Abstract
Backgrounds/Aims
The present study looked at the role of radical surgery in gallbladder cancers (GBC) with limited metastatic disease.
Methods
The retrospective observational study was conducted to screen the database from 1st January 2010 to 31st December 2019. Patients of GBC found to have low-volume metastatic disease upon surgical exploration were included.
Results
Of the 1,040 patients operated for GBC, 234 patients had low-volume metastatic disease (microscopic disease in station 16b1 node or N2 disease isolated port-site metastases, or low burden peritoneal disease with deposits less than 1 cm, in adjacent omentum or adjacent diaphragm or Morrison’s pouch or a solitary discontinuous liver metastasis in adjacent liver parenchyma) detected intraoperative. Of these, 62 patients underwent radical surgery for R-0 metastatic disease followed by systemic therapy, while the remaining 172 patients did not undergo radical surgery and were given palliative systemic chemotherapy. Patients who underwent radical surgery had significantly superior overall survival (19 months versus 12 months, p < 0.01) and superior progression-free survival (10 months versus 5 months, p < 0.01) when compared to the rest. This difference in survival was more significant amongst patients when operated on after neoadjuvant chemotherapy. Regression analysis showed that a sub-group of patients with incidental GBC with limited metastases showed more favorable outcomes with radical surgery.
Even in the era of multi-modality treatment, survival outcomes from gallbladder cancer (GBC) remain dismal. GBC is a relatively chemo-resistant disease without any robust options for systemic therapy against it. The best chance of cure is offered by an optimal combination of surgery with systemic therapy, depending upon the stage of the disease. The reported 5-year overall survival (OS) for GBC is around 75%, 35%, and less than 5% for early-stage disease, locally advanced disease, and metastatic disease, respectively [1]. The 5% OS of patients with metastatic disease (M1) ranges between 2% to 5% depending upon the site of metastatic disease, disease burden, and the treatment received [2,3]. The role of radical surgery for primary tumors in metastatic disease has been seldom studied because of the diseasés inherently aggressive nature with poor outcomes. In a recent analysis of the National Cancer Database, Casabianca et al. [4] have suggested the place of surgery in the treatment algorithm for advanced GBCs. While the place of radical surgery in solid metastatic cancers like colorectal and breast cancers is evolving, the same needs to be defined in patients with metastatic GBC [5]. The present study looked at the subset of patients with cM0 GBC who underwent radical R-0 surgery in the presence of low-volume metastatic disease. Authors hypothesized that R-0 surgical resection might be beneficial in appropriately selected patients with advanced disease.
A retrospective observational cohort study design was used to analyze a prospectively maintained GBC database from 1st January 2010 to 31st December 2019.
i. GBC patients with cM0 disease (AJCC 8th edition) before radical surgery (early disease, cT1/2 N0 M0) or after neoadjuvant chemotherapy (NACT) (locally advanced disease, cT3/4 N1/2 M0)
ii. Patients diagnosed with low volume metastatic disease at the time of surgery. The authors define these:
a) Microscopic disease in station 16b1 node
b) N2 disease
c) Isolated port-site metastases
d) Low-burden peritoneal disease with deposits less than 1 cm, adjacent omentum or adjacent diaphragm or Morrison’s pouch only
e) A solitary discontinuous liver metastasis in the adjacent liver parenchyma
iii. Patients who completed at least three months of follow-up
i. Patients with non-adenocarcinoma histology
ii. A Patient with suspected metastases on preoperative radiological staging
iii. A Patient who had disseminated intra-operatively diagnosed metastases (disease beyond the above definition)
i. To examine the survival outcomes of patients diagnosed with aggressively treated low-volume metastatic GBC
i. To study the role of NACT in selected patients with advanced disease for radical treatment
ii. To identify the prognostic factors affecting the survival in this cohort of patients with low volume metastatic disease.
Data collection followed the ethical guidelines of the declaration of Helsinki [6]. Institutional ethical clearance was obtained for data analyzed in the study.
Treatment naïve GBC patients were staged using Contrast-enhanced computed tomography (CECT) scan or FDG-18 positron emission tomography (PET) scan of thorax, abdomen, and pelvis. Clinico-radiologically staged patients with early GBC (cT1 or cT2, N0) underwent open surgery. Those labelled as locally advanced GBC (LAGBC) as per the published “TMH Criteria” (cT3 with > 5 cm of contiguous liver involvement, cT4 or node positive disease) were scheduled for neoadjuvant therapy (NAT) followed by a reassessment for surgery [7]. Patients with incidental GBC (iGBC) were first reassessed with whole body PET-CT or CECT scan, depending on the presentation time after index surgery [8]. Patients without residual disease were operated directly and those with residual disease in the gallbladder fossa or periportal nodes were offered NAT, followed by a re-evaluation for revision surgery [9]. The standard protocol for NAT consisted of 3 cycles of gemcitabine and oxaliplatin followed by a PET CECT scan or re-evaluation with a CECT scan. Patients with radiological progressive or metastatic disease were given palliative systemic therapy. Palliative systemic therapy consisted of six cycles of gemcitabine and cisplatin/oxaliplatin.
Radical cholecystectomy, as standardized by the authors, consists of the removal of the gallbladder with a liver wedge with periportal lymphadenectomy (station 12, 13 and station 8 lymph nodes) [10,11]. The authors follow the NCCN guidelines of removing a minimum of 6 lymph nodes for adequate lymph node staging. After staging laparoscopy, a thorough search is performed to exclude peritoneal or visceral metastatic disease. Kocherisation followed by inter-aortocaval nodal sampling (station 16b) is then performed. Patients with metastases confirmed on frozen section are not offered radical surgery. For iGBC, routine port or scar site excisions are not performed [12-14].
Patients were staged as per latest guidelines on GBC staging by the AJCC manual (8th edition) [15]. Adjuvant treatment was decided based on clinical profile and tumor characteristics at a tumor board meeting [16]. All patients with stage pT2 and above and node positive status (N1, 1 to 3 nodes; N2, 4 or more nodes) were offered chemotherapy. Adjuvant radiotherapy was reserved for margin-positive resections or locally advanced inoperable disease. Adjuvant therapy consisted of 3 or 6 cycles of gemcitabine and oxaliplatin/cisplatin, depending on the cycles of chemotherapy received as NAT. Patients with metastatic disease were given systemic chemotherapy, 6 cycles of gemcitabine and oxaliplatin/cisplatin with palliative intent.
After completion of adjuvant therapy, patients were followed up with an ultrasonography and serum carbohydrate antigen 19.9 levels (CA 19.9) every three months for the first two years and every six months for next three years. An Annual CT scan was done. An Interim CECT scan or a PET CECT was reserved for suspected cases of recurrence.
Selected patients with low-volume metastases diagnosed intra-operatively with radical intent were considered for treatment and underwent surgery with R-0 resection. Radical cholecystectomy was performed with R-0 resection of metastases as described above.
Resectability of the primary disease, regional nodal burden, use of any neoadjuvant systemic therapy, and patients’ performance status guided the selection of such patients toward curative therapy. Primary disease requiring more than a standard radical cholecystectomy (resection of any other organ) or borderline performance status in the presence of minimal metastatic disease excluded any curative resection. Patients with low-volume metastatic disease who had received neoadjuvant systemic therapy were prioritized for curative resection (if other factors were favorable).
The AJCC 7th edition classified the N stage according to the location of positive lymph nodes [17]. Therefore, patients with lymph node metastases in retroperitoneal nodes beyond the recommended template were excluded from radical treatment. The AJCC 8th edition reclassified the N stage as per the number of positive lymph nodes rather than location [15,18]. Patients with isolated microscopic metastatic disease are offered radical surgery at station 16b1.
Patients with discrete metastases in liver parenchyma adjoining gallbladder fossa are considered low-volume metastatic and offered radical surgery as there is no significant increase in the morbidity profile. Isolated metastases in other liver segments demand surgery, which can potentially increase the morbidity profile and have been excluded from the low-volume metastatic disease definition.
The AJCC 8th edition has differentiated patients with N2 lymph nodes into stage IV because of the equally poor prognosis of other patients with metastatic disease [15]. Such patients are given radical surgery as they are technically M0. Therefore, these patients were considered low volume metastatic and used as a reference in the analyses.
Although there are several proposed pathways for peritoneal dissemination, patients with limited peritoneal disease in the adjacent diaphragm, Morrison’s pouch, or omentum may be dropping metastases or local extension and may benefit from en-bloc R-0 resection along with the primary disease. Hence, the authors propose the role of radical surgery in highly selected patients with adjacent peritoneal involvement.
These patients received systemic therapy in the adjuvant setting (six cycles of gemcitabine and oxaliplatin/cisplatin). Patients were routinely followed up according to the follow-up protocol recommended above.
Analysis was done using SPSS version 25 software (IBM Corp.).
Survival results in patients with low volume metastatic disease were analyzed using Kaplan–Meier analysis, emphasizing disease-free interval (DFS) and recurrence patterns. Demographic, disease related, and treatment related factors were analyzed in univariate and multivariate regression models using Cox proportional hazards analysis.
OS was calculated from the surgery date (primary or revision as applicable) to the date of death or last follow-up. DFS was calculated from the surgery date to the first symptom of recurrence. Progression-free survival (PFS) was calculated from surgical exploration to the date of first progression for patients who received palliative therapy. Follow-up was calculated using the Kaplan–Meier method and reported as median with 95% confidence intervals. The calculation was performed from the surgery date to the last follow-up or death.
Cox-regression univariate and multivariate analyses were performed to analyze treatment variables influencing survival. Variable selection for multivariate analysis was performed using backward elimination methods using log-likelihood ratios for model performance. An exit level of 0.1 was used as an alpha threshold.
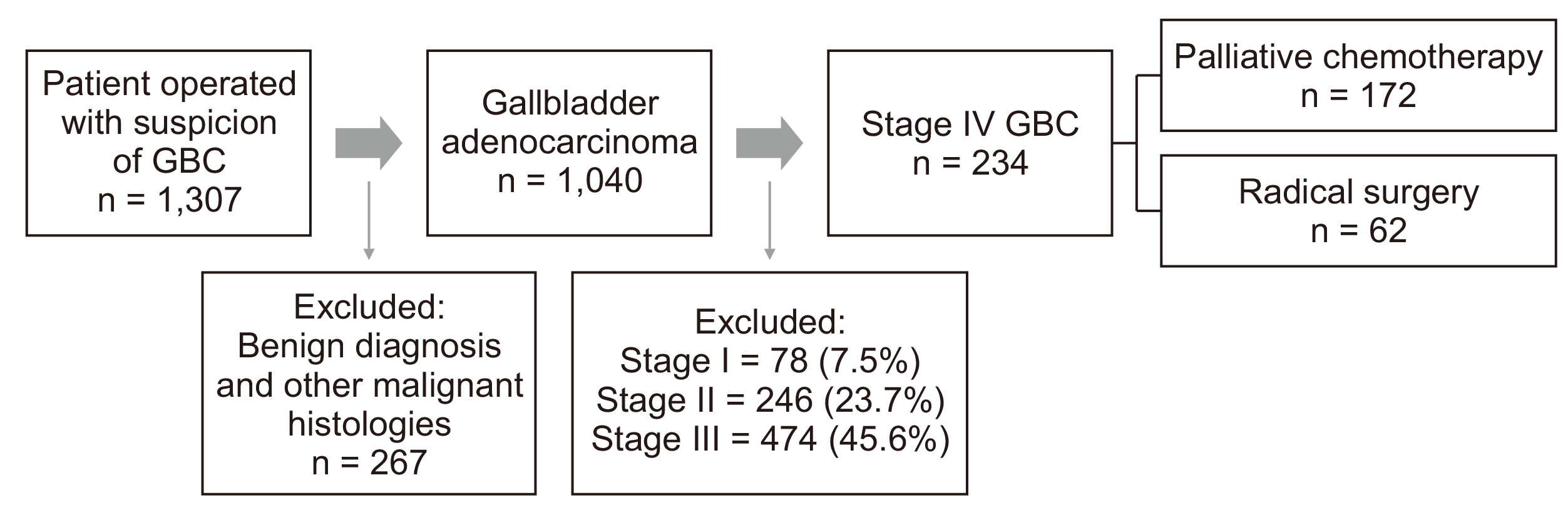
Of the 1,040 patients from the operative database, 234 had a good performance status, were non-metastatic on preoperative workup, and had low-volume metastatic disease detected intraoperative. Of these, 62 patients underwent radical R-0 surgery. Of the remaining 172 patients, ten patients had port-site metastases (after prior NACT) followed by chemotherapy. The remaining 162 patients underwent no surgery for the primary disease and only received palliative chemotherapy. Fig. 1 shows the cohort diagram.
Of the patients who underwent radical surgery, there were 46 patients (19.6%) with N2 nodal disease at final histopathology and 16 patients (6.8%) with other single-site metastatic diseases (frequently omentum, peritoneal nodule, microscopic disease at station 16b1 node)—resected en-bloc.
The remaining 172 patients included patients with low-volume metastatic disease who were not considered suitable for radical surgery as per the aforementioned appropriate criteria by the authors. These included patients with microscopic disease at station 16 node in the frozen section (48, 20.5%), persistent liver metastases (17, 7.3%) or limited peritoneal disease (97, 41.5%) or port-site metastatic disease (10, 4.3%). Table 1 shows the patients’ baseline demographic, clinical, and treatment.
Patients with low burden metastatic disease who underwent radical surgery (n = 62) had significantly superior OS (19 months versus 12 months, p < 0.01) and superior DFS/PFS (10 months vs. 5 months, p < 0.01) in comparison to those who received palliative systemic therapy Fig. 2.
Survival analysis was performed according to the subsite of metastatic disease. Patients who received palliative chemotherapy had a higher median OS, isolated port site metastases, 23 months; en-bloc low-volume metastases, 19 months; N2 disease, 19 months compared to those given palliative chemotherapy alone; peritoneal disease, 12 months; station 16 node, 11 months; liver metastases, 8 months (p = 0.001). A similar advantage was observed regarding DFS in favor of patients treated with curative intent (p < 0.01; Table 2).
The impact of NACT on survival was analyzed among patients’ undergoing curative and palliative treatment subgroups. Of the patients (84, 35.9%), who received NACT, 25 (10.7%) underwent radical surgery, and the remaining 59 patients (25.2%) progressed and received only palliative systemic therapy. Patients who underwent radical treatment had a higher median OS (16.0 vs. 13 months, p = 0.06) and DFS (9 vs. 6 months, p = 0.001) at two years, as seen previously. This difference in survival was more significant among patients who received NACT, as shown by the increased separation of the survival curves (Fig. 3, Table 3).
Univariate and multivariate analysis performed using Cox-regression showed that patients with iGBC (radiologically non-metastatic) with limited metastases who underwent radical surgery had significant survival outcomes (Table 4). The site of low-volume metastases did not affect survival outcomes.
Treatment of advanced GBC focuses on providing relapse-free survival and excellent quality of life. The wide variety of management strategies for advanced GBC has its roots in the heterogeneous geographic distribution with the inherently poor prognosis of GBC that has failed to improve over the past two decades. The 5-year survival rate of stage IV GBC is less than 5%, as cited by Korean, Japanese, and German registries [19,20]. However, this broad “Stage IV GBC” umbrella includes port site limited metastases, single-sided low-volume visceral (liver) metastases, and limited omental/peritoneal metastases, more than four loco-regional nodal metastases (N2 stage), station 16 (inter-aortocaval) lymph node metastases, combined with extensive peritoneal metastases and or visceral metastases or other cases which have a significantly higher disease burden. Given the poor prognosis, this heterogeneous group of patient’s warrants treatment tailored to their disease burden.
Casabianca et al. [4] have analyzed patients of advanced GBC (n = 4,145) from the National Cancer Database and showed that surgery in combination with chemotherapy had superior survival outcomes compared to chemotherapy alone (11.1 months versus 6.8 months, hazard ratio 0.65, p < 0.001). However, the publication lacks to describe sites of metastatic disease and the disease burden amongst the analyzed cohort.
The current AJCC 8th edition places patients with four or more involved regional lymph nodes (N2 disease) in stage IV due to their poor prognosis. Despite a guarded prognosis, patients with advanced local disease undergo radical surgery with curative intent. Subgroup analysis in the present study showed a median OS of 19 months for patients with N2 disease. In comparison, patients with the low-burden oligo-metastatic disease who underwent radical surgery with R-0 resection had a similar OS of 19 months. This latter group included patients with the operable primary disease with metastases limited to the adjacent omentum, peritoneum, or station 16 nodes.
The practice curative intent therapy in the presence of station 16 nodal involvement varies amongst oncologists. Singh et al have suggested a role for routine sampling and frozen section analysis of station 16 nodes and exclude radical surgery in cases with involved nodes [21]. They suggested an inferior prognosis associated with station 16 nodes equivalent to distant metastases. On the contrary, Yang et al. [2] disseminated the role of radical surgery in highly selected patients with limited metastases to distant lymph nodes and limited liver metastases. The present study’s authors followed the intraoperative sampling of station nodes or any other intraoperatively diagnosed metastases. Aggressive surgery was performed only when frozen section analysis of such sites is clear, with exceptions on an individual basis in a highly selected subgroup of patients.
The role of port site or scar site metastases in GBCs is often debated. Because overall prognosis depends on disease biology governed by disease stage and grade, routine port site excision has not been recommended by previous reports on the subjects [14,22]. In the present study, ten patients with limited metastatic disease at the port site underwent only port site excision without any radical surgery for the primary tumor. The OS in this sub-group was 23 months. This sub-group consisted of highly selected patients who received NACT followed by port site excision after discussion at a multi-disciplinary meeting. In well-selected patients with no metastasis elsewhere, the disease can be treated aggressively only at the port or scar site, leading to significantly higher survival.
The authors propose a role for radical treatment in well selected patients with very low burden metastatic disease in the context of multi-modality treatment. This follows the principles and philosophy of treatment of oligometastases as suggested by Weichselbaum and Hellman [23,24]. The approximately 1.5 year OS observed in highly selected patients in the current study is similar to that observed in patients with non-metastatic locally advanced GBC undergoing extensive surgery such as bile duct resection and hepatectomy [25,26]. Prospective studies on this subject will help to make better decisions regarding the role of radical surgery in metastatic disease [5].
NACT has a potential role in selecting patients with favorable tumor biology for radical treatment in metastatic GBC cases. Sub group analysis showed that the difference in survival between patients undergoing radical and palliative treatment becomes more evident in patients operated on after NACT. This follows a similar beneficial trend of NACT observed in LAGBC patients in a previous publication at the author’s institution [7].
iGBCs constitute for about half of the operative case load at the authors’ institution [9]. In a country with a high prevalence of gallstone diseases, it is secondary to referral to a tertiary care center after an index cholecystectomy elsewhere. Regression analysis in the present study showed that patients with iGBCs who were radiologically non-metastatic and found to have limited metastases benefited from radical surgery in terms of survival.
The current study suggests a potential role for radical surgery in patients with radiologically non-metastatic disease, with low-grade metastatic disease detected at the surgery. Radical surgery offers a survival of 19 to 23 months, while patients receiving palliative chemotherapy alone have a survival of 8 to 12 months. There is a survival difference of about 10 to 12 months. As precision medicine continues to evolve, experimental therapies against various molecular targets, such as Her-2-neu, PDL-1, VEGFR, MEK, etc., have shown relapse-free survival that appears to be equivalent to the survival benefit of surgery suggested by the present study [27-29]. However, the affordability of ongoing costs and targeted therapies remain significant limitations, particularly in cost-constrained economies.
Strengths of the study from it being the first report to examine the potential role of radical surgery in selected cases of advanced GBC in context of multi-modality treatment from a center treating high volume of GBCs with a standardized management protocol throughout the study period. Limitations of the study stem from the retrospective study design and inherent selection bias. Objective quantification of disease burden at metastatic sites could potentially increase applicability of results in regular oncological practice. There is a need to define metrics such as the peritoneal carcinomatosis index to assess both peritoneal and visceral metastatic disease burden objectively. It is desirable to include known predictors when performing multicollinearity checks with regression analyses and variance-inflation factors. However, the relatively small cohort and low event rate prevented the authors to perform the same. Standardizing guidelines for patient selection to maintenance therapy in patients with advanced GBC would be desirable.
Results of the study suggest a possible role for surgery in advanced GBC with a limited metastatic burden. NACT can be used for preferentially selecting patients of favorable disease biology for curative treatment. Select cases of advanced GBC can be offered superior survival using varying treatment strategies.
REFERENCES
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021; 71:209–249. DOI: 10.3322/caac.21660. PMID: 33538338.

2. Yang Y, Tu Z, Ye C, Cai H, Yang S, Chen X, et al. 2021; Site-specific metastases of gallbladder adenocarcinoma and their prognostic value for survival: a SEER-based study. BMC Surg. 21:59. DOI: 10.1186/s12893-021-01068-8. PMID: 33485332. PMCID: PMC7825172.

3. Lee AJ, Chiang YJ, Lee JE, Conrad C, Chun YS, Aloia TA, et al. 2018; Validation of American Joint Committee on Cancer eighth staging system for gallbladder cancer and its lymphadenectomy guidelines. J Surg Res. 230:148–154. DOI: 10.1016/j.jss.2018.04.067. PMID: 30100032.

4. Casabianca AS, Tsagkalidis V, Burchard PR, Chacon A, Melucci A, Reitz A, et al. 2022; Surgery in combination with systemic chemotherapy is associated with improved survival in stage IV gallbladder cancer. Eur J Surg Oncol. 48:2448–2454. DOI: 10.1016/j.ejso.2022.06.029. PMID: 35773092.

5. Patel S, Patkar S, Goel M. 2022; Radical treatment for stage IV gallbladder cancers: is surgery a worthwhile exercise in advanced cancers? Eur J Surg Oncol. 48:2572–2573. DOI: 10.1016/j.ejso.2022.08.019. PMID: 36064633.

6. Carlson RV, Boyd KM, Webb DJ. 2004; The revision of the Declaration of Helsinki: past, present and future. Br J Clin Pharmacol. 57:695–713. DOI: 10.1111/j.1365-2125.2004.02103.x. PMID: 15151515. PMCID: PMC1884510.

7. Chaudhari VA, Ostwal V, Patkar S, Sahu A, Toshniwal A, Ramaswamy A, et al. 2018; Outcome of neoadjuvant chemotherapy in "locally advanced/borderline resectable" gallbladder cancer: the need to define indications. HPB (Oxford). 20:841–847. DOI: 10.1016/j.hpb.2018.03.008. PMID: 29706425.

8. Goel M, Tamhankar A, Rangarajan V, Patkar S, Ramadwar M, Shrikhande SV. 2016; Role of PET CT scan in redefining treatment of incidental gall bladder carcinoma. J Surg Oncol. 113:652–658. DOI: 10.1002/jso.24198. PMID: 26847023.

9. Patkar S, Patel S, Gupta A, Ramaswamy A, Ostwal V, Goel M. 2021; Revision surgery for incidental gallbladder cancer-challenging the dogma: ideal timing and real-world applicability. Ann Surg Oncol. 28:6758–6766. DOI: 10.1245/s10434-021-09687-4. PMID: 33625635.

10. Patkar S, Patil V, Acharya MR, Kurunkar S, Goel M. 2019; Achieving margin negative resection-doing less is justified: oncological outcomes of wedge excision of liver in gallbladder cancer (GBC) surgery. Chin Clin Oncol. 8:38. DOI: 10.21037/cco.2019.07.07. PMID: 31431034.

11. Pandey D. 2012; Technical description of a regional lymphadenectomy in radical surgery for gallbladder cancer. HPB (Oxford). 14:216–219. DOI: 10.1111/j.1477-2574.2011.00430.x. PMID: 22321041. PMCID: PMC3371205.

12. Kurahara H, Maemura K, Mataki Y, Sakoda M, Iino S, Kawasaki Y, et al. 2018; Indication of extrahepatic bile duct resection for gallbladder cancer. Langenbecks Arch Surg. 403:45–51. DOI: 10.1007/s00423-017-1620-7. PMID: 28875312.

13. Shukla PJ, Barreto SG. 2010; Systematic review: should routine resection of the extra-hepatic bile duct be performed in gallbladder cancer? Saudi J Gastroenterol. 16:161–167. DOI: 10.4103/1319-3767.65184. PMID: 20616410. PMCID: PMC3003211.

14. Ethun CG, Postlewait LM, Le N, Pawlik TM, Poultsides G, Tran T, et al. 2017; Routine port-site excision in incidentally discovered gallbladder cancer is not associated with improved survival: a multi-institution analysis from the US Extrahepatic Biliary Malignancy Consortium. J Surg Oncol. 115:805–811. DOI: 10.1002/jso.24591. PMID: 28230242. PMCID: PMC5800745.

15. Amin MB, Edge SB, Greene FL, Byrd DR, Brookland RK, Washington MK, et al. AJCC cancer staging manual. 8th ed. Springer;2017.
16. Ostwal V, Swami R, Patkar S, Majumdar S, Goel M, Mehta S, et al. 2018; Gemcitabine-cisplatin (GC) as adjuvant chemotherapy in resected stage II and stage III gallbladder cancers (GBC): a potential way forward. Med Oncol. 35:57. DOI: 10.1007/s12032-018-1115-6. PMID: 29564657.

17. Edge S, Byrd D, Compton C, Fritz A, Greene F, Trotti A. 2010. AJCC cancer staging handbook. 7th ed. Springer-Verlag;p. 97–100.
18. Shirai Y, Sakata J, Wakai T, Ohashi T, Ajioka Y, Hatakeyama K. 2012; Assessment of lymph node status in gallbladder cancer: location, number, or ratio of positive nodes. World J Surg Oncol. 10:87. DOI: 10.1186/1477-7819-10-87. PMID: 22594526. PMCID: PMC3532237.

19. Wi Y, Woo H, Won YJ, Jang JY, Shin A. 2018; Trends in gallbladder cancer incidence and survival in Korea. Cancer Res Treat. 50:1444–1451. DOI: 10.4143/crt.2017.279. PMID: 29370591. PMCID: PMC6192934.

20. Ishihara S, Horiguchi A, Miyakawa S, Endo I, Miyazaki M, Takada T. Biliary tract cancer registry in Japan from 2008 to 2013. J Hepatobiliary Pancreat Sci. 2016; 23:149–157. DOI: 10.1002/jhbp.314. PMID: 26699688.

21. Singh A, Ghosh NK, Rahul R, Kapoor VK, Saxena R. 2021; Retroperitoneal lymph node metastasis in gallbladder cancer: as bad as distant metastasis. Ann Hepatobiliary Pancreat Surg. 25(Suppl 1):S324. DOI: 10.14701/ahbps.EP-126.

22. Maker AV, Butte JM, Oxenberg J, Kuk D, Gonen M, Fong Y, et al. 2012; Is port site resection necessary in the surgical management of gallbladder cancer? Ann Surg Oncol. 19:409–417. DOI: 10.1245/s10434-011-1850-9. PMID: 21698501.

23. Hellman S, Weichselbaum RR. 1995; Oligometastases. J Clin Oncol. 13:8–10. DOI: 10.1200/JCO.1995.13.1.8. PMID: 7799047.

24. Weichselbaum RR, Hellman S. 2011; Oligometastases revisited. Nat Rev Clin Oncol. 8:378–382. DOI: 10.1038/nrclinonc.2011.44. PMID: 21423255.

25. Kuipers H, de Savornin Lohman EAJ, van Dooren M, Braat AE, Daams F, van Dam R, et al. 2021; Extended resections for advanced gallbladder cancer: results from a Nationwide Cohort Study. Ann Surg Oncol. 28:835–843. DOI: 10.1245/s10434-020-08858-z. PMID: 32696306. PMCID: PMC7801314.

26. Goel M, Gupta AM, Patkar S, Parray AM, Shetty N, Ramaswamy A, et al. 2021; Towards standardization of management of gallbladder carcinoma with obstructive jaundice: analysis of 113 cases over 10 years at a single institution. J Surg Oncol. 124:572–580. DOI: 10.1002/jso.26564. PMID: 34106475.

27. De Lorenzo S, Garajova I, Stefanini B, Tovoli F. 2021; Targeted therapies for gallbladder cancer: an overview of agents in preclinical and clinical development. Expert Opin Investig Drugs. 30:759–772. DOI: 10.1080/13543784.2021.1928636. PMID: 33966562.

28. Ahn S, Lee JC, Shin DW, Kim J, Hwang JH. 2020; High PD-L1 expression is associated with therapeutic response to pembrolizumab in patients with advanced biliary tract cancer. Sci Rep. 10:12348. Erratum in: Sci Rep 2020;10:21552. DOI: 10.1038/s41598-020-69366-4. PMID: 32704067. PMCID: PMC7378166.

29. Das CK, Patel A, Raj A, Gupta VG, Mehta P, Bhethanabhotla S, et al. 2020; Anti-Her2neu directed therapy in advanced gall bladder cancer: a prospective, multicenter experience from India. J Clin Oncol. 38(15 Suppl):e16682. DOI: 10.1200/JCO.2020.38.15_suppl.e16682.

Fig. 2
Survival analysis of radical surgery versus systemic therapy alone, in the setting of low-volume metastatic disease. (A) Overall survival (OS); (B) disease-free survival (DFS).
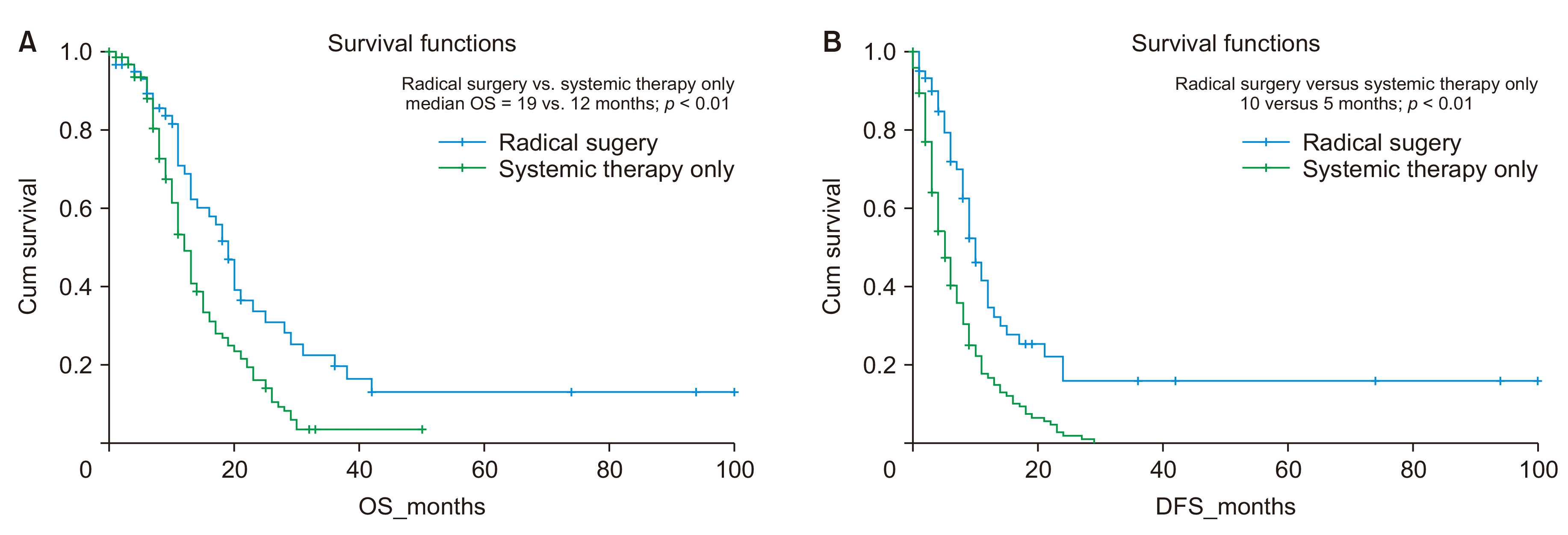
Fig. 3
Survival analysis about the use of neoadjuvant chemotherapy (NACT): Overall survival (OS) (A, B) and disease-free survival (DFS) (C, D).
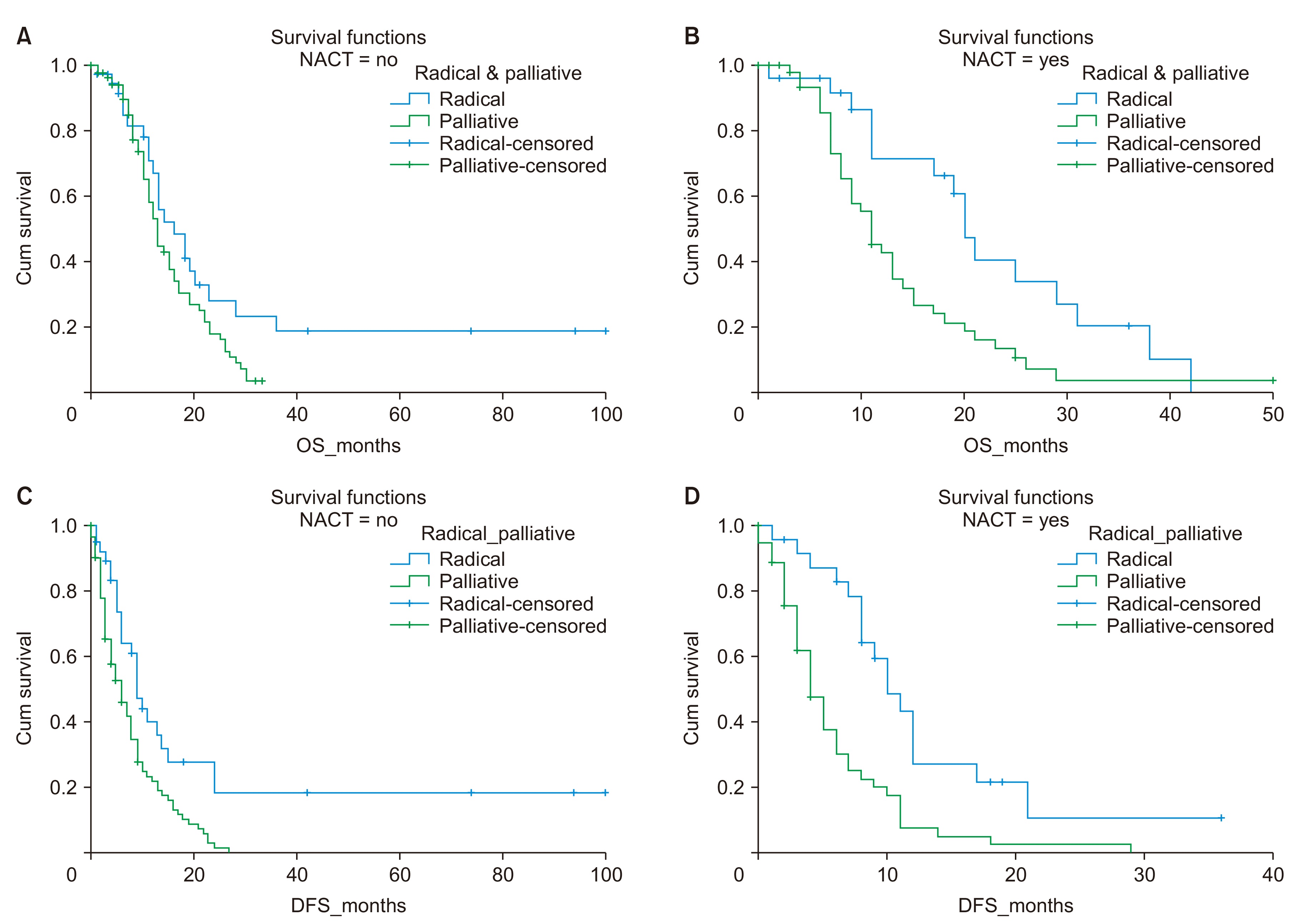
Table 1
Distribution of baseline characteristics in the group of patients undergoing radical surgery and palliative chemotherapy (percentages indicated in a vertical column)
Table 2
Sub-group analysis for overall survival and disease-free survival with respect to site of disease involvement
Table 3
Impact of NACT among patients receiving curative and palliative intent treatment
Table 4
Cox-regression analysis to determine factors affecting survival during treatment of oligo-metastatic GBC




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download