Abstract
It has been shown that external pancreatic ductal stenting (EPDS) can reduce the incidence of clinically relevant postoperative pancreatic fistula. Although studies have described EPDS in open pancreaticoduodenectomy (PD), EPDS in minimally invasive PD has not been reported yet. Thus, the objective of this study was to describe the technique of EPDS in minimally invasive PD. The procedure was performed either laparoscopically or using a robot. Once PD was completed, key steps included triple enterotomy, threading of silk-suture through all enterotomies and exteriorization, completing posterior layer of pancreaticojejunostomy (PJ), railroading stent through preplaced silk-suture, intubation of stent into the pancreatic duct, completion of PJ, followed by hepaticojejunostomy and parietalization of jejunum at the stent exit site. EPDS in PD through a minimally invasive approach can be performed safely in selected cases with either a small-sized pancreatic duct or a soft pancreas.
In the current minimally invasive surgery (MIS) era, pancreatoduodenectomy (PD) remains one of the most complex surgeries performed. MIS demands meticulous surgical techniques. Thus, MIS has a steep learning curve [1]. Although the mortality rate among patients undergoing PD has reduced from 25%–30% to 1%–5% over the past 25 years, it is still a significant number due to the number of cases performed worldwide and the broader application of MIS [2]. Most of the morbidity and mortality related to this procedure are either directly or indirectly attributed to pancreatic anastomotic leak, which remains the "Achilles heel" of Whipplés procedure [3]. Among various strategies proposed to reduce the risk of developing clinically relevant postoperative pancreatic fistula (CR-POPF), stenting of pancreaticojejunal anastomosis is a prominent measure [4,5]. The incidence of POPF in minimally invasive PD has been reported to be 4%–33% [6]. Although external pancreatic ductal stenting (EPDS) technique in open PD has been described, EPDS in minimally invasive PD has not been reported yet. Thus, the objective of this study was to describe our EPDS technique in laparoscopic and robotic PD.
All patients underwent contrast-enhanced CT using a pancreatic protocol to localize and stage the malignancy and to assess the resectability. Patients with clinical or subclinical cholangitis features underwent preoperative endoscopic retrograde cholangiography and biliary drainage using plastic stents. Simultaneously, a biopsy was obtained for cases with apparent tumor growth in the side viewing scope. Otherwise, a diagnosis was made based on imaging findings. Serum tumor markers such as CA19-9 and CEA were routinely evaluated. Positron emission tomography was decided on a case-by-case basis. Definitive surgery was planned at the end of two to three weeks following biliary drainage. Due consent was obtained from all patients who underwent surgical procedures preoperatively.
The patient was placed in the supine position with a leg split (French position). Pneumoperitoneum was created using the open umbilical pillar technique. A 12 mm infraumbilical trocar was placed at the camera port. Port placement for laparoscopic and robotic DaVinci Xi® system for PD is shown in Fig. 1. The Robot was docked from the right side of the patient after port placement was complete.
After opening the gastrocolic ligament, the gastrocolic trunk was identified at its junction with the superior mesenteric vein and ligated. The tunnel of love was entered between the portal vein and the neck of the pancreas. Kocherization of the duodenum was done. Pylorus was dissected and stapled. Lymphadenectomy was completed along the common hepatic artery and the left of hepatoduodenal ligament (HDL) after taking down the right gastric artery. Gastroduodenal artery was identified and ligated. Cholecystectomy was completed. The bile duct was transected and HDL lymphadenectomy was completed. Duodenojejunal flexure was taken down. Proximal jejunum was transected and its mesentery was divided, preserving the first jejunal artery. Pancreatic neck transection was done using electrocautery. An attempt was made to visualize the pancreatic duct during transection. Uncinate dissection was completed after carefully dividing the inferior pancreaticoduodenal artery and securing the posterior superior pancreaticoduodenal vein. The pancreatic stump was not routinely mobilized in order to preserve its vascularity.
The technique of EPDS will be described under the following headings:
1. Identification and dilation of pancreatic duct
2. Triple enterotomy of jejunum and threading
3. Completion of the posterior layer of pancreatojejunostomy (PJ) and ductal cannulation
4. Completion of PJ and hepaticojejunostomy (HJ)
5. Parietalization of jejunum
A large pancreatic duct could be easily identified. However, identifying a pancreatic duct with a small-caliber might be challenging. Maneuvers such as gentle pressure over the pancreas' surface or gentle probing with non-traumatic instruments might extrude pancreatic juice and help identify smaller ducts. After the duct was identified, it could be dilated using the Maryland dissecting forceps tip to facilitate anastomosis.
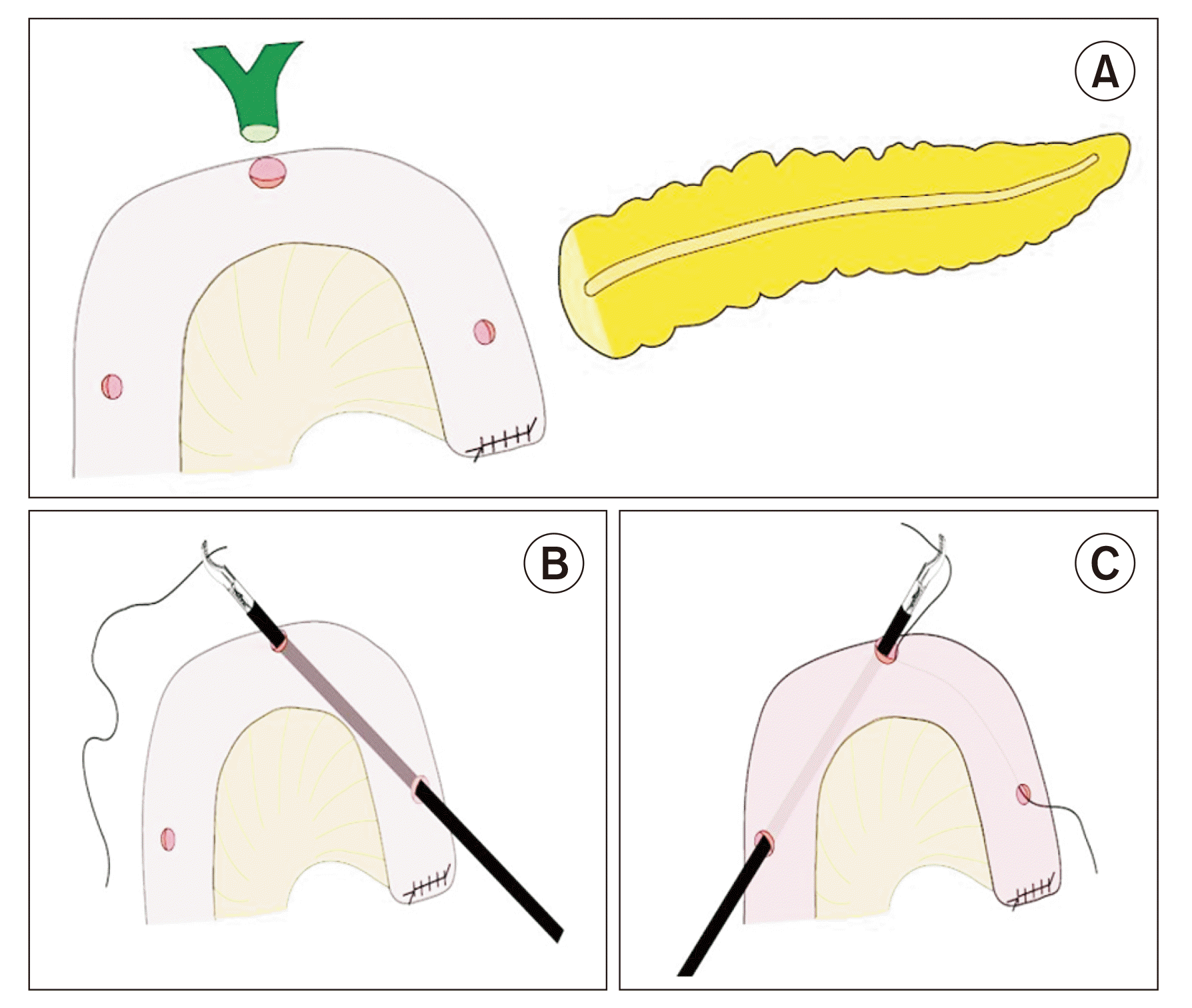
The jejunum was bought through the native mesocolic route for reconstruction. Three enterotomies were made using electrocautery on the jejunal loop. The first enterotomy was made at the planned PJ pancreatic duct-to-mucosa anastomotic site. The second enterotomy was made at the area corresponding to HJ. The third enterotomy was made at the place where the stent was to be exteriorized from the jejunum (Fig. 2A). The enterotomy at the PJ site and stent exteriorization site should not be larger than 0.5 cm. In contrast, the enterotomy at the HJ site could be made in concordance with bile duct diameter. A 1-0 silk suture Ethicon of approximately 100 cm was threaded through each enterotomy using Maryland dissecting forceps after completing the enterotomy. The thread's entry and exit points would be from the PJ site and parietalization site, respectively (Fig. 2B, 2C). After threading, the suture was taken externally using a suture passer at the pre-planned stent exit site. While the operating surgeon performed this step-in laparoscopic procedure, the assistant performed it in robotic PD through assistant ports.
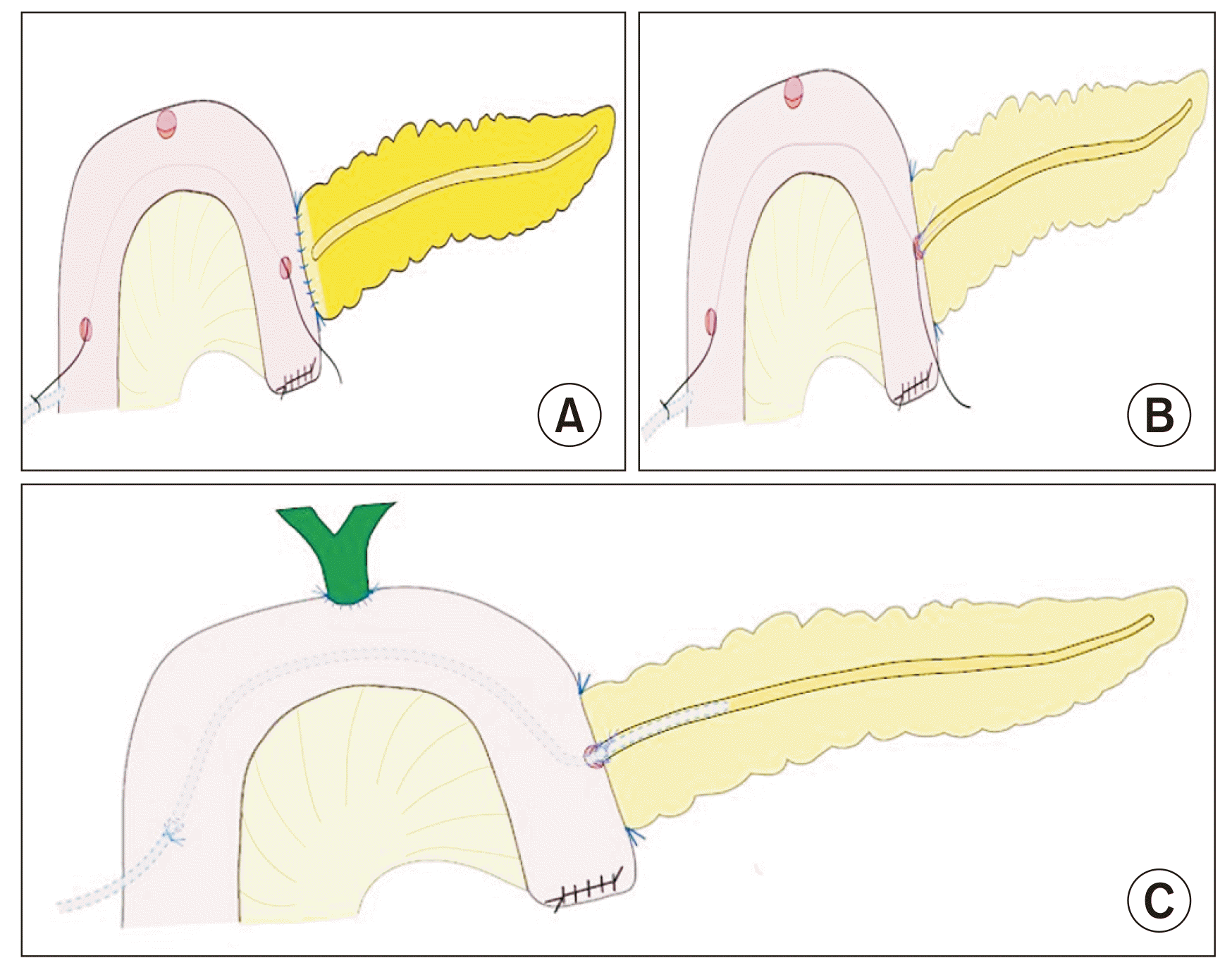
We preferred the modified Blumgart technique for PJ [7]. The posterior layer of PJ was initially taken with 4-0 prolene Ethicon interrupted stitches, which incorporated the pancreas parenchyma with posterior pancreatic capsule to the seromuscular layer of jejunum. Three duct to mucosa sutures were taken using 4-0 PDS Ethicon (absorbable) suture at 11'o clock, 9'o clock, 7'o clock, which completed the posterior layer of duct to mucosa anastomosis (Fig. 3A, 3B). The suture taken at 9'o clock was left long to secure the stent. At this juncture, the temporary plastic stent of 15 cm length placed in the pancreatic duct could guide in taking pancreatic ductal stitches by keeping the duct patent and visualizing the ductal mucosa.
The stent was selected depending on duct size. A polyurethane plastic stent of 5 to 8 Fr was usually used. The stent's length varied from 40 to 50 cm. Subsequently, the plastic stent's tip was secured to the exteriorized end of the silk suture and gently maneuvered inside the abdomen. The stent was railroaded through the jejunum by gently withdrawing the other end of the silk suture at the PJ enterotomy site. The temporary stent was removed and the external plastic stent was cannulated into the pancreatic duct. The stent was introduced into the pancreatic duct until resistance was encountered. After cannulation, the stent was secured using long cut ends of the duct to mucosa stitch at a 9'o clock position.
The anterior layer of the pancreaticojejunal duct to the mucosa was completed by taking 1'o clock, 3'o clock, and 5'o clock positions for suturing using 4-0 PDS Ethicon (absorbable) sutures with corresponding full-thickness jejunal mucosa. The outer (anterior) layer was completed using 3-0 prolene Ethicon interrupted sutures by incorporating the pancreas' anterior capsule with partial pancreatic parenchyma and jejunal seromuscular layer.
Hepaticojejunostomy was performed using 4-0 PDS Ethicon (absorbable) sutures with anterior and posterior layers taken in a continuous manner. The position of the stent after completion of PJ and HJ is shown in Fig. 3C.
The stent was secured at the jejunal enterotomy exit site by placing a purse-string suture using a 3-0 PDS Ethicon (absorbable) suture. Parietalization of jejunal at stent exit site was done using 3-0 Prolene Ethicon suture.
At the same time, the stent was fixed externally to prevent accidental dislodgement. Posterior gastrojejunostomy was done in an ante-colic fashion approximately 40 cm from HJ. A feeding jejunostomy (FJ) was also added. The specimen was extracted through a small periumbilical incision. One abdominal drain was placed near the PJ and another one was placed near the HJ. Fig. 4 shows the abdomen's final appearance after completing the procedure with an external pancreatic ductal stent in situ.
Critical steps of the procedure are demonstrated in the video provided as a Supplementary Video.
We started enteral feeds through the FJ on the first postoperative day (POD) 2. Serum and drain fluid amylases (DFAs) were measured on the 1st, 3rd, and 5th POD as per protocol. Abdominal drains were removed based on drainage and level of DFA. Pancreatic juice output collected from the EPDS was monitored and periodically re-fed through the FJ. EPDS was blocked on POD 5–7 and removed after six weeks during a follow-up visit. A follow-up imaging was routinely done as per protocol to look for any recurrence. The pancreatic ductal diameter was assessed to look for any ductal stenosis.
The morbidity and mortality related to PD are either directly or indirectly related to pancreatic fistulae development. The incidence rate of CR-POPF is 15% in minimally invasive PD and 13% in open PD [8]. Both PJ and pancreaticogastrostomy were performed in minimally invasive PD based on individual preference. We routinely performed PJ in minimally invasive PD. An ideal pancreaticojejunal anastomosis should maintain an unobstructed pancreatic output flow into the jejunum while preserving the pancreatic stump's vascularity, irrespective of pancreatic duct size or consistency of pancreatic parenchyma.
The risk of POPF is increased in patients with a smaller pancreatic duct and soft pancreatic parenchyma [9]. Consistency of the pancreas is one of the well-known risk factors for the development of POPF [10]. However, with widespread adaptation of MIS, where there is a lack of adequate tactile feedback, it is difficult to assess the consistency of the pancreas because routine predictors such as Fistulae risk score cannot be used [11]. Hence, many new predictors for POPF such as visceral fat attenuation and pancreatic attenuation are being explored to reliably predict POPF preoperatively [12-14]. The development of POPF has been attributed to multiple risk factors. Hence, various technical strategies have been tried to address this issue [15].
Stenting the pancreatic duct following PD has been shown to be an effective strategy to reduce the incidence of CR-POPF or decrease the morbidity associated with CR-POPF if it develops in a stented individual [16,17]. A pancreatic duct stent is proposed to direct the pancreatic juice away from anastomosis, decompress residual pancreatic stump despite initial impaired intestinal peristalsis, and maintain the patency of the pancreatic duct by preventing any stenosis, all of which are critical for the healing of PJ anastomosis. While both internal pancreatic ductal stenting (IPDS) and EPDS have been shown to confer these benefits, external stenting tends to offer near-complete diversion of pancreatic secretion away from the anastomosis, showing a lower risk of migration and retention than internal stenting in a few studies [18]. EPDS has been routinely described in open PD. However, the IPDS technique remains widely used during MIS-PD due to its technical ease. The need of the hour is to describe a standardized and reproducible EPDS technique in MIS-PD given the widespread application of a minimally invasive platform for performing PD.
Certain factors are responsible for the scientific community to dichotomize over the concept of stenting the pancreatic duct in PDT [19]. The technical skill that it demands, the difficulty of identifying the pancreatic duct, the additional amount of time required to complete this step, stent-related complications, and the absence of a universally standardized and accepted technical description are main harbingers preventing its widespread adaptation.
Contrary to a common belief that a stent in the pancreatic duct makes reconstruction more complicated, it makes the placement of duct to mucosa sutures more accurate. Although identifying small-sized pancreatic ducts can be challenging at times, with currently available advanced minimally invasive visual systems providing higher resolution, magnification, and 3D vision, identification should never be difficult. Stent-related complications have been reported previously. However, they are infrequent and overrated [20]. None of the patients in our series had stent-related complications such as stent migration, stent-related pancreatitis, peritonitis, kinking, or obstruction of the stent, although these complications are possible in EPDS [21]. The frequency of stent-related complications varies in literature ranging around 4 to 5% [21,22]. Pancreatic ductal stenosis and stricture following stenting were among worrisome complications. Their rates have been reported to be around 20% on long-term follow-up [22]. Pancreatic ductal stenosis is defined when there is a decrease in pancreatic ductal size by 25% compared to preoperative size [22].
Multiple techniques for stenting, albeit described in the open procedure in the literature, are main factors responsible for the low acceptance of EPDS [23]. A method of EPDS through the blind limb of PJ was described previously in open PD [18]. However, replicating the same in MIS is fraught with the risk of transient peritonitis, which is aided by low adhesions and less robust tract maturation. It is not advisable in MIS, although it is technically easier to perform [18,24].
Our EPDS stenting technique is relatively simple, providing near-complete diversion of the fistulogenic pancreatic secretions away from the anastomosis (we usually re-feed through feeding jejunostomy), safe for all patients who have undergone this procedure, showing promising early results.
EPDS of PJ anastomosis is a safe, feasible, and easily reproducible technique following MIS-PD when it is performed either laparoscopically or robotically for a pancreas with a small duct.
Supplementary data related to this article can be found at https://doi.org/10.14701/ahbps.22-098.
ACKNOWLEDGEMENTS
The author would like to acknowledge Prof. Peush Sahni, Prof. Sujoy Pal, and Prof. NR Dash from the Department of GI Surgery, AIIMS New Delhi, for their valuable inputs.
REFERENCES
1. Sharpe SM, Talamonti MS, Wang CE, Prinz RA, Roggin KK, Bentrem DJ, et al. 2015; Early national experience with laparoscopic pancreaticoduodenectomy for ductal adenocarcinoma: a comparison of laparoscopic pancreaticoduodenectomy and open pancreaticoduodenectomy from the national cancer data base. J Am Coll Surg. 221:175–184. DOI: 10.1016/j.jamcollsurg.2015.04.021. PMID: 26095569.

2. Pedrazzoli S. 2017; Pancreatoduodenectomy (PD) and postoperative pancreatic fistula (POPF): a systematic review and analysis of the POPF-related mortality rate in 60,739 patients retrieved from the English literature published between 1990 and 2015. Medicine (Baltimore). 96:e6858. DOI: 10.1097/MD.0000000000006858. PMID: 28489778. PMCID: PMC5428612.
3. Crippa S, Salvia R, Falconi M, Butturini G, Landoni L, Bassi C. 2007; Anastomotic leakage in pancreatic surgery. HPB (Oxford). 9:8–15. DOI: 10.1080/13651820600641357. PMID: 18333107. PMCID: PMC2020778.

4. Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, et al. 2017; The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery. 161:584–591. DOI: 10.1016/j.surg.2016.11.014. PMID: 28040257.

5. Nahm CB, Connor SJ, Samra JS, Mittal A. 2018; Postoperative pancreatic fistula: a review of traditional and emerging concepts. Clin Exp Gastroenterol. 11:105–118. DOI: 10.2147/CEG.S120217. PMID: 29588609. PMCID: PMC5858541.

6. Chen K, Pan Y, Liu XL, Jiang GY, Wu D, Maher H, et al. 2017; Minimally invasive pancreaticoduodenectomy for periampullary disease: a comprehensive review of literature and meta-analysis of outcomes compared with open surgery. BMC Gastroenterol. 17:120. DOI: 10.1186/s12876-017-0691-9. PMID: 29169337. PMCID: PMC5701376.

7. Hirono S, Kawai M, Okada KI, Miyazawa M, Kitahata Y, Hayami S, et al. 2019; Modified blumgart mattress suture versus conventional interrupted suture in pancreaticojejunostomy during pancreaticoduodenectomy: randomized controlled trial. Ann Surg. 269:243–251. DOI: 10.1097/SLA.0000000000002802. PMID: 29697455. PMCID: PMC6325750.

8. Kantor O, Pitt HA, Talamonti MS, Roggin KK, Bentrem DJ, Prinz RA, et al. 2018; Minimally invasive pancreatoduodenectomy: is the incidence of clinically relevant postoperative pancreatic fistula comparable to that after open pancreatoduodenectomy? Surgery. 163:587–593. DOI: 10.1016/j.surg.2017.12.001. PMID: 29454444.

9. Olakowski M, Grudzińska E, Mrowiec S. 2020; Pancreaticojejunostomy-a review of modern techniques. Langenbecks Arch Surg. 405:13–22. DOI: 10.1007/s00423-020-01855-6. PMID: 31975148. PMCID: PMC7036071.

10. Hu BY, Wan T, Zhang WZ, Dong JH. 2016; Risk factors for postoperative pancreatic fistula: analysis of 539 successive cases of pancreaticoduodenectomy. World J Gastroenterol. 22:7797–7805. DOI: 10.3748/wjg.v22.i34.7797. PMID: 27678363. PMCID: PMC5016380.

11. Callery MP, Pratt WB, Kent TS, Chaikof EL, Vollmer CM Jr. 2013; A prospectively validated clinical risk score accurately predicts pancreatic fistula after pancreatoduodenectomy. J Am Coll Surg. 216:1–14. DOI: 10.1016/j.jamcollsurg.2012.09.002. PMID: 23122535.

12. McAuliffe JC, Parks K, Kumar P, McNeal SF, Morgan DE, Christein JD. 2013; Computed tomography attenuation and patient characteristics as predictors of complications after pancreaticoduodenectomy. HPB (Oxford). 15:709–715. DOI: 10.1111/hpb.12037. PMID: 23458275. PMCID: PMC3948539.

13. Tranchart H, Gaujoux S, Rebours V, Vullierme MP, Dokmak S, Levy P, et al. 2012; Preoperative CT scan helps to predict the occurrence of severe pancreatic fistula after pancreaticoduodenectomy. Ann Surg. 256:139–145. DOI: 10.1097/SLA.0b013e318256c32c. PMID: 22609844.

14. Yardimci S, Kara YB, Tuney D, Attaallah W, Ugurlu MU, Dulundu E, et al. 2015; A simple method to evaluate whether pancreas texture can be used to predict pancreatic fistula risk after pancreatoduodenectomy. J Gastrointest Surg. 19:1625–1631. DOI: 10.1007/s11605-015-2855-7. PMID: 25982120.

15. Kawaida H, Kono H, Hosomura N, Amemiya H, Itakura J, Fujii H, et al. 2019; Surgical techniques and postoperative management to prevent postoperative pancreatic fistula after pancreatic surgery. World J Gastroenterol. 25:3722–3737. DOI: 10.3748/wjg.v25.i28.3722. PMID: 31391768. PMCID: PMC6676555.

16. Markar SR, Vyas S, Karthikesalingam A, Imber C, Malago M. 2012; The impact of pancreatic duct drainage following pancreaticojejunostomy on clinical outcome. J Gastrointest Surg. 16:1610–1617. DOI: 10.1007/s11605-012-1852-3. PMID: 22383216.

17. Patel K, Teta A, Sukharamwala P, Thoens J, Szuchmacher M, DeVito P. 2014; External pancreatic duct stent reduces pancreatic fistula: a meta-analysis and systematic review. Int J Surg. 12:827–832. DOI: 10.1016/j.ijsu.2014.06.008. PMID: 25003575.

18. Poon RT, Fan ST, Lo CM, Ng KK, Yuen WK, Yeung C, et al. 2007; External drainage of pancreatic duct with a stent to reduce leakage rate of pancreaticojejunostomy after pancreaticoduodenectomy: a prospective randomized trial. Ann Surg. 246:425–433. discussion 433–435. DOI: 10.1097/SLA.0b013e3181492c28. PMID: 17717446. PMCID: PMC1959348.
19. Dong Z, Xu J, Wang Z, Petrov MS. 2016; Stents for the prevention of pancreatic fistula following pancreaticoduodenectomy. Cochrane Database Syst Rev. 2016:CD008914. DOI: 10.1002/14651858.CD008914.pub3.
20. Helaly M, iwi D Sr, Alkholaidi WS, Almamlouk R, Elshaer A, Allaboon RM, et al. 2019; Retrograde pancreatic duct stent migration into the biliary tract presenting as a rare early complication of pancreaticoduodenectomy (Whipple procedure). Am J Case Rep. 20:1864–1868. DOI: 10.12659/AJCR.917297. PMID: 31831724. PMCID: PMC6930705.

21. Jang JY, Chang YR, Kim SW, Choi SH, Park SJ, Lee SE, et al. 2016; Randomized multicentre trial comparing external and internal pancreatic stenting during pancreaticoduodenectomy. Br J Surg. 103:668–675. DOI: 10.1002/bjs.10160. PMID: 27040594.

22. Shin YC, Jang JY, Chang YR, Jung W, Kwon W, Kim H, et al. 2019; Comparison of long-term clinical outcomes of external and internal pancreatic stents in pancreaticoduodenectomy: randomized controlled study. HPB (Oxford). 21:51–59. DOI: 10.1016/j.hpb.2018.06.1795. PMID: 30093143.

23. Boggi U, Amorese G, Vistoli F, Caniglia F, De Lio N, Perrone V, et al. 2015; Laparoscopic pancreaticoduodenectomy: a systematic literature review. Surg Endosc. 29:9–23. DOI: 10.1007/s00464-014-3670-z. PMID: 25125092.

24. Justo Alonso I, Marcacuzco Quinto A, Caso Maestro O, Jiménez-Romero C. 2018; Anastomotic reconstruction and external drainage of Wirsung's duct as treatment for pancreaticojejunal stenosis following pancreaticoduodenectomy. Cir Esp (Engl Ed). 96:648–652. DOI: 10.1016/j.ciresp.2018.08.003. PMID: 30448151.

Fig. 1
Port positions. (A) Laparoscopic pancreaticoduodenectomy, (B) Robot-assisted pancreaticoduodenectomy. R1–4, robotic port 1 to 4; A1, 2, assistant port 1, 2.
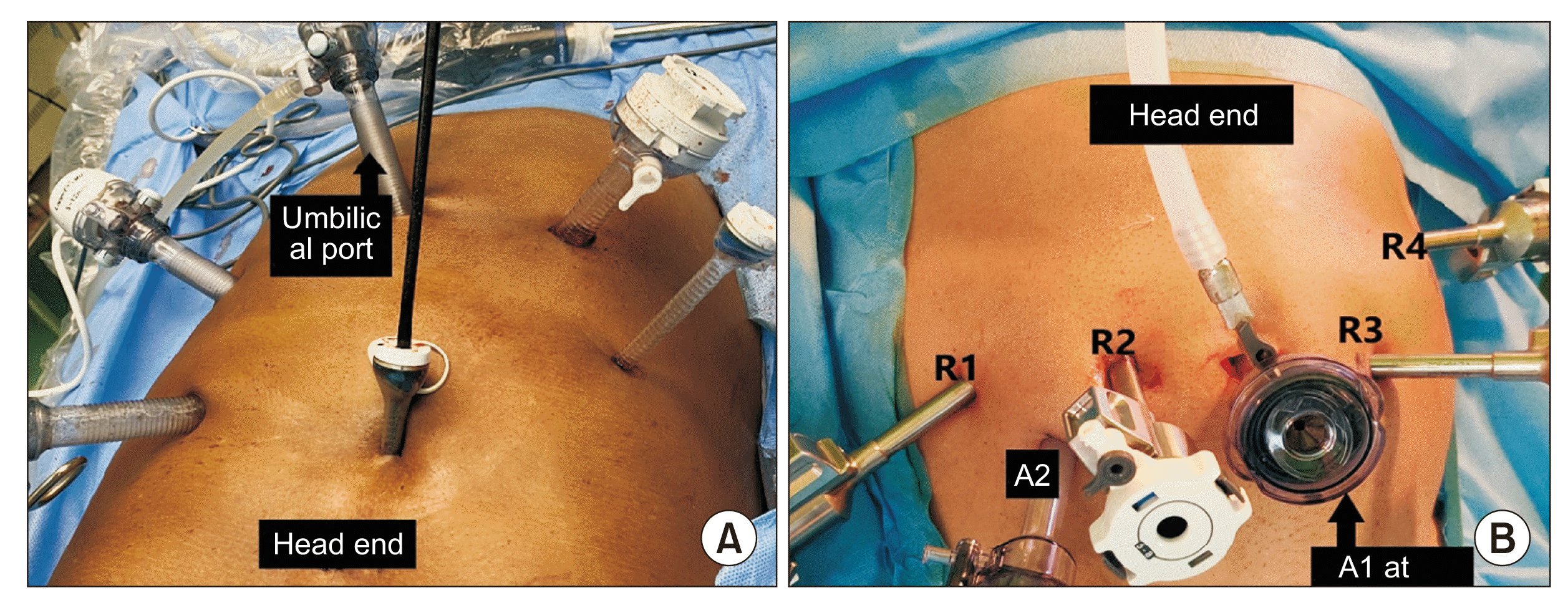
Fig. 2
(A) Triple enterotomy. (B) Threading of suture through hepaticojejunostomy (HJ) and pancreatojejunostomy, (C) Threading of suture through parietalization site and HJ site.




 PDF
PDF Citation
Citation Print
Print





 XML Download
XML Download