Abstract
Backgrounds/Aims
Cancer stigma (CS), a self-inflicted sense of hopelessness, has been identified as a major factor affecting cancer patients’ outcomes. However, few studies have investigated the CS-related outcomes in hepatobiliary and pancreatic (HBP) cancer. Thus, the aim of this study was to investigate effects of CS on quality of life (QoL) of HBP cancer.
Methods
From 2017 to 2018, 73 patients who underwent curative surgery for HBP tumor at a single intuitive were enrolled prospectively. The QoL was measured using the European Organization for Research and Treatment of Cancer QoL score, and CS was evaluated in three categories, “impossibility of recovery,” “cancer stereotypes,” and “social discrimination.” the stigma was defined by higher scores of attitudes compared with the median value.
Results
The stigma group showed a lower QoL (–17.67, 95% confidence interval [CI]: –26.75 to 8.60, p < 0.001) than the no stigma group. Similarly, most function and symptoms of the stigma group showed worse results than the no stigma group. The difference in function scores between the two groups according to CS was highest in cognitive function (–21.20, 95% CI: –30.36 to 12.04, p < 0.001). Fatigue showed the largest difference between the two groups at 22.84 (95% CI: 12.88–32.07, p < 0.001) and was the most severe symptom in stigma group.
Hepatobiliary and pancreatic (HBP) cancer refers to pancreatic, periampullary, gallbladder, and hilar bile duct cancers. It exhibits poorer prognosis than other digestive tract cancers. The five-year survival rate for HBP cancer is low. Deaths due to HBP cancer are increasing in western countries and Korea [1,2]. The social burden for HBP cancer is also increasing [3,4]. Despite advances in surgical and medical treatments for HBP cancer, survival rate of patients with HBP cancer is still lower than that of patients with other malignancies [5]. Additionally, patients with HBP cancer have a quality of life (QoL) similar to or lower than patients diagnosed with esophageal, gastric, or colorectal cancers [6-8].
In cancers with a dismal prognosis, changing the treatment and patient care strategy can increase the number of long-term survivors [9,10]. Due to increased survival rates, it is necessary to evaluate effects of treatment on QoL of patients [10,11]. Furthermore, QoL is a measurable prognostic factor for long-term survival in many chronic diseases including cancer [12-14]. Factors affecting the QoL of cancer survivors include their symptoms [15], depression [10], and cancer stigma (CS) [16]. Stigma is a concept characterized by negative behaviors and social stereotypes, typically seen in patients suffering from chronic diseases [17,18]. In particular, CS is described as a hopeless self-inflicted feeling. It is considered to be a major factor affecting the outcome of cancer patients [19].
Despite advances in medical treatment and improved long-term survival rates, fear and CS remain prevalent in patients with HBP cancer [20]. For patients and their families, the diagnosis alone can negatively affect cancer treatment. Studies analyzing the importance of CS in the long-term prognosis of patients with HBP cancer have not been reported yet. Thus, the purpose of this study was to determine the prevalence of CS in HBP cancer patients and its impact on disease prognosis, including their QoL, physical and psychological functions, and symptoms.
A prospective survey was conducted for patients who visited the outpatient clinic following surgery at a single tertiary hospital from October 2017 to March 2018. Patient aged between 20 and 65 years who underwent surgery for HBP cancer and borderline malignancy diagnosis were recruited for this retrospective study. Most patients underwent a complete pancreatectomy or hepatectomy. Patients with a periampullary cancer underwent a pancreaticoduodenectomy and patients with a left-sided pancreatic cancer underwent a distal pancreatectomy. Patients with a hilar cholangiocarcinoma underwent bile duct resection with or without major hepatectomy. This study was approved by the Institutional Review Board of the Samsung Medical Center (approval no. 2019-02-041). This study was conducted after obtaining IRB approval and informed consent from patients prior to the start of the survey.
Stigma
CS was assessed using the questionnaire described by Cho et al. [20], which consisted of 12 questions in three domains: (1) impossibility of recovery; (2) stereotypes; and (3) experience of discrimination. In terms of impossibility of recovery, four questions were used to assess the impossibility of recovery, likelihood of cure, impossibility of social activities, and impaired task ability at work. The stereotypes about cancer patients were evaluated with the following 4 questions. The questionnaire consisted of questions about perception of cancer patients (identifying a person with cancer by their appearance), cancer patients' sexual intimacy, cancer patients' vulnerability, and social contribution of cancer patients. These questions were assessed using a 4-point Likert scale ranging from 1 point (strongly disagree) to 4 points (strongly agree). We calculated average score of 12 items. We then divided patients into a no stigma group and a stigma group according to the median score (> 1.375).
To assess social discrimination, we used dichotomous questions to ask whether patients’ friends tended to avoid interactions with them, whether neighbors avoided interactions with them, whether they had problems within their family or marriage, and whether employers or coworkers discriminated against them because of their cancer diagnosis.
QoL was measured using the European Organization for Research and Treatment of Cancer (EORTC) Quality of Life Questionnaire Core 30 (QLQ-C30), which was translated into Korean and validated [21]. QoL functional and symptom scores ranged from 0 to 100, with a higher score on the EORTC representing a better QoL or a better level of functioning. A higher score on the EORTC also represented a more severe level of symptom. We scored EORTC QLQ-C30 answers according to a scoring manual [22]. Data were linearly transformed to yield scores ranging from 0 to 100. We analyzed incomplete questionnaires according to developers’ recommendations. A higher score indicated a better status of the functioning domain but a worse status for the symptom domain. QoL mean and the proportion of ‘problematic groups’ in each QoL scale were also determined. Problematic groups were defined as scores that were lower than 66 in global health status/QoL and better functioning and those higher than 33 on the symptom score. To interpret QoL scores, we defined ‘clinically significant’ difference in QoL as a 10-point difference in mean score [22].
Patients’ sociodemographic characteristics including gender, age, marital status, education, current employment status, and current monthly family income were obtained for this study. Clinical data such as primary cancer subtype, stage, type of surgery, adjuvant treatment, and treatment-related complications were obtained from each patient’s electronic medical records.
To assess stigma, the mean and standard deviation were calculated for each item in each domain for impossibility of recovery and stereotypes of cancer patients. Descriptive statistics were used to report social discrimination and prevalence of unemployment.
We used univariable and multivariable linear regression models to identify the association between CS and QoL, including functions and symptoms. All statistical analyses were performed using the Stata 14 software (StataCorp LP). We used two-sided p-values. p < 0.05 was considered statistically significant.
Among 75 recruited patients, two participants who did not undergo surgery were excluded. Thus, we performed analysis for the remaining 73 patients. The median score of CS was 1.375. Compared to the no stigma group, patients with stigma were more likely to be females (p = 0.05). The number of working patients throughout the data collection period was significantly higher in the no stigma group (p = 0.014). Among the total patients, 62 (84.9%) were diagnosed with cancer, including 22 (35.5%) who had pancreatic ductal adenocarcinoma. A total of 40 (64.6%) patients had advanced stage II or higher. There were no significant differences in rates of postoperative complications or survival between the no stigma group and the stigma group (Table 1).
Among the four items related to impossibility of recovery, more than 85% of patients were positive for at least three items. However, for questions related to job performance at the workplace, 14 (19.2%) patients believed that their ability to work efficiently decreased even after successful cancer treatment (Table 2). According to the type of discrimination endured, 15 (20.5%) patients reported that they experienced discrimination from both employers and coworkers. Forty-two (57.5%) patients disclosed cancer diagnosis to a colleague and 25 (34.2%) patients disclosed cancer diagnosis to customers (Table 3). Results of subgroup analysis for cancer patients also showed a similar trend to that for all patients (Supplementary Table 1, 2).
Using an unadjusted model of linear regression, we found that the stigma group was more likely to report a lower score in overall QoL (78.51 vs. 62.62, p < 0.01) and in all functional scales, particularly the cognitive scale (92.11 vs. 69.52, p < 0.001). In addition, patients in the stigma group were significantly more likely to exhibit higher levels of all symptoms, particularly fatigue (23.10 vs. 46.98, p = 0.005). After adjusting for age, sex, and disease stage, this association remained statistically and clinically significant (Table 4, Fig. 1). In subgroup analysis, QoL was lower in the stigma group of cancer patients (77.94 vs. 60.12, p < 0.01). In the advanced stage, the stigma group showed significantly lower QoL (82.14 vs. 58.33, p < 0.01). Furthermore, the QoL of the stigma group was significantly lower in patients who received adjuvant chemotherapy (80.70 vs. 61.67, p < 0.01). There were also significant differences in adjusted models. Moreover, according to coefficient of the adjusted models 1 and 2, the association between CS and overall QoL was stronger in cancer patients (–19.23, –13.25) and advanced stage (–22.58, –16.20) than in patients with borderline disease and early stage, respectively (Table 5).
We found that the stigma group of HBP cancer patients scored lower scores for all functional scales than the no stigma group. Cognitive functions showed the largest differences between the two groups. Other studies have also found that CS might be a key factor in reducing cognitive functions in cancer patients [19,23-25]. Therefore, modifying CS might be the key in order to prevent cognitive decline in cancer patients, including those with HBP cancer.
In this study, we also found that the stigma group had lower functions other than cognition than the no-stigma group. Physical function showed the second largest difference between the two groups (Table 4, Fig. 1). Emotional, social, and other functions were also lower in the stigma group. These results were consistent with other studies investigating other types of cancers. Some studies have reported that stigmatization can affect physical functions of breast and prostate cancer patients [19,26]. Emotional function and social function in lung cancer patients can be significantly affected by stigmatization [19,26]. Therefore, it is important to actively modify CS in order to improve all functional scales in cancer patients.
Fatigue has been reported as one of the most serious symptoms associated with cancer and its treatment. It is also a strong and independent predictor of reduced overall patient contentment and QoL [15,24,27,28]. Here, we also found that fatigue was the most common symptom among all 73 patients. It also had the largest difference among all symptoms in relation to CS. In previous studies, the highest prevalence of fatigue was observed in patients receiving treatment and long-term survivors [29]. Further investigation of this symptom in follow-up patients is important to find ways to improve fatigue through CS modification.
Pain is one of the most serious complications of cancer [30]. In previous studies, pain was strongly associated with CS [31]. Pain is a complex phenomenon wherein physiological, sensory, emotional, and cognitive components interact to affect its recognition and expression [30,32]. Our results showed that the severity of pain was lower than in those with other symptoms of HBP cancer. However, these results might have been influenced by the nature of our cross-sectional study and the type of surgery performed in each patient. Pain should be consistently controlled in cancer patients. In addition, CS correction may improve symptoms, including pain in HBP cancer patients.
In our study, the ratio of maintaining a job in the stigma group was lower than that in the no stigma group. When evaluating patients’ CS, according to ‘impossibility of recovery’ items, the percentage of patients who responded negatively to the question about decline in ability at the workplace was the highest. In addition, upon evaluating ‘experience of social discrimination’, the proportion of patients who experienced discrimination at the workplace was the highest. Additionally, patients disclosed their cancer diagnosis to their families more than to their co-workers or clients (Table 2, 3). These results showed that CS negatively affected cancer patients in terms of their personal outlook, relationships, and perceptions [20]. Therefore, changing the social perception of cancer survivors must be done along with correcting the stigma of cancer patients.
So far, studies directly linking CS to long-term survival have not been reported yet. Despite this, evidence linking QoL to survival in chronic diseases is emerging [33-37]. Several studies have examined the association between QoL and survival in patients with cancer [12,38,39]. Here, we did not find a direct association between survival rate and CS or QoL. However, another study has shown that modifying CS can increase the number of long-term survivors by improving symptoms and QoL, restoring the patient’s ability to perform daily activities [33]. The small patient cohort of our study made it difficult to understand the exact relationship between CS and survival. There might be a causal relationship between CS and survival. To define this relationship better, larger cohort studies will be necessary.
CS is typically linked to a negative stereotype and hopeless feeling for oneself. It has been recently found to influence outcomes of cancer patients [14]. This study clarified the relationship between QoL and CS in a group of HBP cancer patients. The stigma group had a significantly lower QoL than the no stigma group (Table 4, Fig. 1). In order to find out whether CS and QoL showed a correlation while minimizing the influence of surgical complications, subgroup analysis was performed by equalizing surgical groups by type of surgery. In patients who underwent pancreaticoduodenectomy, QoL was lower in the stigma group than in the no-stigma group. Furthermore, after adjusting for pain, diarrhea, nausea, and constipation, the stigma group had a significantly lower QoL than the no stigma group (Table 5). Several studies have reported an association between CS and QoL among patients with other cancer types and found that reducing stigmatization can decrease the risk of both psychological and physiological issues [10,13,16,19,26,40]. This is a rare study that presents the relation between CS and QoL in HBP cancer patients. It shows results consistent with several previous studies on other cancers and new clinical outcomes.
This study had some limitations. First, our study group was composed of a small and heterogeneous group of patients. Second, this study was conducted with a cross-sectional design, which was limited in accurately asserting a causal relationship between CS and QoL because neither questionnaire administered to patients before surgery nor information on preoperative clinical symptoms of patients were investigated. Therefore, further large cohort and longitudinal studies are needed. A prospective study on HBP cancer that determines the difference in QoL according to the treatment plan of patients will give more insight regarding the relationship between CS and long-term survival.
In summary, CS is an important factor that can negatively affect overall QoL and clinical symptoms of HBP cancer patients despite its lack of effect on cancer survival. Appropriate treatment selection and patient management, especially if intensive psychological support therapy is performed for cancer patients diagnosed in an advanced stage who have received chemotherapy, it will be helpful to improve their overall QoL and long-term prognosis after surgery.
Supplementary data related to this article can be found at https://doi.org/10.14701/ahbps.22-084.
ACKNOWLEDGEMENTS
The authors would like to thank Hyemin Kim (Data Manager, Department of Surgery, Samsung Medical Center, Sungkyunkwan University School of Medicine) for help with data collection.
REFERENCES
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016; 66:7–30. DOI: 10.3322/caac.21332. PMID: 26742998.

2. Pulte D, Weberpals J, Schröder CC, Emrich K, Holleczek B, Katalinic A, et al. 2018; Survival of patients with hepatobiliary tract and duodenal cancer sites in Germany and the United States in the early 21st century. Int J Cancer. 143:324–332. DOI: 10.1002/ijc.31322. PMID: 29479701.

3. Carrato A, Falcone A, Ducreux M, Valle JW, Parnaby A, Djazouli K, et al. 2015; A systematic review of the burden of pancreatic cancer in Europe: real-world impact on survival, quality of life and costs. J Gastrointest Cancer. 46:201–211. DOI: 10.1007/s12029-015-9724-1. PMID: 25972062. PMCID: PMC4519613.

4. Jung KW, Won YJ, Oh CM, Kong HJ, Lee DH, Lee KH. Prediction of cancer incidence and mortality in Korea, 2017. Cancer Res Treat. 2017; 49:306–312. DOI: 10.4143/crt.2017.130. PMID: 28301926. PMCID: PMC5398390.

5. McGuigan A, Kelly P, Turkington RC, Jones C, Coleman HG, McCain RS. 2018; Pancreatic cancer: a review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol. 24:4846–4861. DOI: 10.3748/wjg.v24.i43.4846. PMID: 30487695. PMCID: PMC6250924.

6. Jia L, Jiang SM, Shang YY, Huang YX, Li YJ, Xie DR, et al. 2010; Investigation of the incidence of pancreatic cancer-related depression and its relationship with the quality of life of patients. Digestion. 82:4–9. DOI: 10.1159/000253864. PMID: 20145402.

7. Petzel MQ, Parker NH, Valentine AD, Simard S, Nogueras-Gonzalez GM, Lee JE, et al. 2012; Fear of cancer recurrence after curative pancreatectomy: a cross-sectional study in survivors of pancreatic and periampullary tumors. Ann Surg Oncol. 19:4078–4084. DOI: 10.1245/s10434-012-2566-1. PMID: 22875648.

8. Bauer MR, Bright EE, MacDonald JJ, Cleary EH, Hines OJ, Stanton AL. 2018; Quality of life in patients with pancreatic cancer and their caregivers: a systematic review. Pancreas. 47:368–375. DOI: 10.1097/MPA.0000000000001025. PMID: 29521939.

9. Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009; 59:225–249. DOI: 10.3322/caac.20006. PMID: 19474385.

10. Cataldo JK, Jahan TM, Pongquan VL. 2012; Lung cancer stigma, depression, and quality of life among ever and never smokers. Eur J Oncol Nurs. 16:264–269. DOI: 10.1016/j.ejon.2011.06.008. PMID: 21803653. PMCID: PMC3360805.

11. Montazeri A, Milroy R, Hole D, McEwen J, Gillis CR. 2001; Quality of life in lung cancer patients: as an important prognostic factor. Lung Cancer. 31:233–240. DOI: 10.1016/S0169-5002(00)00179-3. PMID: 11165402.
12. Montazeri A. 2009; Quality of life data as prognostic indicators of survival in cancer patients: an overview of the literature from 1982 to 2008. Health Qual Life Outcomes. 7:102. DOI: 10.1186/1477-7525-7-102. PMID: 20030832. PMCID: PMC2805623.

13. Phelan SM, Griffin JM, Jackson GL, Zafar SY, Hellerstedt W, Stahre M, et al. 2013; Stigma, perceived blame, self-blame, and depressive symptoms in men with colorectal cancer. Psychooncology. 22:65–73. DOI: 10.1002/pon.2048. PMID: 21954081. PMCID: PMC6000725.

14. Stergiou-Kita M, Pritlove C, Kirsh B. 2016; The "Big C"-stigma, cancer, and workplace discrimination. J Cancer Surviv. 10:1035–1050. DOI: 10.1007/s11764-016-0547-2. PMID: 27170116.

15. Pinto AC, de Azambuja E. 2011; Improving quality of life after breast cancer: dealing with symptoms. Maturitas. 70:343–348. DOI: 10.1016/j.maturitas.2011.09.008. PMID: 22014722.

16. Chambers SK, Dunn J, Occhipinti S, Hughes S, Baade P, Sinclair S, et al. 2012; A systematic review of the impact of stigma and nihilism on lung cancer outcomes. BMC Cancer. 12:184. DOI: 10.1186/1471-2407-12-184. PMID: 22607085. PMCID: PMC3517321.

17. Heijnders M, Van Der Meij S. 2006; The fight against stigma: an overview of stigma-reduction strategies and interventions. Psychol Health Med. 11:353–363. DOI: 10.1080/13548500600595327. PMID: 17130071.

18. Weiss MG, Ramakrishna J, Somma D. 2006; Health-related stigma: rethinking concepts and interventions. Psychol Health Med. 11:277–287. DOI: 10.1080/13548500600595053. PMID: 17130065.
19. Ernst J, Mehnert A, Dietz A, Hornemann B, Esser P. 2017; Perceived stigmatization and its impact on quality of life - results from a large register-based study including breast, colon, prostate and lung cancer patients. BMC Cancer. 17:741. DOI: 10.1186/s12885-017-3742-2. PMID: 29121876. PMCID: PMC5680772.

20. Cho J, Smith K, Choi EK, Kim IR, Chang YJ, Park HY, et al. 2013; Public attitudes toward cancer and cancer patients: a national survey in Korea. Psychooncology. 22:605–613. DOI: 10.1002/pon.3041. PMID: 22344743.

21. Yun YH, Bae SH, Kang IO, Shin KH, Lee R, Kwon SI, et al. 2004; Cross-cultural application of the Korean version of the European Organization for Research and Treatment of Cancer (EORTC) Breast-Cancer-Specific Quality of Life Questionnaire (EORTC QLQ-BR23). Support Care Cancer. 12:441–445. DOI: 10.1007/s00520-004-0632-3. PMID: 15088137.

22. Fayers PM, Aaronson NK, Bjordal K, Groenvold M, Curran D, Bottomley A. EORTC QLQ-C30 scoring manual. 3rd ed. Brussels: European Organisation for Research and Treatment of Cancer;2001.
23. Ahles TA, Saykin AJ, McDonald BC, Li Y, Furstenberg CT, Hanscom BS, et al. 2010; Longitudinal assessment of cognitive changes associated with adjuvant treatment for breast cancer: impact of age and cognitive reserve. J Clin Oncol. 28:4434–4440. DOI: 10.1200/JCO.2009.27.0827. PMID: 20837957. PMCID: PMC2988635.

24. Jacobs W, Das E, Schagen SB. 2017; Increased cognitive problem reporting after information about chemotherapy-induced cognitive decline: the moderating role of stigma consciousness. Psychol Health. 32:78–93. DOI: 10.1080/08870446.2016.1244535. PMID: 27701901.

25. Burstein HJ. 2007; Cognitive side-effects of adjuvant treatments. Breast. 16 Suppl 2:S166–S168. DOI: 10.1016/j.breast.2007.07.027. PMID: 17719225.

26. Brown Johnson CG, Brodsky JL, Cataldo JK. 2014; Lung cancer stigma, anxiety, depression, and quality of life. J Psychosoc Oncol. 32:59–73. DOI: 10.1080/07347332.2013.855963. PMID: 24428251. PMCID: PMC4634635.

27. Curt GA, Breitbart W, Cella D, Groopman JE, Horning SJ, Itri LM, et al. 2000; Impact of cancer-related fatigue on the lives of patients: new findings from the Fatigue Coalition. Oncologist. 5:353–360. DOI: 10.1634/theoncologist.5-5-353. PMID: 11040270.

28. Daniell HW. 2004; Cancer-related fatigue: evolving concepts in evaluation and treatment. Cancer. 100:2484author reply 2484–2485. DOI: 10.1002/cncr.20246. PMID: 15160357.

29. Wang XS, Zhao F, Fisch MJ, O'Mara AM, Cella D, Mendoza TR, et al. 2014; Prevalence and characteristics of moderate to severe fatigue: a multicenter study in cancer patients and survivors. Cancer. 120:425–432. DOI: 10.1002/cncr.28434. PMID: 24436136. PMCID: PMC3949157.

30. Money S, Garber B. 2018; Management of cancer pain. Curr Emerg Hosp Med Rep. 6:141–146. DOI: 10.1007/s40138-018-0170-9.

31. Cataldo JK, Brodsky JL. 2013; Lung cancer stigma, anxiety, depression and symptom severity. Oncology. 85:33–40. DOI: 10.1159/000350834. PMID: 23816853.

32. Caffo O, Amichetti M, Ferro A, Lucenti A, Valduga F, Galligioni E. 2003; Pain and quality of life after surgery for breast cancer. Breast Cancer Res Treat. 80:39–48. DOI: 10.1023/A:1024435101619. PMID: 12889597.

33. Steel JL, Geller DA, Robinson TL, Savkova AY, Brower DS, Marsh JW, et al. 2014; Health-related quality of life as a prognostic factor in patients with advanced cancer. Cancer. 120:3717–3721. DOI: 10.1002/cncr.28902. PMID: 25104581. PMCID: PMC4239171.

34. Kramer JA, Curran D, Piccart M, de Haes JC, Bruning P, Klijn J, et al. 2000; Identification and interpretation of clinical and quality of life prognostic factors for survival and response to treatment in first-line chemotherapy in advanced breast cancer. Eur J Cancer. 36:1498–1506. DOI: 10.1016/S0959-8049(00)00144-1. PMID: 10930797.

35. Meyers CA, Hess KR, Yung WK, Levin VA. 2000; Cognitive function as a predictor of survival in patients with recurrent malignant glioma. J Clin Oncol. 18:646–650. DOI: 10.1200/JCO.2000.18.3.646. PMID: 10653880.

36. Poon RT, Fan ST, Yu WC, Lam BK, Chan FY, Wong J. 2001; A prospective longitudinal study of quality of life after resection of hepatocellular carcinoma. Arch Surg. 136:693–699. DOI: 10.1001/archsurg.136.6.693. PMID: 11387012.

37. Lis CG, Gupta D, Granick J, Grutsch JF. 2006; Can patient satisfaction with quality of life predict survival in advanced colorectal cancer? Support Care Cancer. 14:1104–1110. DOI: 10.1007/s00520-006-0100-3. PMID: 16819630.

38. Coates A, Porzsolt F, Osoba D. 1997; Quality of life in oncology practice: prognostic value of EORTC QLQ-C30 scores in patients with advanced malignancy. Eur J Cancer. 33:1025–1030. DOI: 10.1016/S0959-8049(97)00049-X. PMID: 9376182.

39. Dancey J, Zee B, Osoba D, Whitehead M, Lu F, Kaizer L, et al. 1997; Quality of life scores: an independent prognostic variable in a general population of cancer patients receiving chemotherapy. The National Cancer Institute of Canada Clinical Trials Group. Qual Life Res. 6:151–158. DOI: 10.1023/A:1026442201191. PMID: 9161115.
40. Steffen LE, Vowles KE, Smith BW, Gan GN, Edelman MJ. 2018; Daily diary study of hope, stigma, and functioning in lung cancer patients. Health Psychol. 37:218–227. DOI: 10.1037/hea0000570. PMID: 29172604. PMCID: PMC5837918.

Fig. 1
Mean quality of life, function (A), and symptoms (B) by the presence of stigma. Patients with higher scores than median (1.375 out of 3) of stigma score were assigned to the stigma group. Quality of life, function and symptom scores ranged from 0 to 100, with higher scores indicating better general health status/quality of life and better functioning but higher symptoms.
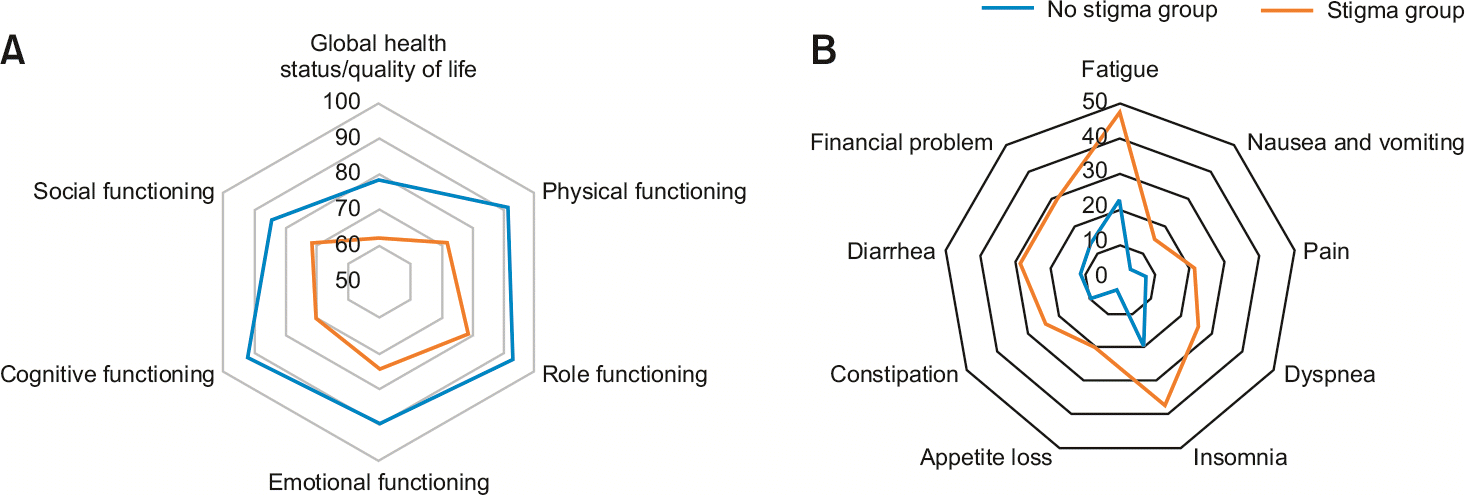
Table 1
Patients characteristics and clinical outcomes (n = 73)
| Overall | Cancer stigma | p-value | ||
|---|---|---|---|---|
|
|
||||
| No (n = 38) | Yes (n = 35) | |||
| Socio-demographic factor | ||||
| Age (yr) | 55.3 ± 5.5 | 55.6 ± 5.2 | 55.1 ± 5.8 | 0.68 |
| Sex | 0.05 | |||
| Female | 14 (19.2) | 4 (10.5) | 10 (28.6) | |
| Male | 59 (80.8) | 34 (89.5) | 25 (71.4) | |
| Marital status, married | 65 (89.0) | 36 (94.7) | 29 (82.9) | 0.11 |
| Monthly family income ($) | 0.53 | |||
| < 5,500 | 19 (26.0) | 12 (31.6) | 7 (20.0) | |
| ≥ 5,500 | 52 (71.2) | 25 (65.8) | 27 (77.1) | |
| Unknown | 2 (2.7) | 1 (2.6) | 1 (2.9) | |
| Education | 0.51 | |||
| < High school | 34 (46.6) | 19 (50.0) | 15 (42.9) | |
| ≥ High school | 38 (52.1) | 19 (50.0) | 19 (54.3) | |
| Unknown | 1 (1.4) | 0 (0) | 1 (2.7) | |
| Current work status | 0.014 | |||
| No work | 16(21.9) | 4(10.5) | 12 (34.3) | |
| Current work | 57 (78.1) | 34(89.5) | 23 (65.7) | |
| Clinical factor | ||||
| Median survival year | 2.5 (1.7–3.8) | 2.9 (1.7–4.1) | 2.4 (1.4–3.7) | 0.37 |
| Survival year | 0.30 | |||
| < 1 | 4 (5.5) | 1 (2.6) | 3 (8.6) | |
| 1 to < 2 | 21 (28.8) | 11 (28.9) | 10 (28.6) | |
| 2 to < 3 | 18 (24.7) | 8 (21.1) | 10 (28.6) | |
| 3 to < 4 | 12 (16.4) | 8 (21.1) | 4 (11.4) | |
| 4 to < 5 | 11 (15.1) | 8 (21.1) | 3 (8.6) | |
| ≥ 5 | 7 (9.6) | 2 (5.3) | 5 (14.3) | |
| Disease characteristic | 0.26 | |||
| Benign | 11 (15.1) | 4 (10.5) | 7 (20.0) | |
| Cancer | 62 (84.9) | 34 (89.5) | 28 (80.0) | |
| Cancer subtypea) | 0.20 | |||
| Pancreatic ductal adenocarcinoma | 22 (35.5) | 10 (29.4) | 12 (42.9) | |
| Distal common bile duct | 14 (22.6) | 9 (26.5) | 5 (17.9) | |
| Hilar cholangiocarcinoma | 6 (9.7) | 2 (5.9) | 4 (14.3) | |
| Carcinoma of the Ampulla of Vater | 11 (17.7) | 9 (26.5) | 2 (7.1) | |
| Others | 9 (14.5) | 4 (11.8) | 5 (17.9) | |
| Disease stage at diagnosisa) | 0.27 | |||
| Stage I | 21 (33.9) | 14 (41.2) | 7 (25.0) | |
| Stage II | 36 (58.1) | 19 (55.9) | 17 (60.7) | |
| Stage III | 4 (6.5) | 1 (2.9) | 3 (10.7) | |
| Unknown | 1 (1.6) | 0 (0.0) | 1 (3.6) | |
| Type of surgery | 0.34 | |||
| Pancreatectomy | 60 (82.2) | 32 (84.2) | 28 (80.0) | |
| Hepatectomy | 9 (12.3) | 3 (7.9) | 6 (17.1) | |
| Others | 4 (5.5) | 3 (7.9) | 1 (2.9) | |
| Surgical method | 0.92 | |||
| Laparoscopic surgery | 6 (8.2) | 3 (7.9) | 3 (8.6) | |
| Open surgery | 67 (91.8) | 35 (92.1) | 32 (91.4) | |
| Adjuvant treatment | 0.26 | |||
| No | 30 (41.1) | 18 (47.4) | 12 (34.3) | |
| Yes | 43 (58.9) | 20 (52.6) | 23 (65.7) | |
| Adjuvant treatment (≥ stage II) | 40 | 20 | 20 | 0.63 |
| No | 5 (12.5) | 2 (10.0) | 3 (15.0) | |
| Yes | 35 (87.5) | 18 (90.0) | 17 (85.0) | |
| Complication | 0.31 | |||
| No or C-D classification I–II | 59 (80.8) | 29 (76.3) | 30 (85.7) | |
| C-D classification ≥ IIIa | 14 (19.2) | 9 (23.7) | 5 (14.3) | |
Table 2
Postoperative stigma of patients
Table 3
Postoperative experience of discrimination by patients
Table 4
Association between cancer stigma and quality of life, function, and symptoms
| No stigma (n = 38) | Stigma (n = 35) | p-value | No stigma vs. stigma Coef (95% CI)a) | p-valuea) | |
|---|---|---|---|---|---|
| Global health status/quality of life | 78.51 (16.96) | 62.62 (21.04) | < 0.001 | –17.67 (–26.75, –8.60) | < 0.001 |
| Functional scales | |||||
| Physical functioning | 91.58 (8.30) | 72.00 (17.47) | < 0.001 | –19.35 (–26.04, –12.67) | < 0.001 |
| Cognitive functioning | 92.11 (12.70) | 69.52 (23.39) | < 0.001 | –21.20 (–30.36, –12.04) | < 0.001 |
| Emotional functioning | 89.25 (11.11) | 76.51 (16.31) | < 0.001 | –11.47 (–17.91, –5.03) | 0.001 |
| Social functioning | 84.65 (23.05) | 71.90 (23.14) | 0.002 | –13.30 (–24.60, –1.99) | 0.022 |
| Role functioning | 92.98 (15.32) | 79.04 (22.27) | 0.002 | –14.94 (–24.53, –5.35) | 0.003 |
| Symptoms | |||||
| Fatigue | 23.10 (16.21) | 46.98 (22.56) | 0.005 | 22.48 (12.88, 32.07) | < 0.001 |
| Nausea and vomiting | 4.39 (10.10) | 15.23 (20.76) | 0.002 | 8.94 (1.49, 16.39) | 0.019 |
| Pain | 7.46 (11.43) | 20.48 (22.17) | 0.013 | 11.20 (2.49, 19.91) | 0.012 |
| Dyspnea | 8.77 (25.33) | 24.76 (28.40) | 0.005 | 16.87 (3.35, 30.41) | 0.015 |
| Insomnia | 19.30 (24.05) | 37.14 (28.89) | 0.002 | 13.87 (1.55, 26.18) | 0.028 |
| Appetite loss | 2.63 (11.96) | 19.82 (27.73) | < 0.001 | 11.68 (2.36, 21.00) | 0.015 |
| Constipation | 9.65 (24.39) | 24.76 (30.62) | 0.022 | 13.63 (–0.00, 27.27) | 0.050 |
| Diarrhea | 11.40 (19.42) | 30.48 (33.70) | 0.003 | 18.02 (4.76, 31.28) | 0.009 |
| Financial problem | 13.16 (26.33) | 28.57 (30.40) | 0.023 | 17.85 (3.72, 31.98) | 0.014 |
Table 5
Subgroup analysis in association between cancer stigma and global health status/quality of life
| No stigma | Stigma | p-value | No stigma vs. stigma Coef (95% CI) | ||
|---|---|---|---|---|---|
|
|
|||||
| Model 1a) | Model 2b) | ||||
| Disease characteristics (n = 73) | |||||
| Borderline | 83.33 (11.79) | 72.62 (9.27) | 0.13 | –7.80 (–32.05, 16.45) | –6.49 (–30.07, 17.09) |
| Cancer | 77.94 (17.51) | 60.12 (22.49) | < 0.01 | –19.23 (–28.98, –9.47) | –13.25 (–23.62, –2.88) |
| Stage (n = 62) | |||||
| Early stage | 71.15 (20.59) | 70.83 (25.00) | 0.98 | –3.06 (–28.22, 22.10) | 5.60 (–20.03, 31.23) |
| Advanced stage | 82.14 (14.26) | 58.33 (22.12) | < 0.01 | –22.58 (–34.61, –10.56) | –16.20 (–29.15, –3.23) |
| Type of cancer (n = 62) | |||||
| Others cancer | 78.47 (19.65) | 59.90 (23.22) | < 0.01 | –23.18 (–36.20, –10.17) | –18.33 (–31.74, –4.92) |
| Pancreatic cancer | 76.67 (11.65) | 60.42 (22.51) | 0.05 | –10.89 (–28.37, 6.59) | –3.25 (–21.46, 14.95) |
| Type of surgery (n = 62) | |||||
| No PD | 83.33 (13.82) | 60.61 (21.11) | 0.01 | –25.98 (–44.34, –7.61) | –21.40 (–39.92, –2.87) |
| PD | 76.00 (18.52) | 59.80 (23.98) | 0.01 | –16.08 (–28.53, –3.62) | –8.78 (–22.22, 4.66) |
| Adjuvant chemotherapy (n = 62) | |||||
| No | 74.44 (21.24) | 56.25 (17.68) | 0.05 | –27.29 (–45.70, –8.89) | –18.29 (–38.29, 1.71) |
| Yes | 80.70 (13.90) | 61.67 (24.39) | < 0.01 | –14.57 (–27.88, –1.27) | –10.46 (–24.12, 3.20) |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download