This article has been
cited by other articles in ScienceCentral.
Abstract
A 50-year-old male presented gradually growing pancreatic body mass. An abdominal computed tomography showed a 9.9-cm mass, larger than the 8.9-cm mass one year ago. As the patient did not have complaints for any symptomatic problems, the gastroenterologist decided to check it with regular follow-up. However, as the tumor grew faster than expected, the patient was recommended for surgical resection. Laparoscopic pylorus preserving pancreaticoduodenectomy was done. Since the tumor abutted to the superior mesenteric vein and the portal vein, wedge resection of vessel was inevitable. Pathology was serous cystadenoma. The patient was discharged without postoperative complications. Herein, we report this case with asymptomatic large serous cystic neoplasm treated by laparoscopic approach. The appropriateness of current guidelines for surgery in serous cystic neoplasm is also discussed.
Go to :

Keywords: Pancreatic cyst, Serous cystic neoplasm, Pancreatectomy
INTRODUCTION
Serous cystic neoplasm (SCN) accounts for a relatively smaller proportion than other tumorous lesions of pancreas [
1]. Since its malignancy potential is low, surgery is required only if it is symptomatic, if it is difficult to differentiate it from malignant tumor, or if it has features of serous cystadenocarcinoma. Otherwise, radiological follow-up might be appropriate for management of this tumor.
Patients visiting hospitals with large, asymptomatic SCN are not rare. For these cases, since surgeons must decide whether to perform a surgery or not, the extent of surgery, surgical options (open or minimally invasive) based on surgical risk-benefit. In the past, we presented three cases of SCN treated by surgical extirpation, suggesting not only symptomatic but also asymptomatic SCN could be managed by minimally invasive approach based on the growth rate of the tumor and its potential clinical consequences [
2].
Herein, we present a case with successful laparoscopic pancreaticoduodenectomy (LPD) for a huge asymptomatic SCN of the pancreas. This report is not only suggesting technical feasibility of LPD for a huge pancreatic tumor, but also questioning if the current practice guideline of SCN, no symptom, no surgery policy, is really appropriate for all patients with SCN.
Go to :

CASE
A 50-year-old male with a history of hypertension presented to outpatient department with a gradually growing pancreatic body mass without showing any symptoms. All routine laboratory tests were within normal values, with CA19-9 level of 6.1 U/mL and carcinoembryonic antigen level of 1.58 ng/mL, which were also normal. An abdominal computed tomography (CT) scan showed a huge pancreatic body mass causing upstreaming pancreatic duct dilatation. The size of the mass increased compared with that in CT scan one year ago (7.7 cm × 8.9 cm to 8.3 cm × 9.9 cm) (
Fig. 1). With enhancing septations without specific invasion of nearby tissue under radiologic and clinical impression of serous cystadenoma, the patient underwent laparoscopic pylorus preserving pancreatoduodenectomy. Since the tumor was abutting to the superior mesenteric vein (SMV) and the portal vein (PV) (
Fig. 2A,
2B), patient underwent wedge resection of the SMV-PV complex (
Fig. 2C,
2D). Operation time was 718 minutes. The operation was finished without massive bleeding or open conversion. The patient’s postoperative course had no specific events such as postoperative pancreatic fistula or bile leakage. Reported pathology was serous cystadenoma of a microcystic type with a size of 11.0 cm × 9.0 cm × 8.0 cm. The resection margin was free from tumor. There was no lymph node metastasis or vascular invasion. Postoperative course was not eventful. The patient was discharged on the 11th day postoperatively.
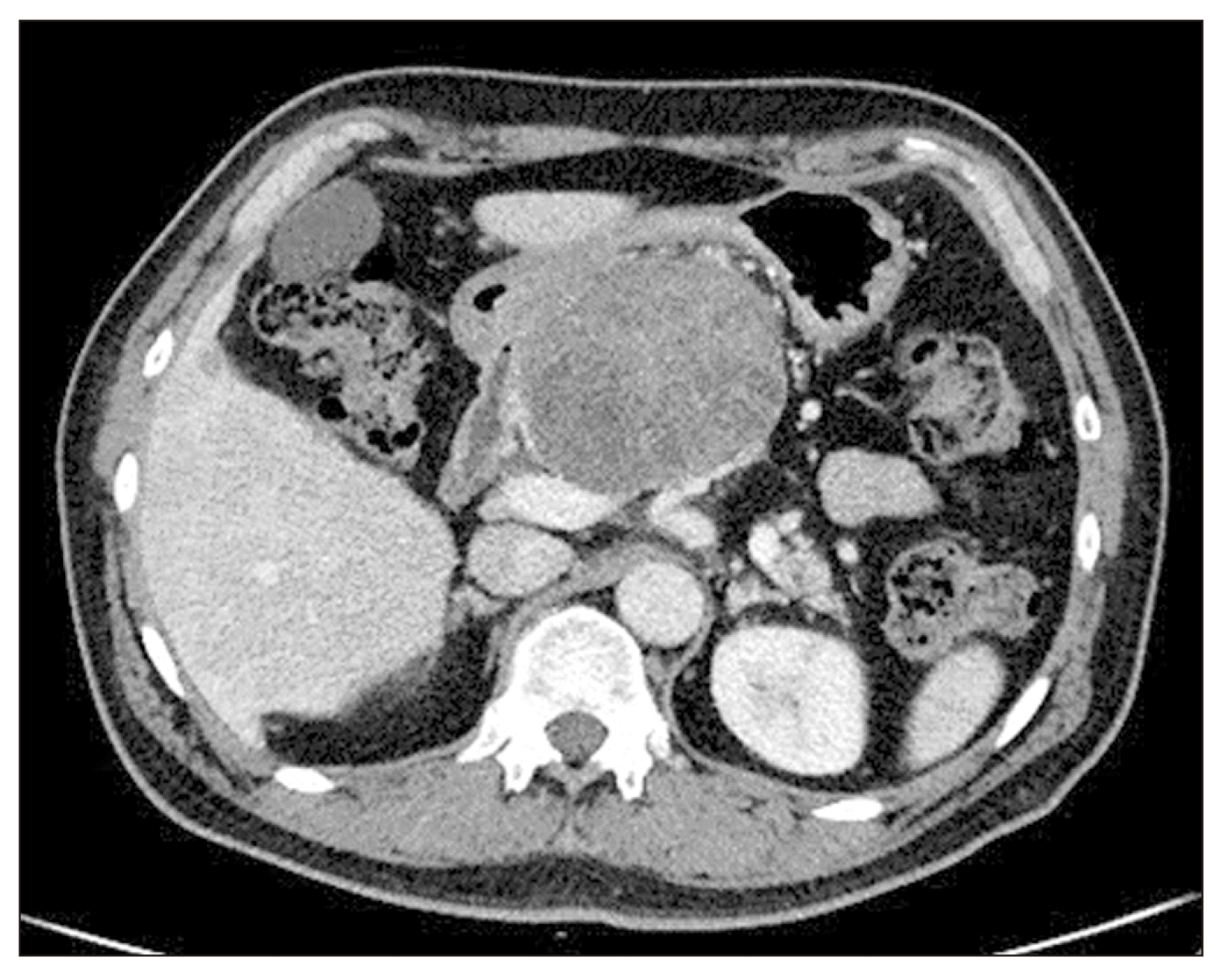 | Fig. 1Preoperative abdominal computed tomography showing a 9.9 cm sized pancreatic body mass that is annually increasing in size. 
|
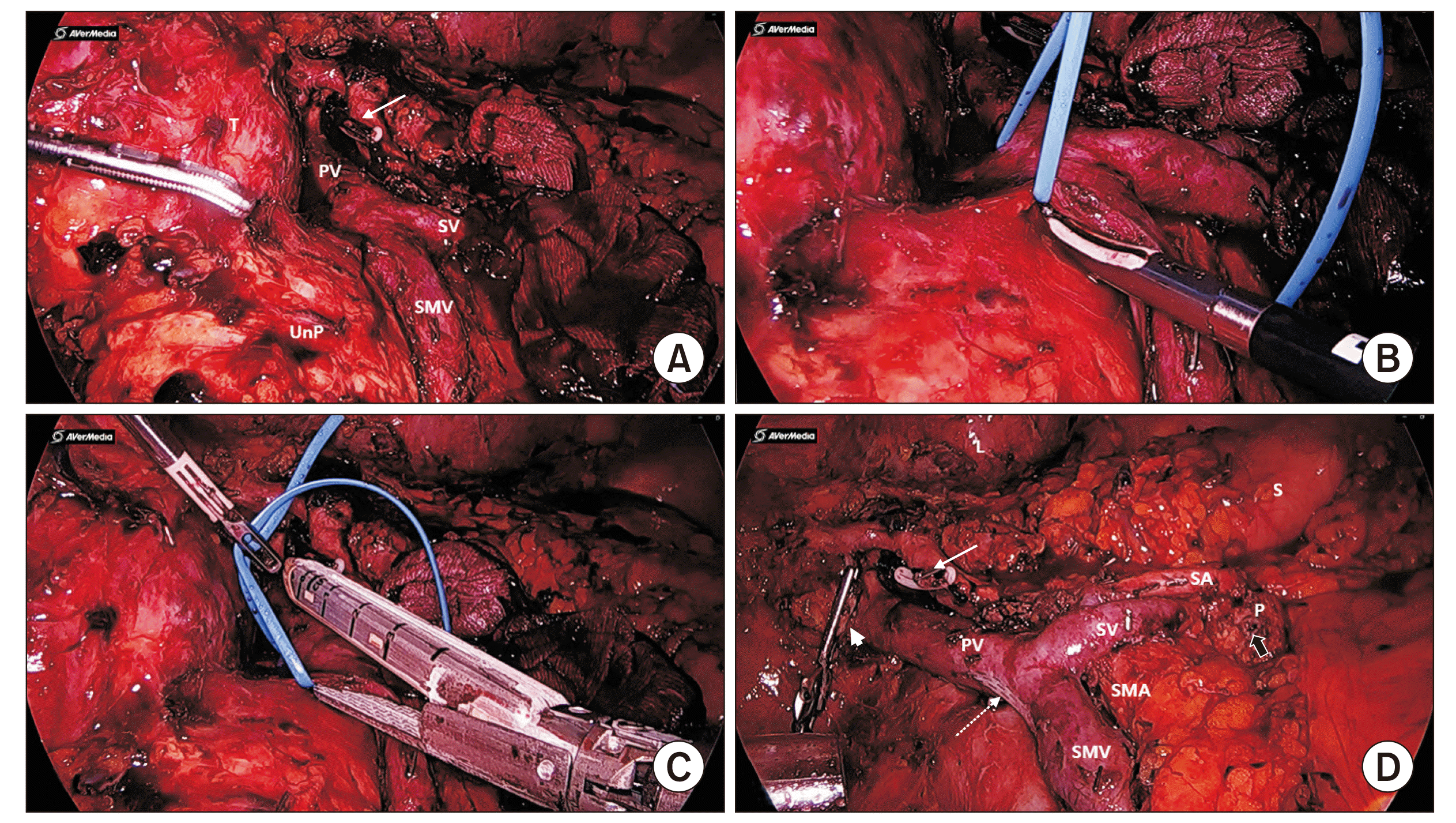 | Fig. 2Operative finding showing that the tumor is not involving the SA. (A) After division of the pancreas over the SA, a gastroduodenal artery stump (white long arrow) is found. (B) Severe adhesion between the tumor and the SMV lateral border was found. (C) Vascular endo-GIA was applied. (D) Final operative field after removal of the specimen. Note stapled line (dotted white arrow), gastroduodenal artery stump (white long arrow), resected bile duct (thick white arrow), and resected pancreatic duct (thick black arrow). T, tumor; PV, portal vein; SV, splenic vein; SMV, superior mesenteric vein; UnP, uncinated process; P, remnant pancreas; L, liver; S, stomach; SA, splenic artery; SMA, superior mesenteric artery. 
|
Go to :

DISCUSSION
Issues on surgery only when it causes trouble: As previously reported, SCN is known to have a very low malignant potential [
3]. In addition, with the advance of technology for image modality and increasing radiologists’ clinical experiences, surgery due to uncertain diagnosis is thought to be rare. Thus, most cases of SCN treated by surgical extirpation are those causing real clinical symptoms.
However, there are several concerning points when determining a surgery for a symptomatic SCN of the pancreas. First, tumor-related issues should be considered. Usually, SCN is a slowly growing tumor. There is a high chance of chronic inflammation around tumors and potential malignant changes, resulting in abutment to major vascular structures or adjacent organ that might require potential combined resection when considering resection of a large symptomatic SCN [
4].
Basically, tumor biology of SCN is benign in nature. However, above-mentioned clinical circumstances are thought to be the same as those of a malignant disease. According to several studies reporting surgical extirpation of symptomatic SCNs [
5], the size of the tumor is relatively large. Combined adjacent organ resection has been frequently reported [
6]. In addition, a recent meta-analysis has shown that although pancreatoduodenectomy combined with venous vascular resection is safe, morbidity and mortality are still high [
7]. In the present case, there was severe adhesion noted between tumor and SMV, leading to tangential vascular resection using endo-GIA. Second, patient-related issue should be also considered. Usually, SCNs grow very slowly, resulting in symptoms at late age of patients’ life time. In addition to the potential of combined organ or vascular resection, old age-associated with severe co-morbidity is another obstacle for safe resection of a symptomatic SCN. Although this clinical situation is not the final goal of appropriate follow-up strategy, it might be a consequence of neglecting patients under the guideline saying that
“Do not surgery! till it causes trouble.”
“Surgery before it causes troubles.“ Taking the following issues into consideration, a surgical strategy before it causes troubles can be proposed [
2,
8]. Once the SCN begins to grow, the growth rate is known to be increasing [
9,
10]. As patients get older, comorbidities and patients’ systemic conditions might not appropriate for an extended surgical intervention for large symptomatic SCNs. With the advance of laparoscopic and robotic surgery, minimally invasive pancreatectomy can be safely performed in selected patients by experts [
11]. In particular, long-term survival is highly expected for most benign and low-grade malignant tumors of the pancreas. Thus, a minimally invasive pancreatectomy is the optimal treatment for these patients.
Although opinions about the maximum size of a tumor suitable for minimally invasive pancreatectomy are different from surgeon to surgeon, it is generally advisable to decide surgery within an acceptable range of tumor size. In the present case, LPD was performed for a larger SCN of more than 10 cm in diameter. It showed that even huge SCN could be managed by LPD. However, surgical technique was difficult and combined tangential PV resection was necessary. In our previous report, 5 cm was suggested as the maximum size of a minimally invasive approach for an enlarged SCN [
2,
8]. A multicenter collaborative study is needed to propose an appropriate strategy for SCN in clinical practice.
It would be an appropriate strategy for surgeons to accurately inform patients about the natural course of SCNs, allowing them to benefit from minimally invasive surgery at an appropriate time (not too late as shown in the present study) based on close follow-up. However, it is still questionable whether surgical resection would improve the quality of life of patients with SCNs in the long run. Pros and cons of an early surgery in asymptomatic patients with SCN remain unclear. The strategy of waiting until symptoms occur is still debatable. Thus, further study and experience are needed.
In conclusion, minimally invasive pancreatectomy is now regarded as safe and effective in well selected patients according to recent literature and experiences. Timely surgical intervention by minimally invasive pancreatectomy can be one of the options for an annually growing asymptomatic SCN before it is too late.
Go to :







 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download