Abstract
Laparoscopic cholecystectomy is associated with a higher incidence of biliary/vasculobiliary injuries than open cholecystectomy. Anatomical misperception is the most common underlying mechanism of such injuries. Although a number of strategies have been described to prevent these injuries, critical view of safety method of structural identification seems to be the most effective preventive measure. The critical view of safety can be achieved in the majority of cases during laparoscopic cholecystectomy. It is highly recommended by various guidelines. However, its poor understanding and low adoption rates among practicing surgeons have been global problems. Educational intervention and increasing awareness about the critical view of safety can increase its penetration in routine surgical practice. In this article, a technique of achieving critical view of safety during laparoscopic cholecystectomy is described with the aim to enhance its understanding among general surgery trainees and practicing general surgeons.
Laparoscopic cholecystectomy (LC) is the current standard of care for symptomatic cholelithiasis. However, it is associated with higher incidence of complications such as bile duct injury (BDI) and vasculobiliary injury (VBI) than open cholecystectomy [1].
The most common underlying mechanism of major post-cholecystectomy BDI/VBI involves misidentification of anatomical structures [2-4]. Due to various factors (misperception, altered anatomy, local pathological alterations, etc.), misidentification of structures can lead to division/occlusion of wrong targets (e.g., common bile/hepatic duct, aberrant right sectoral bile duct, right hepatic artery) instead of correct targets (i.e., cystic duct and artery), resulting in BDI/VBI [2,3].
Various preventive strategies have been described to reduce the incidence of post-cholecystectomy BDI/VBI [4-9]. The critical view of safety (CVS) method of target identification was described by Strasberg et al. in 1995 [10]. It is considered as the one of the most important critical factors for overall safety during LC by the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) expert group [11] and international expert groups from Japan, Korea, Taiwan, the USA, India, and other nations [4]. It is an essential component of the culture of safety in cholecystectomy concept (COSIC) as proposed by SAGES [9]. Because of its overall proven safety, the CVS method is uniformly recommended by various societies and guidelines as a target identification method during LC [1,12-15].
Despite the description of CVS many decades back and uniform recommendation for its use, its poor understanding and low adoption rates among surgeons and trainees in routine general surgical practice remain global problems [16-21]. In a survey of 374 practicing surgeons belonging to the Midwest Surgical Association and SAGES, only 27% of the respondents practiced the CVS method, whereas many of them could not identify the CVS descriptively (75%) or visually (21%) [17]. In a recent study conducted in China, the correct execution and cognition of the CVS were found to be deficient in the majority of cases with very low CVS achievement rates (18.18% in a non-inflammatory group and 9.84% in an inflammatory group) [19]. In a Mexican study involving both practicing surgeons and surgical residents, although 95% (n = 708) participants reported that they knew the concept of CVS, only half (51%) could define the concept correctly [20]. In our recent study, the majority (88.3%) of general surgery trainees claimed to know the CVS. However, only 11.5% knew it correctly, 15% described more than three components, and one third (34.9%) considered exposure of the cystic duct-common bile duct junction as a component of the CVS [21].
Despite poor understanding and dismal adoption rates of CVS in routine practice at present, educational intervention and increasing awareness of CVS might improve its achievement rates during LC effectively [19,22]. Herein, a technique of achieving CVS during LC utilizing various anatomical landmarks is described with the aim to enhance its understanding among general surgery trainees and practicing general surgeons.
Technique of achieving the CVS is described here with standard four-port method of LC with a setup in American position (Supplementary Video 1). Camera port (10 mm) is placed at the umbilicus by open method. One 10 mm port is placed in the epigastrium (surgeon’s right hand main working port). One 5 mm port is placed in the right lumbar region for gallbladder fundal retraction (by an assistant). The last port (5 mm) is placed in the right subcostal area for gallbladder neck retraction (surgeon’s left hand working port).
The CVS consists of three essential component or steps: 1) dissection of the hepatocystic triangle (HCT); 2) exposure of at least lower one third of the cystic plate (CP); and 3) demonstration of only two tubular structures (cystic duct and cystic artery) that remain attached to the gallbladder (after components 1 and 2 are achieved) [2,23]. All these three steps must be completed before considering that CVS has been achieved.
The surgical field of dissection during LC is HCT. Adjoining structures e.g., liver segments 4 and 5, hepatoduodenal ligament, hepatic hilum, umbilical fissure, duodenum, etc. are the anatomical areas of interest (Fig. 1). Proper exposure of these areas is imperative for a safe completion of the surgical procedure. Adequate abdominal distension (intrabdominal pressure ~12 mm Hg), proper patient positioning (reversed Trendelenburg with left lateral rotation), adequate gastric decompression (through properly positioned and patent nasogastric tube), optimal port positioning with appropriate modifications (e.g., for obese patients), proper gallbladder retraction, and perihepatic and pericholecystic adhesiolysis if required are some of the important steps to ensure a good exposure of the surgical field.
For proper gallbladder retraction, fundus is retracted towards the patient’s right shoulder. The infundibulum/neck is retracted infero-laterally during anterior dissection in the HCT and supero-medially towards the umbilical fissure/left shoulder during posterior dissection in the HCT (Fig. 1–3). Besides correct direction of retraction, traction force should be sufficient enough to open up the HCT and facilitate dissection. However, excessive traction can distort the anatomy, align the cystic duct with the common bile duct, increases the risk of avulsion of the cystic duct or tear the cystic duct-common bile duct junction, and may injure the diaphragm after accidental slippage of fundal grasper.
After exposing the surgical field and anatomical area of interest, certain fixed anatomical landmarks around the gallbladder are identified (Fig. 1). These landmarks can be used for proper orientation of the surgical field before and during the procedure, especially during difficult conditions.
Important anatomical landmarks are the Rouviere’s sulcus (RS), segment 4b and its base, umbilical fissure, hepatic artery proper, cystic lymph node, duodenum, and stomach (Fig. 1). The RS is the most important anatomical landmark. It provides the basis for the R4U line and planes as described by us previously [23,24]. Briefly, the R4U line is an imaginary line passing from the roof of the RS across the base of the segment 4b till the umbilical fissure (Fig. 1). This safety line provides the basis for two planes (the ‘A’ plane that passes along the anterior surface of the RS and the ‘R’ plane that passes along the roof the RS). These planes divide the surgical field into four zones: anterosuperior (safe zone), posterosuperior (potentially unsafe zone), anteroinferior (unsafe), and posteroinferior (unsafe) zones [24].
Once various landmarks are identified and safe and unsafe zones are demarcated, dissection is started in the anterosuperior zone and remained confined to superior zones only to avoid injury to vitals structures present in inferior zones.
Posterior (lateral/right) peritoneal division
After ascertaining the anatomy, the gallbladder neck is gently pulled in the ventral direction (i.e., elevated). Putative junction of the gallbladder neck and cystic duct is then identified and the peritoneal layer overlying this junction is opened using a monopolar cautery or bluntly (Fig. 2).
Posterior aspect of the HCT is then exposed by retracting gallbladder neck medially. The peritoneal layer is gently elevated by blunt dissection using Maryland forceps inserted through the opening created earlier (Fig. 2). Seepage of CO2 under pressure into the sub-serosal space further dissects planes (pneumo/capno-dissection) in the HCT, especially in cases with un-obliterated planes (i.e., cases with no or minimal inflammation) (Fig. 3). The peritoneal layer is then divided close to the gallbladder with a monopolar hook cautery starting from the gallbladder neck. It is then extended onto the body as far as possible (Fig. 3). The peritoneal division is then continued towards the cystic duct along the neck.
Once the posterior peritoneal attachment is divided, the anterior aspect of the HCT is exposed by retracting the neck inferolaterally while retaining the fundal retraction in the original direction (i.e., towards the right shoulder of the patient). Starting from the gallbladder neck-cystic duct junction, peritoneal attachment on the anterior aspect of the gallbladder is divided as distally as possible towards the fundus (Fig. 4). The cystic artery (sometimes with more than one branch) and cystic lymph node are usually encountered during this step (Fig. 4). Peritoneal division line remains close to the gallbladder wall and passes on the right (lateral) of or over the cystic lymph node. Peritoneal division is then continued along the cystic duct between the cystic duct and artery.
Once anterior and posterior peritoneal attachments are divided, actual (deeper) dissection in the HCT is performed. It is initially done from the posterior aspect. The gallbladder neck is retracted medially during this step (Fig. 5).
HCT is gradually cleared of fibrous and fatty tissues while remaining close to the gallbladder wall. Our preferred dissection technique is to use a monopolar hook cautery. Basic principles of safe use of energy source in the HCT, i.e., elevation and division of small amount of tissues at a time with small bursts of low wattage (≤ 25 W) current, that I refer to as HPB maneuver (Hook→Pull→Burn), are followed during this step [23]. Blunt dissection technique is an option. Sometimes a combination of these techniques can be used to create a window across the Calot’s triangle or dissect any tubular structures such as the cystic duct and artery.
As division of tissues progresses, the HCT gradually opens up. A window may be created across the gallbladder bed while exposing the CP and elevating the gallbladder from the liver, which can facilitate further dissection (Fig. 5, 6).
Once the posterior dissection seems adequate, dissection from the anterior aspect of the HCT is performed in a similar fashion (Fig. 6). Dissection in the HCT can also be performed alternatively from posterior and anterior aspects.
Dissection is then continued towards the gallbladder pedicle. The cystic duct is then circumferentially dissected (Fig. 6). For this, a window is created between the cystic duct and artery either by blunt dissection (Fig. 6) or with a hook cautery. Tissues are cleared along the cystic duct by alternate dissection from the anterior and posterior aspects of the HCT (Fig. 6). Once a sufficient extent of the cystic duct is exposed, the cystic artery is dissected which is already exposed after division of the anterior peritoneal attachment and during dissection in the HCT. If not, a window is created on either side of the cystic artery close to the gallbladder wall. Dissection is then continued proximally and distally along the artery. A portion of the cystic artery lateral to the cystic node is exposed (Fig. 6, 7).
Dissection along the lateral aspect of the cystic artery clears all tissues between the cystic duct and artery. A large window in the Calot’s triangle is created while exposing the cystic duct inferomedially, the cystic artery superomedially, and the gallbladder neck/body on the lateral side with the liver seen across this window (Fig. 6, 7). Similarly, dissection on the medial aspect of the cystic artery completes tissue clearance in the HCT.
Once exposed, both the cystic duct and artery are dissected for an extent sufficient enough to allow secure occlusion and division. No attempt is made to expose them in their entire extent (i.e., till the junction of the cystic duct with the bile duct or till the origin of the cystic artery). No attempt is made to excise any tissues or divide any luminal structures while dissecting in the HCT. Optimal dissection in HCT allows clear visualization of the liver across windows created (Fig. 6).
The CP is normally not visible during LC [23]. To expose it, dissection in the HCT is continued in the gallbladder bed to separate the gallbladder from the liver. Adequate traction and countertraction allow dissection in the safe plane that lies between the gallbladder and the CP. Dissection is performed alternatively from lateral and medial aspects of the gallbladder till optimal portion of the CP is exposed (at least the lower third) (Fig. 5–7). Effort should be made to expose the maximum possible portion of the CP while dissecting towards the fundus and leaving a sufficient portion of fundus/body still attached to the liver (Fig. 8). This step can be performed using a monopolar hook cautery (our preferred technique) or by blunt dissection.
Once optimal dissection in the HCT is completed and the CP is exposed adequately, the CVS is confirmed by looking at the anatomy obtained after the dissection. This anatomical assessment is done from the anterior/medial aspect (anterior view of the CVS) and the posterior/lateral aspect (posterior view of the CVS) (Fig. 8).
For the anterior view of the CVS, adequate gallbladder retraction is obtained at the fundus and the neck is retracted towards the right lower abdomen. With this maneuver, segment 5 of the liver is seen clearly across the window between the gallbladder and the liver. The gallbladder is seen attached to the hepatoduodenal ligament/hepatic hilum only through the cystic duct and the cystic artery (Fig. 8). In addition, these two pedicular structures are seen separate from each other.
For the posterior view of the CVS, the gallbladder is flipped on the left side by retracting the neck towards the umbilical fissure/left shoulder of the patient while maintaining adequate traction on the fundus towards the right shoulder. With this repositioning of the gallbladder, segment 4b of the liver is seen clearly across the window between the gallbladder and the liver. The gallbladder is seen attached to the hepatoduodenal ligament/hepatic hilum only through the cystic duct and the cystic artery which were identified on the anterior view (Fig. 8).
After apparent achievement of the CVS, time-out is adopted before proceeding with clipping and division of the cystic duct and cystic artery. During this time-out, the quality of the CVS is ascertained by looking at both the views (doublet view) (Fig. 8) and discussing them with the surgical team/colleagues. With a suboptimal CVS, necessary dissection is performed further. Once a satisfactory CVS is achieved, final views of the CVS are captured for documentation purpose. The cystic duct and artery are clipped and divided.
The CVS has been described as a preventive measure against misidentification injuries [3]. The concept of CVS is based on the technique used during an open cholecystectomy where the gallbladder is completely separated from the liver after an initial dissection in the Calot’s triangle to isolate the cystic duct and cystic artery, thereby providing conclusive evidence that the two structures entering the gallbladder are only cystic duct and cystic artery [2]. This technique has been modified for LC to allow the same principles to be followed but without complete detachment of the gallbladder from the liver as complete gallbladder detachment may pose technical difficulty in handling a free ptotic gallbladder.
The CVS technique is very effective in preventing misidentification injuries compared to other techniques of anatomic identification during LC (pooled incidence of BDI 2 in 1 million cases when CVS is employed vs. pooled incidence of 1.5 in 1000 cases when an infundibular technique is used) [1]. Based on its proven safety, CVS is uniformly recommended by various guidelines including the latest multi-society guideline for BDI prevention [1,12-15].
Despite its description many decades ago, correct understanding and wide adoption of CVS in real practice are limited worldwide. This might result from the fact that the CVS technique follows more stringent criteria of structural identification and requires more extensive dissection, which might take more time to complete than a conventional technique such as an infundibular technique. In addition, it has a low awareness among surgeons.
A few considerations pertaining to each component are worth discussion. The first and second components of CVS involve active dissection to expose the cystic duct, cystic artery, and CP. The last component, on the other hand, involves active visual assessment of the anatomy thus exposed. All these three components are essential steps. All of them must be fulfilled before labeling satisfactory CVS attainment.
As the first component, dissection in the HCT should be limited but sufficient enough to delineate both the cystic duct and artery and any other structures abnormally present in the HCT. It is not necessary to perform a total dissection (skeletonization) in the HCT to expose the common hepatic duct or trace the entire cystic duct till its junction with extrahepatic bile duct or trace the cystic artery till its origin. This step is not necessary. Rather, it is potentially harmful. Contrary to general (mis)perception, it is not a component of the CVS [21]. At the same time, it is important to know that just creating only two windows in the HCT with limited delineation of the cystic duct and artery without exposing the CP is not a CVS (Fig. 9).
Another important consideration is to start and confine the dissection in the anterosuperior safe zone as described by us earlier [24]. Dissection in the posterosuperior (a potentially unsafe) zone should be performed more carefully. The surgeon should be mindful of the presence of anomalous structures (e.g., aberrant sectoral duct or artery) in this zone. Remaining cognizant of various anatomical landmarks, especially the RS and other B-SAFE anatomical landmarks, throughout the dissection phase can lead to correct orientation. It may also prevent entering into an unsafe zone (i.e., inferior to the R4U line or medial to the cystic lymph node) [23,24]. Adequate dissection in the HCT can facilitate subsequent dissection in the gallbladder bed to expose the CP to achieve the second component of CVS.
The second component of the CVS (i.e., exposure of the CP) remains the least understood, most neglected, and the most difficult part of the CVS. Awareness about this component is also poor among surgeons [21]. The purpose of adequate exposure of the CP is to ensure that no structures other than the cystic duct and the cystic artery are entering the gallbladder. Cholecystohepatic ducts may also become apparent and can be properly secured during this step. Wider exposure of the CP has two advantages: 1) it increases the confidence of correct structural identification; and 2) it saves time for subsequent step of gallbladder separation from the liver after pedicle division.
HCT dissection and CP exposure can be performed in two ways. In the first way (the Calot’s triangle first approach), dissection in the Calot’s triangle is completed first to expose and delineate the cystic duct and artery. Dissection is then continued towards the fundus in the plane between the gallbladder body and CP by gently elevating and flipping the gallbladder in the right and left direction alternatively to expose the required portion of the CP (Fig. 7). In the second way (the CP first approach), an initial limited dissection is performed in the Calot’s triangle to expose the cystic duct and artery, dissection is then started separately along the mid portion of the gallbladder body in the gallbladder bed to expose the required portion of the CP. Finally, dissection is completed in the Calot’s triangle (Fig. 6, Supplementary Video 1).
The CP first approach is our preferred approach as it facilitates subsequent dissection in the HCT. This is because early separation of the gallbladder from the liver gives more leverage to the gallbladder for easy maneuvering and further dissection. Occasionally, a hybrid approach is adopted if some difficulty is encountered in either approach.
The last component of the CVS (i.e., two and only two tubular structures should be seen entering the gallbladder) involves active visual assessment as the CVS is the final view obtained after the dissection phase. For this, the surgeon needs to take a pause (time-out) to look at the anatomy that is exposed after completion of the dissection. The aim of this assessment is to ensure that a good quality CVS has been obtained. This can be objectively assessed by the Strasberg score [25]. A score of at least 5 out of 6 is required to label the CVS as satisfactory [25]. Assessment needs visualization of the CVS from both anterior and posterior aspects (doublet view) as it allows circumferential assessment of the cystic duct and artery from different angles, thus ensuring conclusive identification of these two structures before their division (Fig. 10, Supplementary Video 1).
It is important to realize that dissection close to the gallbladder sometimes can result in exposure of two branches of the cystic artery (anteroinferior/superficial and posterosuperior/deeper branches). Thus, three, instead of two, tubular structures seem to enter the gallbladder. Another situation includes uncommon anatomical variation of two or more separate cystic arteries [26]. Still, it may be safe to consider the anatomy as the classical CVS under these exceptional situations.
With proper understanding and training, the CVS can be attained in 85%–95% of cases in routine practice [1]. Certain preoperative factors e.g., male gender, higher age (> 60 years), emergency admission, past or current acute cholecystitis, previous biliary intervention (endoscopic or surgical), and so on and intraoperative factors e.g., extensive and/or dense pericholecystic adhesions, contracted/obliterated Calot’s triangle due to severe fibrosis and scarring (vanishing Calot’s syndrome [27]), impacted stone in the Hartmann’s pouch, contracted gallbladder, empyema, Mirizzi syndrome and cholecystoenteric fistula, etc. can help the surgeon to predict difficulty in achieving the CVS with a higher failure rate (Fig. 11) [4,28]. In the presence of one of more of adverse factors, the surgeon should strongly consider alternate options early to complete the procedure safely.
Intraoperatively, if the surgeon finds it difficult to achieve the CVS on an initial attempt, proper exposure of the surgical field, reorientation (with anatomical landmarks), and reassessment of the situation during a time-out session, gentle, precise, and patient dissection, and addressing underlying cause(s) of difficulty can facilitate achieving the CVS. Troubleshooting with simple maneuvers such as adequate and proper gallbladder retraction (which may require suture retraction of a thick fundus), decompression of an overdistended gallbladder (e.g., in mucocele, empyema), milking out the impacted stone from the neck, inserting additional port for liver retraction (for overhanging /floppy left lateral section or segment 4), and so on can be helpful. They might increase the probability of successful completion of dissection to achieve a satisfactory CVS. Performing a time-out can significantly improve the achievement rate of CVS [29]. If difficulty still persists, opinions from more experienced surgical colleagues and intraoperative imaging (conventional cholangiography, near infrared fluorescence cholangiography, intraoperative ultrasonography) might be helpful if the later facility is available and the surgeon is well acquainted with these technologies. If the CVS still could not be achieved, the surgeon should strongly consider resorting to alternate options (bailout/salvage options) such as subtotal cholecystectomy for safe completion of the procedure as ongoing dissection in such conditions is fraught with danger and a very high risk of biliary/vascular or other visceral injury [13,23,28,30].
In conclusion, CVS technique is an important and essential component of COSIC. It should be an inherent component of structured training curriculum for general surgery trainees. The practicing surgeon should strive to use it in every case as it can be attained in the majority of cases. However, a good quality CVS achieved after a careful dissection is imperative for it to be effective in preventing post-cholecystectomy BDI/VBI. This requires correct understanding, adequate training, regular practice, and knowledge of factors that prohibit its safe attainment.
Supplementary data related to this article can be found at https://youtu.be/VkM_gLDy7_Q.
REFERENCES
1. Brunt LM, Deziel DJ, Telem DA, Strasberg SM, Aggarwal R, Asbun H, et al. 2020; Safe cholecystectomy multi-society practice guideline and state of the art consensus conference on prevention of bile duct injury during cholecystectomy. Ann Surg. 272:3–23. DOI: 10.1097/SLA.0000000000003791. PMID: 32404658.

2. Strasberg SM, Brunt LM. 2010; Rationale and use of the critical view of safety in laparoscopic cholecystectomy. J Am Coll Surg. 211:132–138. DOI: 10.1016/j.jamcollsurg.2010.02.053. PMID: 20610259.

3. Strasberg SM. 2017; A perspective on the critical view of safety in laparoscopic cholecystectomy. Ann Laparosc Endosc Surg. 2:91. DOI: 10.21037/ales.2017.04.08.

4. Iwashita Y, Hibi T, Ohyama T, Umezawa A, Takada T, Strasberg SM, et al. 2017; Delphi consensus on bile duct injuries during laparoscopic cholecystectomy: an evolutionary cul-de-sac or the birth pangs of a new technical framework? J Hepatobiliary Pancreat Sci. 24:591–602. DOI: 10.1002/jhbp.503. PMID: 28884962.

5. Strasberg SM. 2002; Avoidance of biliary injury during laparoscopic cholecystectomy. J Hepatobiliary Pancreat Surg. 9:543–547. DOI: 10.1007/s005340200071. PMID: 12541037.

6. Way LW, Stewart L, Gantert W, Liu K, Lee CM, Whang K, et al. 2003; Causes and prevention of laparoscopic bile duct injuries: analysis of 252 cases from a human factors and cognitive psychology perspective. Ann Surg. 237:460–469. DOI: 10.1097/01.SLA.0000060680.92690.E9. PMID: 12677139. PMCID: PMC1514483.
7. Callery MP. 2006; Avoiding biliary injury during laparoscopic cholecystectomy: technical considerations. Surg Endosc. 20:1654–1658. DOI: 10.1007/s00464-006-0488-3. PMID: 17063288.

8. Strasberg SM. 2008; Error traps and vasculo-biliary injury in laparoscopic and open cholecystectomy. J Hepatobiliary Pancreat Surg. 15:284–292. DOI: 10.1007/s00534-007-1267-9. PMID: 18535766.

9. Society of American Gastrointestinal and Endoscopic Surgeons (SAGES). The SAGES safe cholecystectomy program [Internet]. Available from: https://www.sages.org/safe-cholecystectomy-program/. SAGES;cited 2022 Jul 31.
10. Strasberg SM, Hertl M, Soper NJ. 1995; An analysis of the problem of biliary injury during laparoscopic cholecystectomy. J Am Coll Surg. 180:101–125.
11. Pucher PH, Brunt LM, Fanelli RD, Asbun HJ, Aggarwal R. 2015; SAGES expert Delphi consensus: critical factors for safe surgical practice in laparoscopic cholecystectomy. Surg Endosc. 29:3074–3085. DOI: 10.1007/s00464-015-4079-z. PMID: 25669635.

12. Conrad C, Wakabayashi G, Asbun HJ, Dallemagne B, Demartines N, Diana M, et al. 2017; IRCAD recommendation on safe laparoscopic cholecystectomy. J Hepatobiliary Pancreat Sci. 24:603–615. DOI: 10.1002/jhbp.491. PMID: 29076265.

13. Wakabayashi G, Iwashita Y, Hibi T, Takada T, Strasberg SM, Asbun HJ, et al. Tokyo Guidelines 2018: surgical management of acute cholecystitis: safe steps in laparoscopic cholecystectomy for acute cholecystitis (with videos). J Hepatobiliary Pancreat Sci. 2018; 25:73–86. DOI: 10.1002/jhbp.517. PMID: 29095575.
14. Eikermann M, Siegel R, Broeders I, Dziri C, Fingerhut A, Gutt C, et al. 2012; Prevention and treatment of bile duct injuries during laparoscopic cholecystectomy: the clinical practice guidelines of the European Association for Endoscopic Surgery (EAES). Surg Endosc. 26:3003–3039. DOI: 10.1007/s00464-012-2511-1. PMID: 23052493.

15. Bansal VK, Misra M, Agarwal AK, Agrawal JB, Agarwal PN, Aggarwal S, et al. 2021; SELSI consensus statement for safe cholecystectomy - prevention and management of bile duct injury - Part A. Indian J Surg. 83(Suppl 3):592–610. DOI: 10.1007/s12262-019-01993-2.

16. Nijssen MA, Schreinemakers JM, Meyer Z, van der Schelling GP, Crolla RM, Rijken AM. 2015; Complications after laparoscopic cholecystectomy: a video evaluation study of whether the critical view of safety was reached. World J Surg. 39:1798–1803. DOI: 10.1007/s00268-015-2993-9. PMID: 25711485.

17. Daly SC, Deziel DJ, Li X, Thaqi M, Millikan KW, Myers JA, et al. 2016; Current practices in biliary surgery: do we practice what we teach? Surg Endosc. 30:3345–3350. DOI: 10.1007/s00464-015-4609-8. PMID: 26541721.

18. van de Graaf FW, van den Bos J, Stassen LPS, Lange JF. 2018; Lacunar implementation of the critical view of safety technique for laparoscopic cholecystectomy: results of a nationwide survey. Surgery. 164:31–39. DOI: 10.1016/j.surg.2018.01.016. PMID: 29525733.

19. Jin Y, Liu R, Chen Y, Liu J, Zhao Y, Wei A, et al. 2022; Critical view of safety in laparoscopic cholecystectomy: a prospective investigation from both cognitive and executive aspects. Front Surg. 9:946917. DOI: 10.3389/fsurg.2022.946917. PMID: 35978606. PMCID: PMC9377448.

20. Alanis-Rivera B, Rangel-Olvera G. 2022; Evaluation of the knowledge of the critical view of safety and recognition of the transoperative complexity during the laparoscopic cholecystectomy. Surg Endosc. 36:8408–8414. DOI: 10.1007/s00464-022-09120-1. PMID: 35233656.

21. Gupta V, Lal P, Vindal A, Singh R, Kapoor VK. 2021; Knowledge of the culture of safety in cholecystectomy (COSIC) among surgical residents: do we train them well for future practice? World J Surg. 45:971–980. Erratum in: World J Surg 2021;45:1602. DOI: 10.1007/s00268-020-05911-6. PMID: 33454794.

22. Nakazato T, Su B, Novak S, Deal SB, Kuchta K, Ujiki M. 2020; Improving attainment of the critical view of safety during laparoscopic cholecystectomy. Surg Endosc. 34:4115–4123. DOI: 10.1007/s00464-019-07178-y. PMID: 31605213.

23. Gupta V, Jain G. 2019; Safe laparoscopic cholecystectomy: adoption of universal culture of safety in cholecystectomy. World J Gastrointest Surg. 11:62–84. DOI: 10.4240/wjgs.v11.i2.62. PMID: 30842813. PMCID: PMC6397793.

24. Gupta V, Jain G. 2021; The R4U planes for the zonal demarcation for safe laparoscopic cholecystectomy. World J Surg. 45:1096–1101. DOI: 10.1007/s00268-020-05908-1. PMID: 33491141.

25. Sanford DE, Strasberg SM. 2014; A simple effective method for generation of a permanent record of the Critical View of Safety during laparoscopic cholecystectomy by intraoperative "doublet" photography. J Am Coll Surg. 218:170–178. DOI: 10.1016/j.jamcollsurg.2013.11.003. PMID: 24440064.

26. Andall RG, Matusz P, du Plessis M, Ward R, Tubbs RS, Loukas M. 2016; The clinical anatomy of cystic artery variations: a review of over 9800 cases. Surg Radiol Anat. 38:529–539. DOI: 10.1007/s00276-015-1600-y. PMID: 26698600.

27. Gupta V. 2020; Vanishing Calot syndrome: common face of many problems. J Am Coll Surg. 231:187–188. DOI: 10.1016/j.jamcollsurg.2020.04.002. PMID: 32389576.

28. Nassar AHM, Ng HJ, Wysocki AP, Khan KS, Gil IC. 2021; Achieving the critical view of safety in the difficult laparoscopic cholecystectomy: a prospective study of predictors of failure. Surg Endosc. 35:6039–6047. DOI: 10.1007/s00464-020-08093-3. PMID: 33067645. PMCID: PMC8523408.

29. Mascagni P, Rodríguez-Luna MR, Urade T, Felli E, Pessaux P, Mutter D, et al. 2021; Intraoperative time-out to promote the implementation of the critical view of safety in laparoscopic cholecystectomy: a video-based assessment of 343 procedures. J Am Coll Surg. 233:497–505. DOI: 10.1016/j.jamcollsurg.2021.06.018. PMID: 34325017.

30. Strasberg SM. 2019; A three-step conceptual roadmap for avoiding bile duct injury in laparoscopic cholecystectomy: an invited perspective review. J Hepatobiliary Pancreat Sci. 26:123–127. DOI: 10.1002/jhbp.616. PMID: 30828991.

Fig. 1
Surgical field of interest for laparoscopic cholecystectomy showing important anatomical landmarks. Anterior/medial view (A) and posterior/lateral view (B) of the hepatocystic triangle and adjoining areas. CBD, common bile duct; Du, duodenum; HA, hepatic artery; RS, Rouviere’s sulcus; Sg4, segment 4; UF, umbilical fissure.
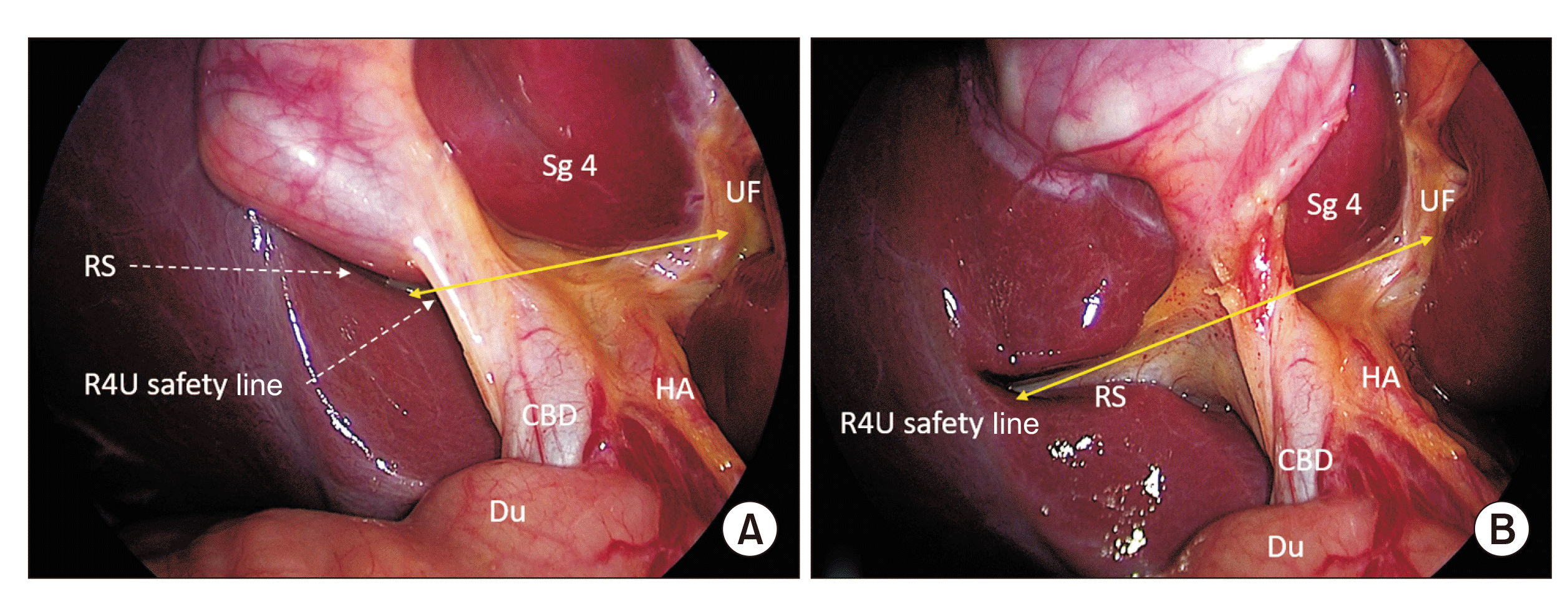
Fig. 2
Initial dissection: (A) peritoneal fold at the putative cystic duct-gallbladder infundibulum junction is opened, and (B) posterior peritoneal layer is gently dissected bluntly. Dissection remains above the R4U line.
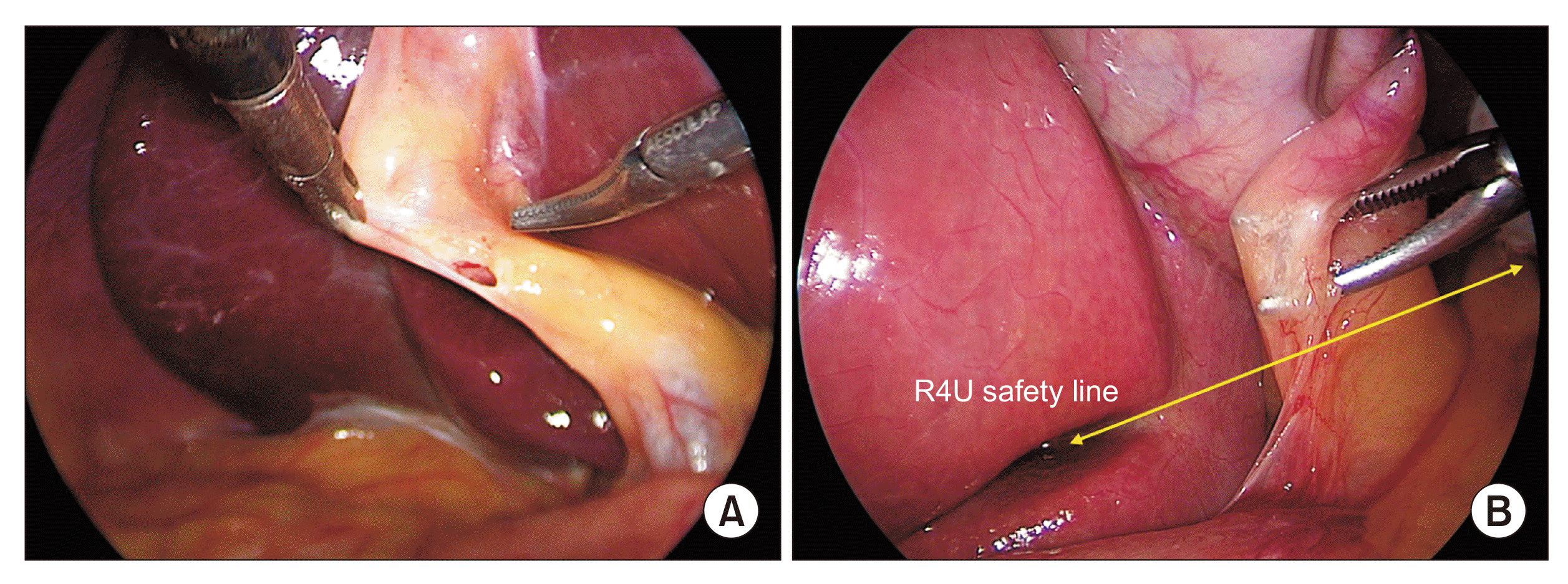
Fig. 3
Dissection of the hepatocystic triangle from the lateral/right side (posterior dissection): Peritoneal fold division. (A) Peritoneal fold is divided close to the gallbladder (broken line). (B) Hook cautery tip is introduced beneath the peritoneal fold and gently elevated with side to side sweeping movements to open up the space and (C) to allow CO2 gas to enter beneath the peritoneal layer for facilitating (pneumo-) dissection (arrowheads), and (D) the peritoneal fold is then gradually divided towards the fundus. Dissection starts above the R4U safely line. To facilitate proper dissection, gallbladder infundibulum is retracted in left-cephalad direction.
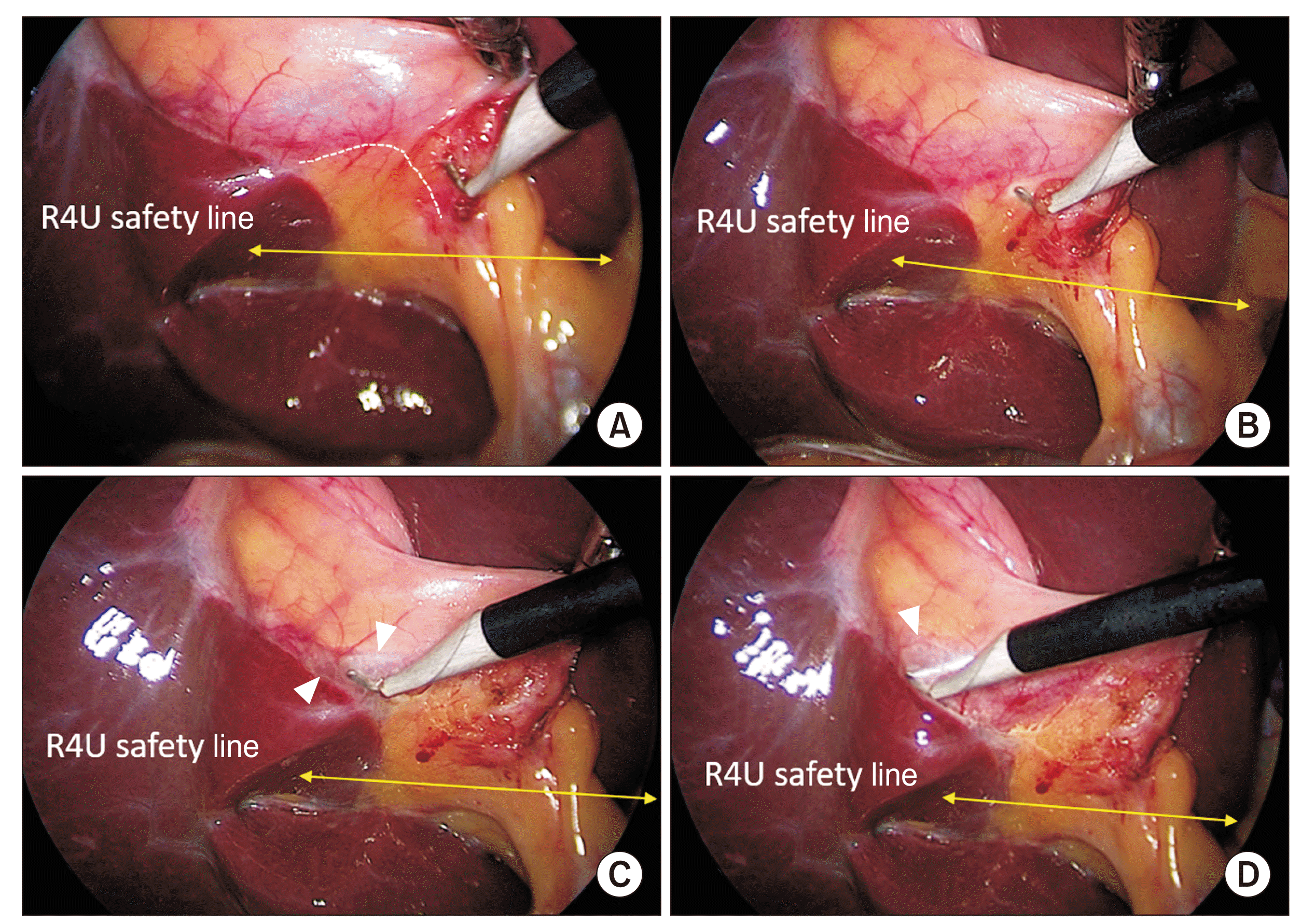
Fig. 4
Dissection of the hepatocystic triangle from the medial/left side (anterior dissection): peritoneal fold division. After opening the peritoneal fold (see Fig. 2), (A) anterior peritoneal fold is gently dissected off the underlying tissue by blunt dissection followed by (B–D) its division close to the gallbladder (broken line) while exposing the cystic lymph node (marked with circle; broken line in ‘B’). To facilitate proper dissection, gallbladder infundibulum is retracted in right-caudal direction.
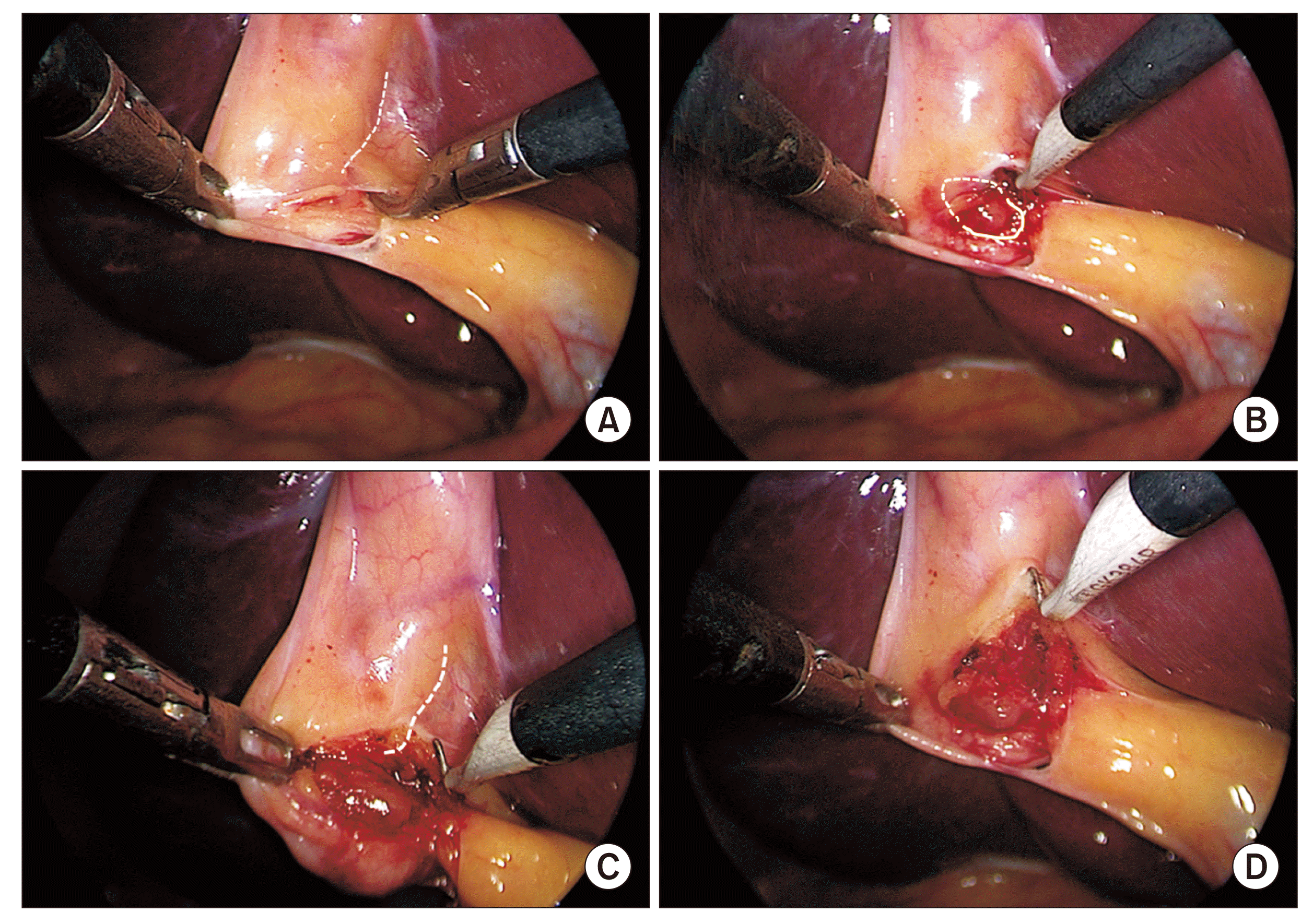
Fig. 5
Dissection of the hepatocystic triangle from the lateral/right side (posterior dissection): deeper dissection. (A) Showing dissection close to the gallbladder (broken line) in the direction (arrowheads) towards and on to the cystic duct across the infundibulum. (B) Dissection close to the gallbladder (broken line) towards the fundus (arrowhead). (C) With an ongoing dissection along the gallbladder (broken line) towards the fundus (arrowhead), a part of the cystic plate is exposed (encircled with broken line). (D) With further dissection, a window is created in the hepatocystic triangle, showing part of segment 4 across it. Larger extent of lower portion of the cystic plate is now exposed (encircled with broken line). Dissection remains confined to areas above the R4U safety line.
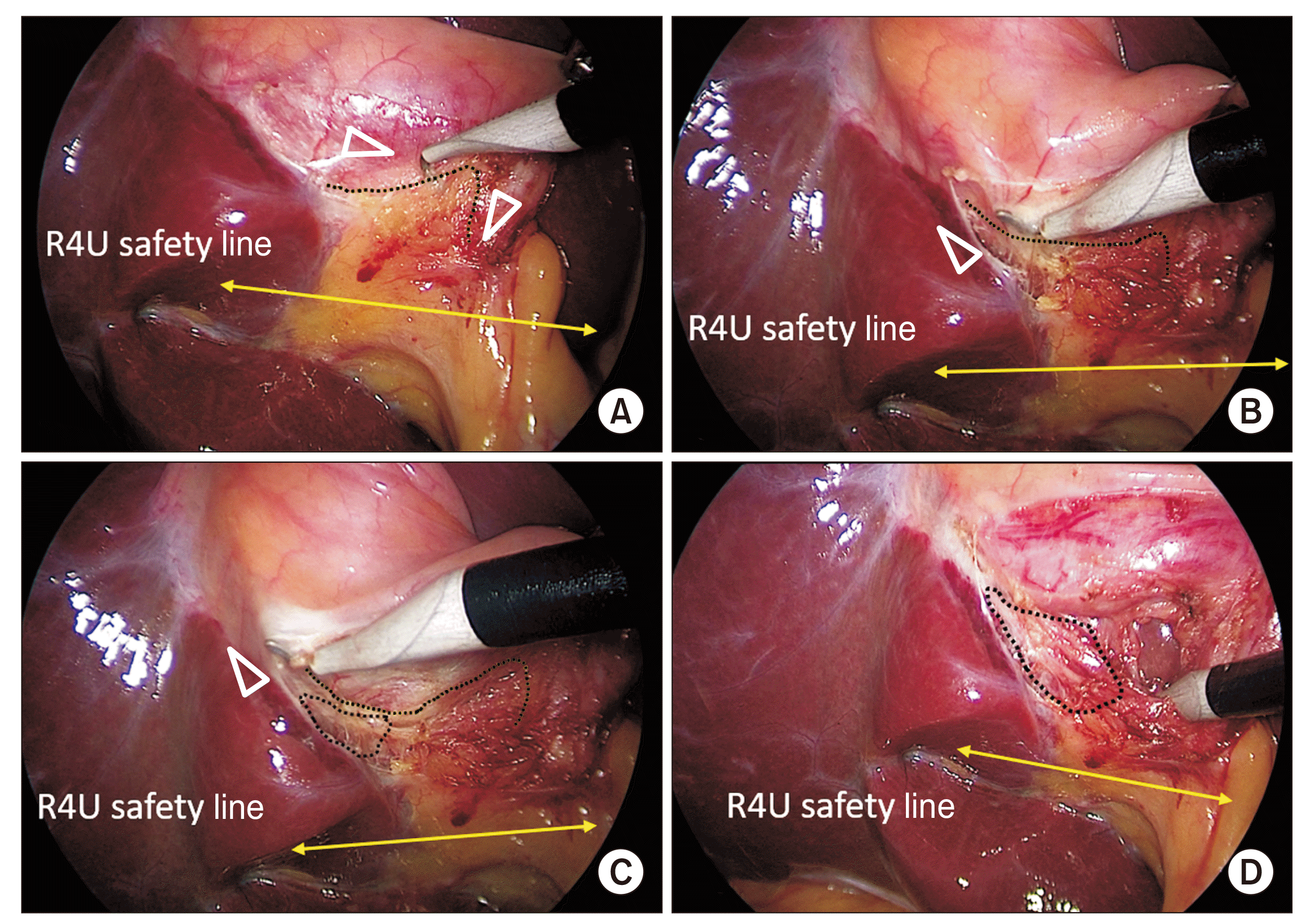
Fig. 6
Dissection of the hepatocystic triangle and exposure of the cystic plate. Cystic plate is being exposed by the alternate dissection from (A) medial/anterior and (B) lateral/posterior aspects with (C, E) subsequent exposure of the necessary extent of the plate (marked with broken line). Calots’ triangle is subsequently dissected from (D) anterior and (E) posterior aspects to (F) finally achieve the critical view of safety (CVS). This figure also illustrates the cystic plate first approach to achieve the CVS (also see Supplementary Video 1).

Fig. 7
Dissection of the hepatocystic triangle: Calot’s triangle first approach. (A) Calot’s triangle is dissected to expose and delineate the cystic duct and cystic artery. CP is not exposed yet (broken line). (B) CP is being exposed. (C) CP is exposed adequately (broken line). White arrowhead indicates cystic duct and black arrowhead indicates cystic artery. CP, cystic plate; N, node.
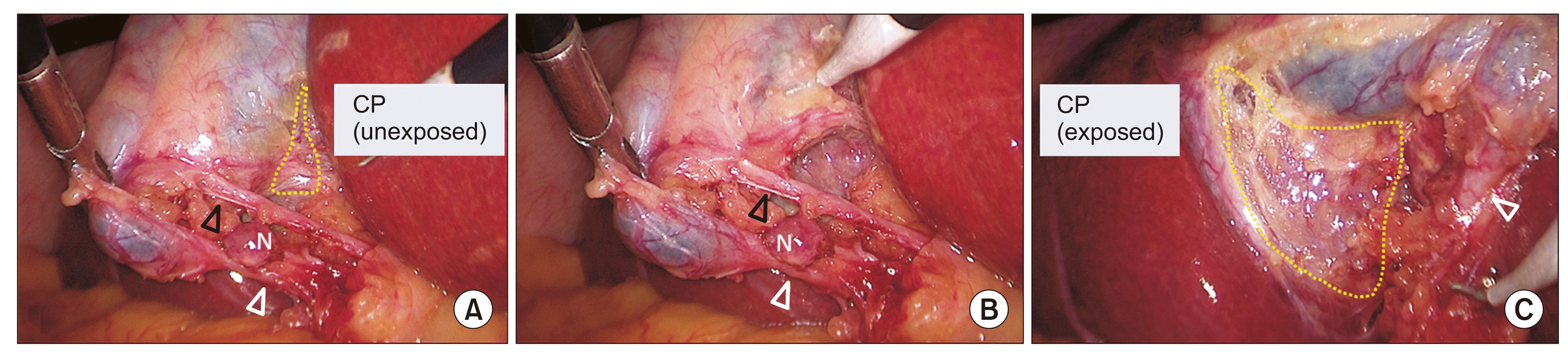
Fig. 8
A critical view of safety. (A) Anterior view. (B) Posterior view. The hepatocystic triangle is adequately dissected and the cystic plate is exposed adequately (broken line). Only two tubular structures (i.e., cystic duct [white arrowheads] and cystic artery [black arrowheads]) can be seen entering the gallbladder. Strasberg score is 6/6. Completed dissection is above the R4U safety line.
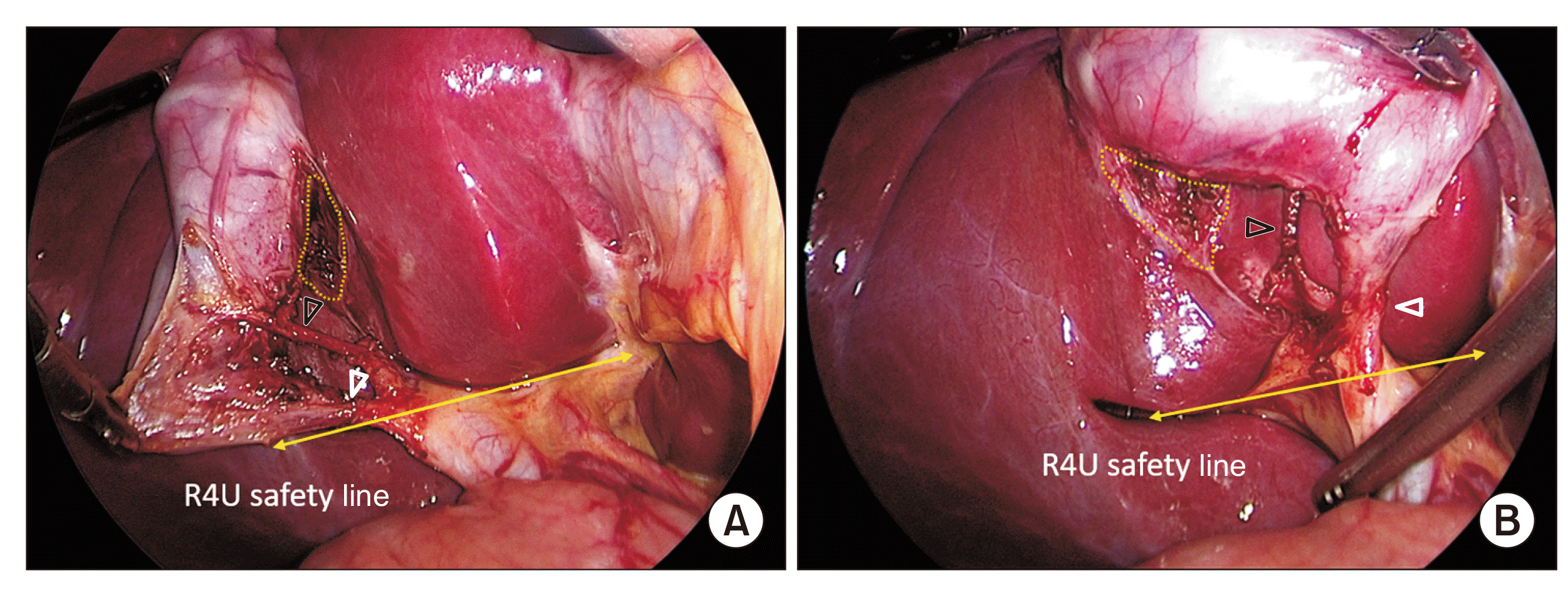
Fig. 9
Just creating two windows in the hepatocystic triangle with limited dissection to expose the cystic duct and artery in their limited extents is not taken as a critical view of safety.
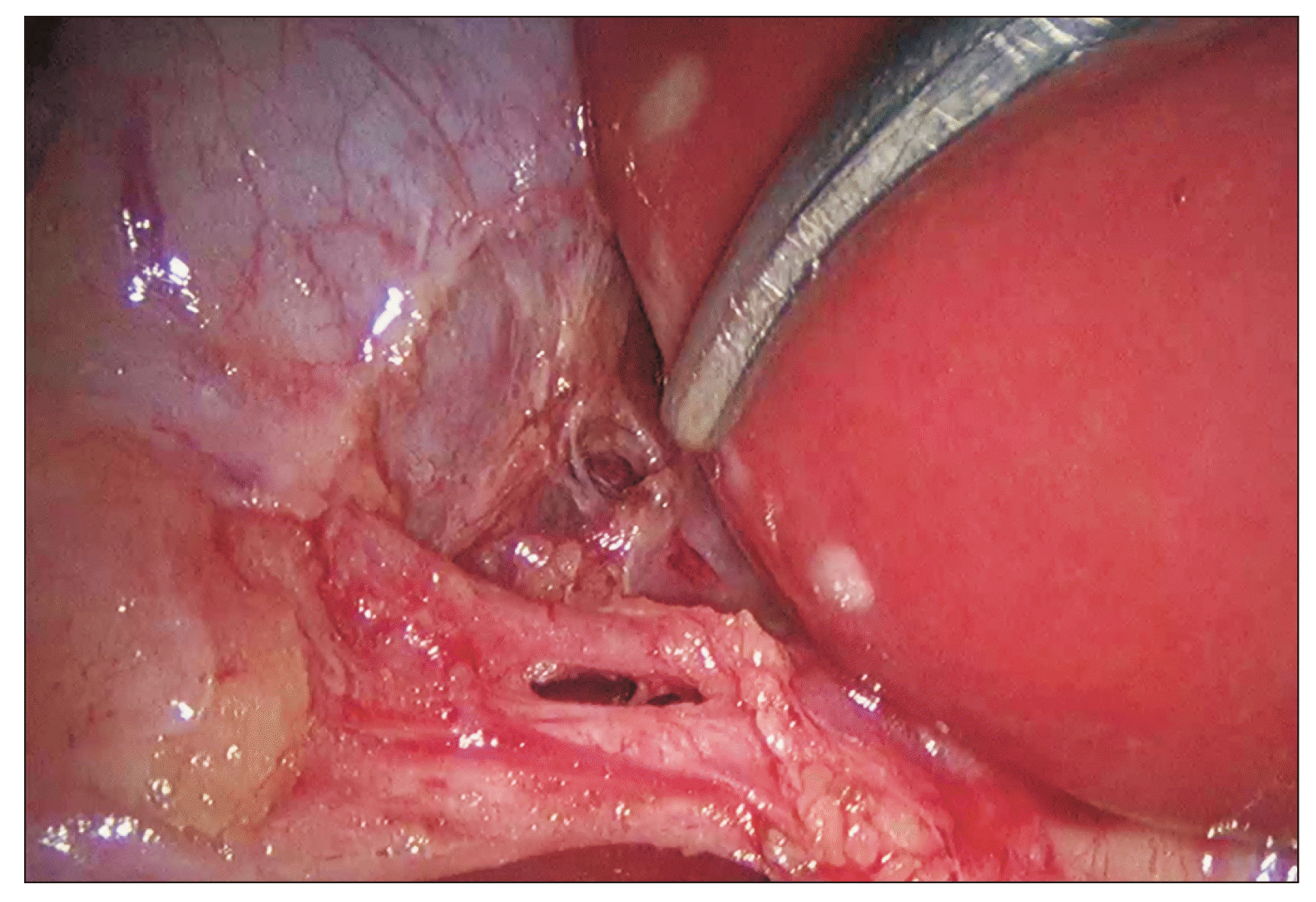
Fig. 10
Significance of a doublet view of critical view of safety. (A) Cystic artery (white arrowhead) is very well delineated as seen from the anterior aspect. (B) However, the posterior (deeper) branch (black arrowhead) of the cystic artery that is supplying the liver and its junction with anterior branch (white arrowhead) is seen only on a posterior view. The posterior branch would be at risk of injury without a posterior view assessment.
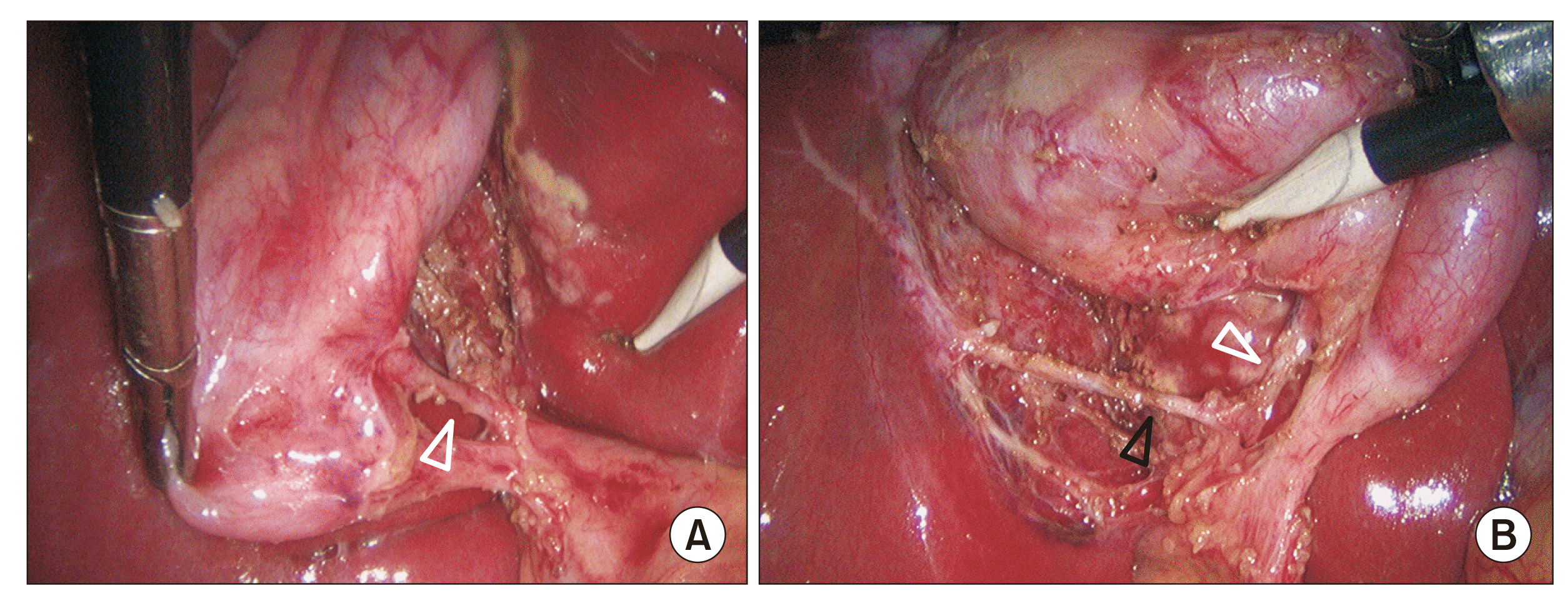
Fig. 11
Adverse intraoperative conditions with low success rates of achieving critical view of safety (CVS). (A, B) Case of acute cholecystitis with a large stone impacted in the Hartmann’s pouch. Stone could not be dislodged. Still, the CVS could be achieved after a careful dissection. (C) Acute cholecystitis with distended gallbladder and omental adhesions over the neck and Calot’s triangle. (D) Vanishing Calot’s syndrome: Inflamed gallbladder and hepatoduodenal ligament with an obliterated Calot’s triangle. Structure that appears to be a dilated thick cystic duct is actually situated below the R4U line and passing vertically behind the duodenum; this structure (marked as B) is common bile duct. No attempt should be made to achieve the CVS in this situation.




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download