Abstract
Backgrounds/Aims
Routine execution of intraoperative cholangiography (IOC) in laparoscopic cholecystectomy (LC) is considered a good practice to help early identification of biliary duct injuries (BDIs) or common bile duct (CBD) stones. This study aimed to determine the impact of IOC during LC.
Methods
This is a retrospective, monocentric study, including patients with a LC performed from January 2020 to December 2021.
Results
Of 303 patients, 215 (71.0%) were in the IOC group and 88 (29.0%) in the no-IOC group. IOC was incomplete or unclear in 10.7% of patients, with a failure rate of 14.7%. Operating time was 15 minutes longer in the IOC group (p = 0.01), and postoperative complications were higher (5.1% vs. 0.0%, p = 0.03). There were three BDIs (0.99%), all included in the IOC group; only one was diagnosed intraoperatively, and the other two were identified during the postoperative course. Regarding identifying CBD stones, IOC showed a sensitivity of 77%, a specificity of 98%, an accuracy of 97.2%, a positive predictive value of 63% and a negative predictive value of 99%.
Laparoscopic cholecystectomy (LC) is one of the most common surgical procedures. Bile duct injury (BDI) is the most severe complication, with an approximate rate of 0.4% to 1.5% [1,2]. Intraoperative cholangiography (IOC) allows the early identification of BDI and common bile duct (CBD) stones, which is essential to improve medical care. Several surgical associations, such as the French or Swedish ones, recommend systematic IOC performance to reduce the risk of BDI [3,4]. A recent meta-analysis confirmed the usefulness of this procedure [5].
Nevertheless, the consensus is not unanimous, the debate is always open, and the number of proponents who agree with selective cholangiography has grown and continues to grow [6,7].
Five surgical societies (Society of American Gastrointestinal and Endoscopic Surgeons, Americas Hepato-Pancreato-Biliary Association, International Hepato-Pancreato Biliary Association, Society for Surgery of the Alimentary Tract, and The European Association of Endoscopic Surgery) stated that the benefit of IOC in elective non-acute cholecystectomy was inconclusive. Therefore, no recommendation can be made for this procedure could. Nevertheless, IOC has been recommended in situations such as previous acute cholecystitis, uncertainty of biliary anatomy, or BDI [8,9].
Likewise, the Italian recommendations, although acknowledging the importance of IOC in recognizing and treating BDI in selected cases, only recommends its systematic use due to a need for sufficient scientific evidence [10].
Furthermore, the European Association for the Study of Liver does not recommend routine or selective execution of IOC in patients at low risk of CBD stones [11].
This study aimed to retrospectively evaluate the impact of IOC on patients undergoing elective LC at our hospital center.
This retrospective cohort study relies on data obtained from a prospectively maintained database of consecutive patients undergoing elective LC in the Grand Hopital de l’Est Francilien, Meaux France from January 2020 to December 2021. The database includes preoperative, operative, and postoperative data. All patients in the study agreed to participate after providing fully informed consent for publication.
The inclusion criteria were as follows: adults aged over 18 years and diagnosed with gallstones requiring surgical treatment. An exclusion criterion was the need to perform cholecystectomy in an emergency setting. A total of 303 patients were included in this study.
The study was approved by the Institutional Review Board of the Grand Hopital de l’Est Francilien (approval number: 202203).
All patients underwent blood tests with a liver function test, abdominal ultrasound, and/or computed tomography scan.
In patients with significant hepatic impairment, a magnetic resonance cholangiopancreatography (MRCP) was performed, and, if necessary, an endoscopic retrograde cholangiopancreatography (ERCP) was scheduled preoperatively. In case of a minor change in the liver blood test, three different responses were performed: preoperative MRCP, systematic IOC, or selective IOC, only in patients with worsening blood tests 48 hours before surgery.
LC was implemented according to the critical safety view described by Strasberg and Brunt [12]. After dissecting of the cystic duct in a standardized manner, and its partial section was performed using laparoscopic scissors, permitting the insertion of a catheter and the injection of a solution 1:1 dilution of normal saline and iodixanol (Visipaque; GE Healthcare SAS). Biliary anatomy was visualized using a mobile C-arm machine equipped with an image intensifier (Siremobil Compact L; Siemens AG). Without additional personnel, the surgeon and a nurse performed IOC without assistance.
If IOC showed CBD stones, we performed cholecystectomy as planned and performed postoperative echoendoscopy with or without ERCP.
Statistical analysis was performed by dividing the study population into two groups based on the completion of IOC. Continuous variables are presented as mean ± standard deviation and compared using the Student’s t-test or unpaired Mann–Whitney test. Categorical variables are presented as numbers (percentages) and compared across groups using the χ2 test or Fisher exact tests. Furthermore, the sensitivity, specificity, positive predictive value, negative predictive value, and test accuracy were calculated by comparing the intraoperative results of IOC with the confirmed presence of lithiasis of the biliary tract. All statistical analyses were performed with SPSS software (Statistical Package for Social Science, IBM SPSS Statistics, version 23 for Macintosh; IBM Corp.).
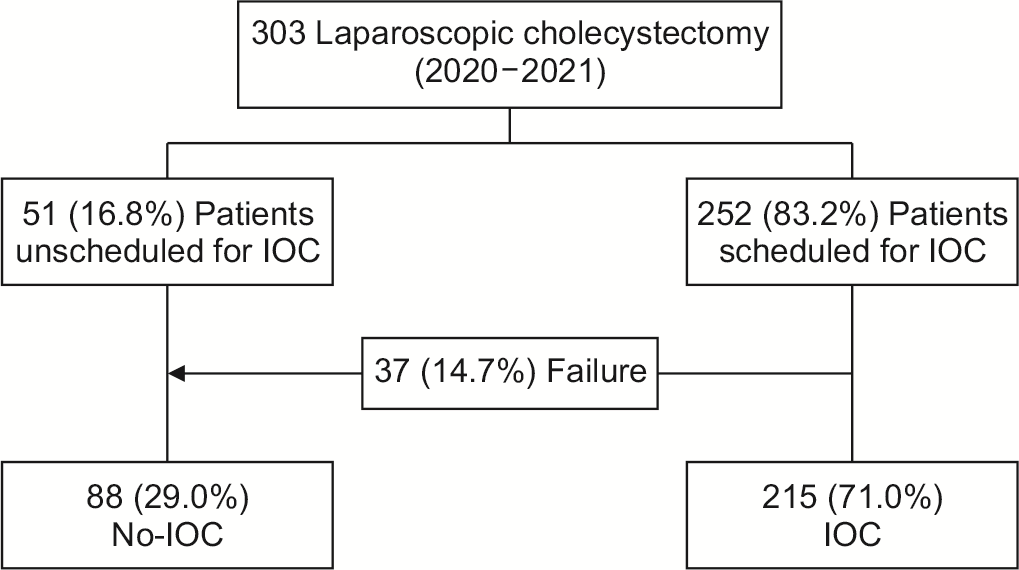
303 elective LC were performed in our hospital center from January 2020 to December 2021: IOC was not scheduled in 51 cases (16.8%). In 37 cases (12.2%), IOC was scheduled but failed, in 215 cases (71.0%), IOC was performed, planned, and realized. Finally, IOC was performed in 215 patients (71.0%) (IOC group), while not in 88 patients (29.0%) (No-IOC group) (Fig. 1).
Both groups were similar in preoperative variables and physical characteristics (Table 1). In the No-IOC group, more preoperative MRCP were performed (17.2% vs. 30.7%, p = 0.01). However, no differences were found in the detection of CBD stones (p = 0.2) or execution of a preoperative ERCP (p = 0.2; Table 1).
Trained surgeons performed IOC more frequently (74.4% vs. 60.2%; p = 0.01). Nevertheless, the IOC group had a longer operative time of approximately 15 minutes (70 min vs. 55 min, p = 0.01; Table 2).
The two groups were similar in terms of intraoperative complications (1.1% vs 1.9%, p > 0.99) and conversion rate (1.1% vs. 0.9%, p > 0.99; Table 2).
The IOC’s success rate is 85.3%. The leading causes of IOC failure were: (1) difficult cannulation of the cystic duct in 28 cases (75.6%); (2) hilar inflammation in 7 cases (19%); (3) cystic duct avulsion in 1 case (2.7%); and (4) other causes in 1 case (2.7%).
IOC was defined as standard in 174 cases (80.9%) and abnormal in the remaining 41 cases (19.1%). The main causes of abnormalities were: (1) an incomplete cholangiogram in 21 cases (51.2%); (2) CBD stones in 11 cases (26.8%); (3) CBD dilation without stones in 5 cases (12.2%); (4) unclear IOC in 2 cases (4.9%); and (5) anatomical anomalies of the biliary tree for two patients (4.9%).
Three BDI were identified, in the IOC group (1.4%): one during surgery and two in the postoperative course.
The first injury was detected in a sclerotic gallbladder patient during cystic duct dissection. The IOC showed an incomplete cholangiogram requiring conversion to open surgery and primary repair.
A second patient had biliary peritonitis two days after LC despite normal IOC, requiring laparoscopic surgical drainage, followed by ERCP and endoscopic biliary stent placement.
Finally, a third patient developed a biliary stricture due to the placement of a clip on a tight cystic after the performance of IOC.
IOC identified 11 cases (5.1%) of suspected CBD stones. Four false-positive cholangiograms (IOC suggesting CBD stones, the postoperative ductal exploration did not find lithiasis) and two false-negative cholangiograms (normal IOC in patients with residual bile duct stones).
Overall, IOC had an accuracy of 97.2%, a sensitivity of 0.77 (95% confidence interval [CI], 0.71–0.83), and a specificity of 0.98 (95% CI, 0.95–0.99) for detecting CBD stones; its positive predictive value was 0.63 (95% CI, 0.56–0.70), and its negative predictive value was 0.99 (95% CI, 0.96–0.99; Table 3).
No differences were observed between the two groups regarding ambulatory surgery rate success (68.8% vs. 63.3%, p = 0.4), hospital stay (p = 0.2), and re-operation (2.3% vs. 0.0%, p = 0.3; Table 2).
Nevertheless, overall 30-day postoperative complications were increased in the IOC group (5.1% vs. 0.0%, p = 0.03) including: (1) bleeding (1.4% vs. 0.0%); (2) surgical site infection (0.9% vs. 0.0%); (3) biliary injuries (1.4% vs. 0.0%); (4) collections (0.5% vs. 0.0%); and (5) postoperative biliary disorders (0.9% vs. 0.0%; Table 2).
In this study, we demonstrated that the failure rate of IOC was 14.7%, it was incomplete or unclear in more than 10% of cases, it had a sensitivity of 77% in diagnosing CBD stones, and IOC failed to detect two out of three BDIs. Moreover, IOC is associated with longer operative time and a higher cumulative complication rate.
Most surgeons in our center systematically perform IOC as recommended by the Fédération de Chirurgie Viscérale et Digestive. Indeed, among patients undergoing LC, 83.2% were scheduled for IOC. Our success rate was approximately 85.3%, confirming results reported in high volume centers (80.4%–98.9%) [6]. On the contrary, according to our experience, 14.7% of IOC are not been completed, but this failure rate may increase to 64% in small centers [13]. The main reason for failure is the difficult of introduction of cholangiography catheter into a small cystic duct. In our series, the patient had a case of cystic duct avulsion without significant consequences. Similar complications were reported by Amott et al. [13] and the BDI case concerning IOC executions. Therefore, the IOC has been a complicated process at times.
Another area for clarification about cholangiogram interpretation is that it depends on the surgeon’s experience. Regarding BDI identification, IOC allowed us to identify only one case in our series, already strongly suspected by the surgeon. We had false-negative cholangiograms and biliary duct stricture after normal IOC. Fletcher et al. [14] showed that anatomy was correctly interpreted in less than 25% of IOCs BDI occurred. Rhaiem et al. [15] suggested performing a systematic MRCP before planning LC to identify “dangerous” anatomic variations and prevent BDI. In theory, this is a desirable solution. However, in actual practice, it is difficult to perform a preoperative MRCP procedure on every patient for several reasons: (1) there are still centers with difficult access to MRCP; and (2) specific skills are required for the interpretation of biliary anatomy on your own; moreover, in most cases, cholecystectomy is performed by surgeons without a real hepatobiliary surgical expertise.
To identify CBD stones, Ford et al. [6], in their systematic review of 8 randomized trials and 1,715 patients, found 24 false-positive cholangiograms. Moreover, in the trials included in the systematic review, the use of IOC did not demonstrate any specific benefit in detecting CBD stones.
Consequently, our data and literature show that IOC interpretation can be challenging to identify BDI or CBD stones.
In case of strong suspicion of CBD stones, we performed a preoperative MRCP. The no-IOC group had preoperative MRCP (30.7% vs. 17.2%), which allowed the identification of 5% of patients with CBD stones. Our results, according to the literature, suggest that preoperative ERCP is an effective and safe tool for evaluating the biliary tree when CBD stones are suspected [16]. Nevertheless 10% to 20% of patients undergoing LC have CBD stones without clinical symptoms or laboratory abnormalities [17]. In this case, the systematic execution of IOC to identify CBD stones is useful. However, there is no unanimous, and the treatment of asymptomatic stones is unclear: the British Society of Gastroenterology recommends their systematic removal [18], suggesting a conservative approach, because of the risk for complications of ERCP [19].
More recently, two other surgical societies have joined forces against the systematic execution of the IOC [20,21]. The Institut de Recherche sur les Cancers de l’Appareil Digestif recommendations on safe LC do not consider the implementation of formal IOC to prevent BDI due to sufficient robust scientific evidence; conversely, IOC is recommended to define unclear anatomy [20]. In this sense, the World Society of Emergency Surgery does not recommend the systematic implementation of IOC for the same reasons, and BDI may occur after IOC misinterpretation [21].
Rystedt et al. [5] address the recent meta-analysis that included eight studies and more than two million patients. The risk of BDI was estimated to be 0.36% vs. 0.53% depending on whether IOC was routine or elective, respectively, suggesting that conventional IOC reduces the risk and prevents BDI in LC. However, several limitations are associated with this study: only significant but retrospectives articles were included, no randomized studies were analyzed, and the criteria of the selected IOC were not adequately investigated.
A randomized study involving 371 patients failed to demonstrate a real advantage of the systematic use of IOC in preventing BDIs or identifying CBD stones [7].
Indocyanine green fluorescence cholangiography (ICG-C) can be a valid alternative to IOC.
ICG-C can identify biliary tree anatomy without adding the additional time and expertise required for IOC [22].
In a randomized study, ICG-C was confirmed to be non-inferior to IOC in identifying biliary anatomy during LC [23]. In another recent randomized study involving 639 patients, Dip et al. [24] reported that the use of ICG-C is statistically superior to classical vision in recognizing extrahepatic biliary structures. ICG-C appears to be a promising tool, but there are some limitations of its systematic use: (1) the need for an appropriate camera; (2) the optimal timing of ICG injection is still debated; (3) ICG-C may fill in certain types of patients, for example, due to visceral adiposity [22]. Nonetheless, the increasing use of fluorography in the colorectal and hepato-biliary surgery could make ICG-C more accessible in the near future.
Our series found a higher overall complication rate in the IOC group (5.1% vs. 0.0%, p = 0.03). One trial, including open cholecystectomies, reported significantly higher morbidity in the IOC group, and another study reported slightly higher surgical site infection in the IOC group [25,26]. The morbidity rate related to IOC is not very detailed in the literature, and there is a lack of data [6].
This study has several limitations: retrospective and single-center study, limited study population size, and procedures performed by young and highly trained surgeons.
In conclusion, routine implementation of IOC during elective LC increased operative time and overall postoperative morbidity without demonstrating a real benefit in the early detection of BDI or CBD stones. The confounding of systematic or selective use of IOC is still unresolved, and further randomized studies are needed.
REFERENCES
1. Flum DR, Cheadle A, Prela C, Dellinger EP, Chan L. 2003; Bile duct injury during cholecystectomy and survival in medicare beneficiaries. JAMA. 290:2168–2173. DOI: 10.1001/jama.290.16.2168. PMID: 14570952.

2. Mangieri CW, Hendren BP, Strode MA, Bandera BC, Faler BJ. 2019; Bile duct injuries (BDI) in the advanced laparoscopic cholecystectomy era. Surg Endosc. 33:724–730. DOI: 10.1007/s00464-018-6333-7. PMID: 30006843.

3. Swedish Agency for Health Technology Assessment and Assessment of Social Services (SBU). Intraoperative cholangiography in cholecystectomy: a systematic review and assessment of medical, economic, social and ethical aspects [Internet]. Stockholm: Swedish Agency for Health Technology Assessment and Assessment of Social Services (SBU);2018. cited 2021 Jun 17. Available from: https://pubmed.ncbi.nlm.nih.gov/34464042/. PMID: 34464042.
4. Fédération de chirurgie viscérale et digestive. 2014; Risk management to decrease bile duct injury associated with cholecystectomy: measures to improve patient safety. J Visc Surg. 151:241–244. DOI: 10.1016/j.jviscsurg.2014.04.003. PMID: 24810713.
5. Rystedt JML, Wiss J, Adolfsson J, Enochsson L, Hallerbäck B, Johansson P, et al. 2021; Routine versus selective intraoperative cholangiography during cholecystectomy: systematic review, meta-analysis and health economic model analysis of iatrogenic bile duct injury. BJS Open. 5:zraa032. DOI: 10.1093/bjsopen/zraa032. PMID: 33688957. PMCID: PMC7944855.

6. Ford JA, Soop M, Du J, Loveday BP, Rodgers M. 2012; Systematic review of intraoperative cholangiography in cholecystectomy. Br J Surg. 99:160–167. DOI: 10.1002/bjs.7809. PMID: 22183717.

7. Ding GQ, Cai W, Qin MF. 2015; Is intraoperative cholangiography necessary during laparoscopic cholecystectomy for cholelithiasis? World J Gastroenterol. 21:2147–2151. DOI: 10.3748/wjg.v21.i7.2147. PMID: 25717250. PMCID: PMC4326152.

8. Michael Brunt L, Deziel DJ, Telem DA, Strasberg SM, Aggarwal R, Asbun H, et al. 2020; Safe cholecystectomy multi-society practice guideline and state-of-the-art consensus conference on prevention of bile duct injury during cholecystectomy. Surg Endosc. 34:2827–2855. DOI: 10.1007/s00464-020-07568-7. PMID: 32399938.
9. Van Dijk AH, De Reuver PR, Besselink MG, Van Laarhoven KJ, Harrison EM, Wigmore SJ, et al. 2017; Assessment of available evidence in the management of gallbladder and bile duct stones: a systematic review of international guidelines. HPB (Oxford). 19:297–309. DOI: 10.1016/j.hpb.2016.12.011. PMID: 28117228.

10. Agresta F, Campanile FC, Vettoretto N, Silecchia G, Bergamini C, Maida P, et al. 2015; Laparoscopic cholecystectomy: consensus conference-based guidelines. Langenbecks Arch Surg. 400:429–453. DOI: 10.1007/s00423-015-1300-4. PMID: 25850631.

11. European Association for the Study of the Liver (EASL). 2016; EASL Clinical Practice Guidelines on the prevention, diagnosis and treatment of gallstones. J Hepatol. 65:146–181. DOI: 10.1016/j.jhep.2016.03.005. PMID: 27085810.
12. Strasberg SM, Brunt LM. 2010; Rationale and use of the critical view of safety in laparoscopic cholecystectomy. J Am Coll Surg. 211:132–138. DOI: 10.1016/j.jamcollsurg.2010.02.053. PMID: 20610259.

13. Amott D, Webb A, Tulloh B. 2005; Prospective comparison of routine and selective operative cholangiography. ANZ J Surg. 75:378–382. DOI: 10.1111/j.1445-2197.2005.03393.x. PMID: 15943720.

14. Fletcher DR, Hobbs MS, Tan P, Valinsky LJ, Hockey RL, Pikora TJ, et al. 1999; Complications of cholecystectomy: risks of the laparoscopic approach and protective effects of operative cholangiography: a population-based study. Ann Surg. 229:449–457. DOI: 10.1097/00000658-199904000-00001. PMID: 10203075. PMCID: PMC1191728.

15. Rhaiem R, Piardi T, Renard Y, Chetboun M, Aghaei A, Hoeffel C, et al. 2019; Preoperative magnetic resonance cholangiopancreatography before planned laparoscopic cholecystectomy: is it necessary? J Res Med Sci. 24:107. DOI: 10.4103/jrms.JRMS_281_19. PMID: 31949458. PMCID: PMC6950362.

16. Zang J, Yuan Y, Zhang C, Gao J. 2016; Elective laparoscopic cholecystectomy without intraoperative cholangiography: role of preoperative magnetic resonance cholangiopancreatography - a retrospective cohort study. BMC Surg. 16:45. DOI: 10.1186/s12893-016-0159-9. PMID: 27411676. PMCID: PMC4944431.

17. Joyce WP, Keane R, Burke GJ, Daly M, Drumm J, Egan TJ, et al. 1991; Identification of bile duct stones in patients undergoing laparoscopic cholecystectomy. Br J Surg. 78:1174–1176. DOI: 10.1002/bjs.1800781008. PMID: 1835665.

18. Williams E, Beckingham I, El Sayed G, Gurusamy K, Sturgess R, Webster G, et al. 2017; Updated guideline on the management of common bile duct stones (CBDS). Gut. 66:765–782. DOI: 10.1136/gutjnl-2016-312317. PMID: 28122906.

19. Hakuta R, Hamada T, Nakai Y, Oyama H, Kanai S, Suzuki T, et al. 2020; Natural history of asymptomatic bile duct stones and association of endoscopic treatment with clinical outcomes. J Gastroenterol. 55:78–85. DOI: 10.1007/s00535-019-01612-7. PMID: 31473828.

20. Conrad C, Wakabayashi G, Asbun HJ, Dallemagne B, Demartines N, Diana M, et al. 2017; IRCAD recommendation on safe laparoscopic cholecystectomy. J Hepatobiliary Pancreat Sci. 24:603–615. DOI: 10.1002/jhbp.491. PMID: 29076265.

21. de'Angelis N, Catena F, Memeo R, Coccolini F, Martínez-Pérez A, Romeo OM, et al. 2021; 2020 WSES guidelines for the detection and management of bile duct injury during cholecystectomy. World J Emerg Surg. 16:30. DOI: 10.1186/s13017-021-00369-w. PMID: 34112197. PMCID: PMC8190978.
22. Goldstein SD, Lautz TB. 2020; Fluorescent cholangiography during laparoscopic cholecystectomy: shedding new light on biliary anatomy. JAMA Surg. 155:978–979. DOI: 10.1001/jamasurg.2020.3003. PMID: 32857120.

23. Lehrskov LL, Westen M, Larsen SS, Jensen AB, Kristensen BB, Bisgaard T. 2020; Fluorescence or X-ray cholangiography in elective laparoscopic cholecystectomy: a randomized clinical trial. Br J Surg. 107:655–661. DOI: 10.1002/bjs.11510. PMID: 32057103.

24. Dip F, LoMenzo E, Sarotto L, Phillips E, Todeschini H, Nahmod M, et al. 2019; Randomized trial of near-infrared incisionless fluorescent cholangiography. Ann Surg. 270:992–999. DOI: 10.1097/SLA.0000000000003178. PMID: 30614881.

25. Hauer-Jensen M, Karesen R, Nygaard K, Solheim K, Amlie EJ, Havig O, et al. 1993; Prospective randomized study of routine intraoperative cholangiography during open cholecystectomy: long-term follow-up and multivariate analysis of predictors of choledocholithiasis. Surgery. 113:318–323.
26. Murison MS, Gartell PC, McGinn FP. 1993; Does selective peroperative cholangiography result in missed common bile duct stones? J R Coll Surg Edinb. 38:220–224.
Table 1
Preoperative characteristics
| Characteristic | All patients (n = 303) | IOC (n = 215, 71.0%) | No-IOC (n = 88, 29.0%) | p-value |
|---|---|---|---|---|
| Age (yr) | 49 (16–89) | 50 (18–89) | 48 (16–86) | 0.06 |
| Male, sex | 114 (37.6) | 74 (34.4) | 40 (45.5) | 0.08 |
| ASA score | 0.20 | |||
| I | 80 (26.4) | 61 (28.4) | 19 (21.6) | |
| II | 182 (60.1) | 125 (58.1) | 57 (64.8) | |
| III | 39 (12.9) | 29 (13.5) | 10 (11.4) | |
| IV | 2 (0.7) | 0 (0.0) | 2 (2.3) | |
| Previous surgery | 97 (32.0) | 72 (33.5) | 25 (28.4) | 0.20 |
| Body mass index | 28.90 ± 5.60 | 28.90 ± 5.65 | 28.90 ± 5.48 | 0.60 |
| Surgical indication | 0.60 | |||
| History of cholecystitis | 107 (35.3) | 75 (34.9) | 32 (36.4) | |
| Hepatic colic | 128 (42.2) | 95 (44.2) | 33 (37.5) | |
| Gallstones | 29 (9.6) | 21 (9.8) | 8 (9.1) | |
| Pancreatitis | 36 (11.9) | 22 (10.2) | 14 (15.9) | |
| Polyps | 3 (1.0) | 2 (0.9) | 1 (1.1) | |
| MRCP | 64 (21.1) | 37 (17.2) | 27 (30.7) | 0.01* |
| Preoperative CBD stones | 33 (10.9) | 20 (9.3) | 13 (14.8) | 0.20 |
| Preoperative ERCP | 31 (10.2) | 19 (8.8) | 12 (13.6) | 0.20 |
Table 2
Intraoperative and postoperative course
| Variable | All patients (n = 303) | IOC (n = 215, 71.0%) | No-IOC (n = 88, 29.0%) | p-value |
|---|---|---|---|---|
| Trained surgeon | 213 (70.3) | 160 (74.4) | 53 (60.2) | 0.01* |
| Operative time | 60 (35–210) | 70 (45–210) | 55 (35–130) | 0.01* |
| Drainage | 44 (14.5) | 31 (14.4) | 13 (14.8) | > 0.99 |
| Intraoperative complications | 5 (1.7) | 4 (1.9) | 1 (1.1) | > 0.99 |
| Bleeding | 4 (1.3) | 3 (1.4) | 1 (1.1) | |
| Biliary injury | 1 (0.3) | 1 (0.5) | 0 (0.0) | |
| Conversion | 3 (1.0) | 2 (0.9) | 1 (1.1) | > 0.99 |
| Bleeding | 1 (0.3) | 0 (0.0) | 1 (1.1) | |
| Biliary injury | 1 (0.3) | 1 (0.5) | 0 (0.0) | |
| Adhesions | 1 (0.3) | 1 (0.5) | 0 (0.0) | |
| Ambulatory surgery | 203 (67.0) | 147 (68.4) | 56 (63.6) | 0.40 |
| Hospital stay (day) | 0.64 ± 1.74 | 0.75 ± 1.90 | 0.64 ± 1.17 | 0.20 |
| 30-Day postoperative complications | 11 (3.6) | 11 (5.1) | 0 (0.0) | 0.03* |
| Bleeding | 3 (1.0) | 3 (1.4) | 0 (0.0) | |
| Surgical site infections | 2 (0.7) | 2 (0.9) | 0 (0.0) | |
| Biliary injury | 3 (1.0) | 3 (1.4) | 0 (0.0) | |
| Collection | 1 (0.3) | 1 (0.5) | 0 (0.0) | |
| Gallstones biliary disorder | 2 (0.7) | 2 (0.9) | 0 (0.0) | |
| Re-operation | 5 (1.7) | 5 (2.3) | 0 (0.0) | 0.30 |
| Bleeding | 3 (1.0) | 3 (1.4) | 0 (0.0) | |
| Biliary injury | 1 (0.3) | 1 (0.5) | 0 (0.0) | |
| Surgical site infections | 1 (0.3) | 1 (0.5) | 0 (0.0) | |
| Postoperative ERCP | 9 (3.0) | 9 (4.2) | 0 (0.0) | 0.06 |
| Programmed | 7 (2.3) | 7 (3.3) | 0 (0.0) | |
| Non-programmed | 2 (0.7) | 2 (0.9) | 0 (0.0) | |
| 90-Day biliary injury | 3 (1.0) | 3 (1.4) | 0 (0.0) | 0.50 |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download