pancreatic anastomosis versus total pancreatectomy after pancreatoduodenectomy: postoperative outcomes and quality of life analysis. Ann Surg. 276:e905–e913. DOI: 10.1097/SLA.0000000000004840. PMID: 33914471.
Fig. 1
Scatter plot showing logarithmic distribution. DFA1, drain fluid amylase values on the first postoperative day; IFA, intraoperative fluid amylase.
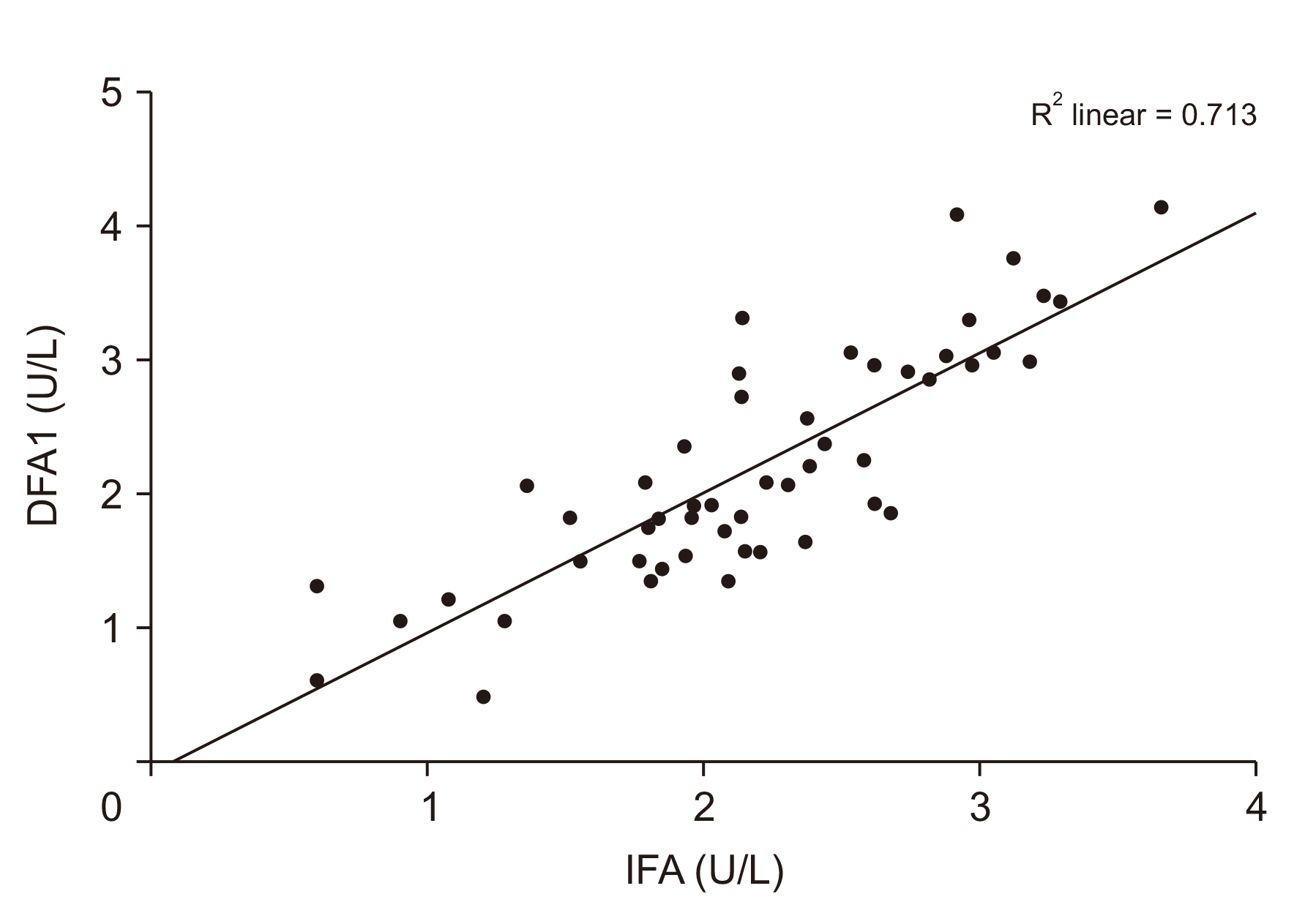
Fig. 2
Receiver operating characteristic (ROC) curve for intraoperative fluid amylase (IFA) and DFA1 > 350. AUC of 0.921 with a 95% confidence interval of 0.848 to 0.994, p < 0.001. DFA1, drain fluid amylase values on the first postoperative day; AUC, area under the ROC curve.
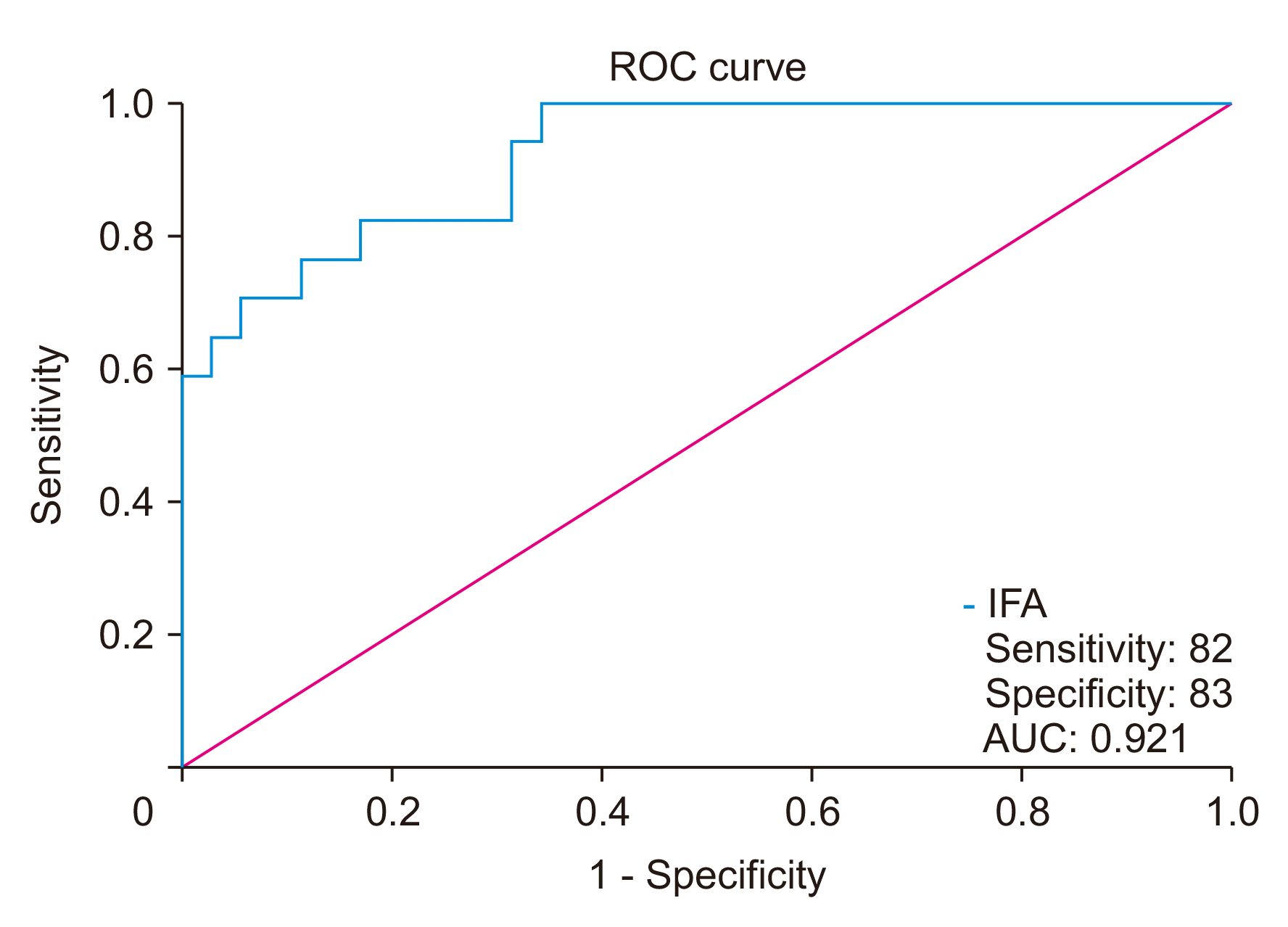
Fig. 3
Receiver operating characteristic (ROC) curve for intraoperative fluid amylase (IFA) and development of POPF. AUC of 0.905 with a 95% confidence interval of 0.794 to 1.000, p < 0.001. POPF, postoperative pancreatic fistula; AUC, area under the ROC curve.
Table 1
Patient demographics
| Demographic | All patients (n = 52) | Raised intraoperative fluid amylase (≥ 260 U/L) (n = 18) | Low intraoperative fluid amylase (< 260 U/L) (n = 34) | p-value |
|---|---|---|---|---|
| Age (yr) | 68 ± 8 | 68 ± 8 | 68 ± 9 | 0.963 |
| Male | 24 (46.2) | 7 (38.9) | 17 (50.0) | 0.562 |
| Charlson comorbidity score | - | 5 (2) | 5 (1) | 0.17 |
| Indication for surgery | 0.390 | |||
| Pancreatic adenocarcinoma | 30 (57.7) | 12 (66.7) | 18 (52.9) | |
| Other | 22 (42.3) | 6 (33.3) | 16 (47.1) | |
| Resection type | 0.543 | |||
| Classic Whipple | 3 (5.8) | 0 (0) | 3 (8.8) | |
| PPPD | 49 (94.2) | 18 (100) | 31 (91.2) | |
| Anastomosis type | 0.329 | |||
| Duct-Mucosa | 38 (73.1) | 15 (83.3) | 23 (67.6) | |
| Dunking | 14 (26.9) | 3 (16.7) | 11 (32.4) | |
| Preoperative haemoglobin (g/L) | - | 117.5 ± 23.6 | 115.8 ± 20.0 | 0.78 |
| Preoperative bilirubin (µmol/L) | - | 96.2 ±112.4 | 188.0 ± 181.0 | 0.05 |
| Preoperative albumin (g/L) | - | 30.2 ± 9.6 | 29.2 ± 7.9 | 0.69 |
| Preoperative creatinine (µmol/L) | - | 72.6 ± 25.8 | 66.0 ± 27.2 | 0.46 |
| BMI (kg/m2) | - | 27.4 ± 4.6 | 26.9 ± 6.0 | 0.77 |
| Pancreatic duct diameter (mm) | - | 2.56 ± 1.54 | 3.65 ± 1.32 | 0.01* |
| Pancreatic texture | 0.046* | |||
| Firm | - | 7 (38.9) | 23 (67.6) | |
| Soft | - | 11 (61.1) | 11 (32.4) | |
| Alternative fistula risk score | - | 17.5 (18) | 7.15 (12.98) | 0.022* |
| Operative time (min) | - | 310 ± 60 | 331 ± 73 | 0.31 |
| Perioperative blood transfusion | - | 2 (11.1) | 5 (14.7) | 0.718 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download