Abstract
Backgrounds/Aims
Cholangiocarcinoma (CCA) can be classified as intrahepatic CCA or extrahepatic CCA (eCCA). We intended to analyze and reports the survival outcomes for eCCA.
Methods
Surveillance, epidemiology, and end results (SEER) registry, site recode C24.0, was used to select cases of eCCA from 2000 to 2018. Patients with incomplete data or ages <18 years were excluded.
Results
Male (52.69%) and White race (77.99%) predominated. Compared with 2000–2006, survival increased in 2013 (adjusted hazard ratio [HRadj]: 0.68, 95% confidence interval [CI] 0.58–0.70; p < 0.01). Surgery with chemoradiotherapy (HRadj: 0.69, 95% CI 0.60–0.7; p < 0.01) and surgery with chemotherapy (HRadj: 0.72, 95% CI 0.62–0.83; p < 0.01) improved survival over surgery alone. Compared with surgery without lymph node (LN) removal, surgery of four or more regional LN reduced the risk of death by 58% (HRadj: 0.42, 95% CI 0.36–0.51; p < 0.01). Compared with patients without surgery, patients who underwent bile duct excision (HRadj: 0.82, 95% CI 0.72–0.94; p < 0.01), simple or extended lobectomy (HRadj: 0.85, 95% CI 0.75–0.95; p = 0.009), and hepatectomy (HRadj: 0.80, 95% CI 0.72–0.88; p < 0.01) significantly improved survival. Patients with distal CCA had a 17% higher survival than perihilar CCA (HRadj: 0.83, 95% CI 0.74–0.92; p < 0.01) and LN dissection was equally beneficial for both subgroups (p < 0.01).
Go to : 
Cholangiocarcinoma (CCA) is a bile duct cancer that arises from the epithelial cells of bile ducts. CCA is rare in the United States and has high mortality rates. CCAs can be further classified based on their location as intrahepatic cholangiocarcinomas (iCCA) or extrahepatic cholangiocarcinomas (eCCA) [1,2]. Approximately five to ten percent of CCA are intrahepatic, arising from small intrahepatic ducts or large intrahepatic ducts proximal to the bifurcation of the right and left intrahepatic ducts [1]. On the other hand, extrahepatic biliary ducts divide into perihilar and distal segments, with the transition occurring proximal to the cystic duct. eCCA account for approximately 70%–90% of CCA [1], with 60% to 70% of eCCA arising in the perihilar region [3]. Patients with eCCA typically have a five-year survival between 10%–30%, influenced by factors such as vascular invasion, lymph node, and distant metastases [2,4]. In the United States, approximately 23,000 cases of CCA are diagnosed annually. Unfortunately, diagnosis is usually made at advanced stages due to non-specific early-stage symptoms and the lack of screening strategies [5]. Furthermore, in the United States, the incidence of CCA is relatively low and has decreased by 0.50% per year between 1999–2011 among women, while male incidence remained stable [5]. Among racial categories, American Indians and Alaskan Natives have higher rates of biliary tract carcinomas than non-Hispanic whites [5]. However, in recent years, there has been a scarcity of survival analysis for eCCA [6]. We intend to extend the knowledge about eCCA by analyzing factors that may improve survival in these patients. Before 2000, the Surveillance, Epidemiology, and End Results (SEER) registry used ICD-O-2, leading to misclassification and overlap of iCCA and eCCA. This overestimated the incidence of iCCA and eCCA in prior studies, affecting the prior survival analysis [7]. We examined survival based on the SEER 18 research plus (2000–2018) registry, focusing on factors affecting survival. The study utilized SEER registry 18 research plus (2000–2018).
Go to : 
The present study used the SEER 18 research database, which has 28% of the US per 2010 census [8]. Additional registries included in SEER 18 can be found at https://seer.cancer.gov/data/. Within the SEER database, the International Classification of Diseases (ICD-0–3/WHO 2008): Extrahepatic bile duct (site C24.0) was used via the SEER*Stat program to select cases of extrahepatic bile duct cancer alone [9]. Patients were excluded if they aged less than 18 years of age, had no microscopically confirmed diagnosis, and had missing mortality status (alive/dead). The years of diagnosis were categorized as 2000–2006, 2007–2012, and 2013+. Regrading age, the four groups include 18–39 years, 40–59 years, 60–79 years, and above 80 years. As in previous studies, the race was categorized into whites, blacks, Asian/the race was classified as Whites, Blacks, Asian/Pacific Islander, and American Indian/Alaskan Natives [10,11]. Data of cancer stage and therapeutic interventions were grouped accordingly.
The outcomes of interest included overall survival, defined by the variables of interest from eCCA diagnosis (in months) to the date of death from any cause. Additional outcomes of interest included, but are not limited to lymph node resection, diagnosis, staging of carcinoma, sex, and race.
Patient characteristics were compared using the chi-squared test for categorical variables or Wilcoxon rank-sum (Mann–Whitney) for continuous variables (if applicable). Cox regression models were developed to estimate the hazard ratios (HRs) for survival at 60 months and reported as adjusted hazard ratios (HRadj) with 95% confidence intervals (CIs) and p-values. The Kaplan–Meier curve represented the survival function, and a stratified Cox regression-based test for the equality of the survival curves was executed for the difference in survival between the two patient groups. Similarly, quantile regression was used to report adjusted median survival time. All biographical (age, sex, and race) and disease specific (TNM Staging and treatment) variables were used to adjust the aforementioned regression analyses. Perihilar cholangiocarcinoma (pCCA) cases were identified using ICD-O-3 histology codes 8000–8152, 8154–8231, 8243–8245, 8250–8576, 8940–8950, and 8980–8981 [12]; and CS Site-Specific Factor 25 (Schema Discriminator: BileDuctsDistal/BileDuctsPerihilar/CysticDuct) codes 010,020,050,060,100 to further define the location of the tumor in the hilum [13]. As cited, both methods have been used previously in the published literature. The threshold for statistical significance was 0.05, with 2-sided p-values in the analysis. We used statistical software for data science (STATA) version 16.0 software (StataCorp LLC, Station, TX, USA) for statistical analysis.
The SEER 18 database consists of an identified database, which is publicly available; therefore, it was deemed exempt from the institutional review board. The patient consent was also waived because the SEER site is publically available.
Go to : 
Our study included 12,747 cases with complete mortality data. Male (52.69%) and White race (77.99%) predominated, followed by Asian or Pacific Islander (12.81%). The median age at diagnosis was 71 years (interquartile range [IQR] 62–81 years). The highest frequency of cases was in the age group 60–79 years (52.35%), with male predominance (Table 1). The median survival time was 18 months (IQR 9.50–43 months). The survival at 12, 36, and 60 months was 36.80%, 12.70%, and 8.50%, respectively. Median survival was longer in female than in male (23 vs. 25 months, p < 0.01). The median survival was longer in Black 27 months (IQR 10.50–48.50) (Table 2). Surgical intervention (overall) was performed in 26.78% of cases, chemotherapy (overall) in 35.38%, and radiotherapy (overall) in only 17.54% of the cases. About 12.54% of cases underwent surgery alone, followed by chemoradiotherapy (7.50%), surgery with chemotherapy (5.68%), and surgery with radiotherapy (1.04%). About 15.93% of cases underwent chemotherapy alone, followed by radiotherapy alone (2.75%). About 6.24% of patients underwent chemotherapy with radiotherapy. The median survival was higher in the 2013+ cohort (Table 2). Patients with surgery and adjuvant chemoradiotherapy and chemotherapy alone had a longer median survival time of 28 months (Table 2), followed by surgery with radiotherapy 22 months (IQR 10–50 months) (Table 2). Survival analysis based on adjusted AJCC 6th edition T Stage staging is presented in Table 2, with the highest median survival time at diagnosis in patients with T1 stage being 36 months (IQR 15–69 months). In addition, the median time was longer for patients with N0 and M0 stages (Table 2). Four or more regional lymph node removal was associated with a longer median survival time of 29 months (IQR 13.50–56.5 months). Table 2 shows the additional median survival times.
Biodemographic characteristics between males and females diagnosed with extrahepatic cholangiocarcinoma in SEER registry 18 (2000–2018)
Mean survival times (months) of patients diagnosed with extrahepatic cholangiocarcinoma in the SEER registry 18 (2000–2018)
HRs did not reveal significant survival in female compared with male (HRadj: 0.96, 95% CI 0.86–1.10; p = 0.58). Patients aged 40–59 years (HRadj: 0.95, 95% CI 0.61–1.46; p = 0.81), 60–79 years (HRadj: 0.97, 95% CI 0.63–1.47; p = 0.80), and above 80 years (HRadj: 1.008, 95% CI 0.65–1.54; p = 0.97), and there was no significant difference in survival compared with those under 40 years. Asians or Pacific Islanders (HRadj: 0.95, 95% CI 0.61–1.46; p = 0.89), Black (HRadj: 0.95, 95% CI 0.61–1.46; p = 0.81) or American Indian/Alaska Native (HRadj: 1.01, 95% CI 0.65–1.54; p = 0.62) did not significantly differ in mortality compared with whites. Survival increased in 2013 (HRadj: 0.68, 95% CI 0.58–0.70; p < 0.001) but not in 2007–2012 (HRadj: 0.95, 95% CI 0.59–0.749; p < 0.52) compared with 2000–2006.
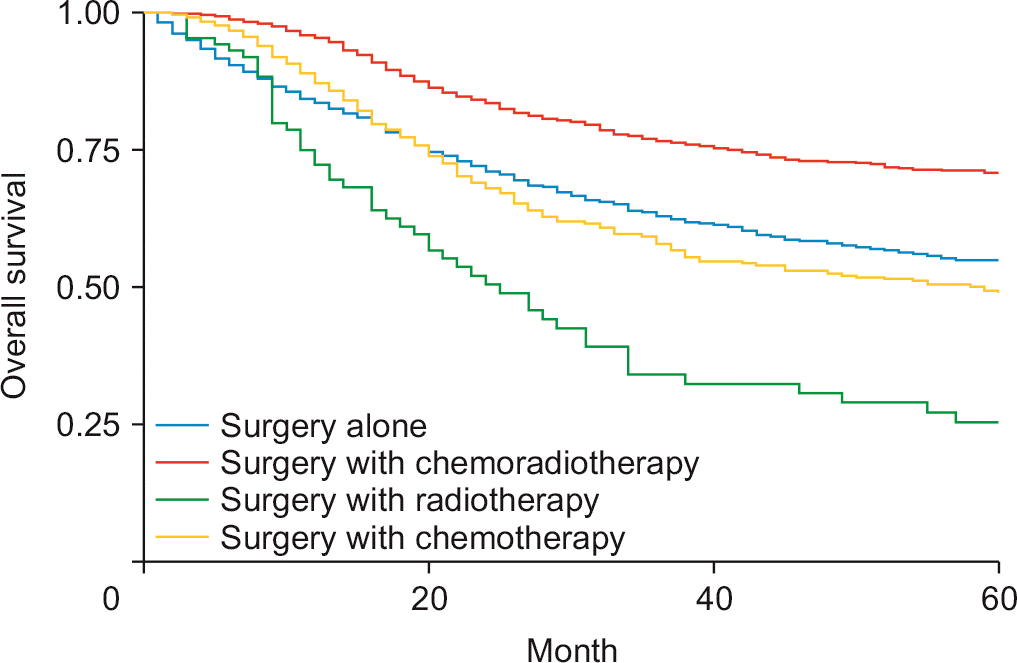
Surgery was associated with improved survival compared to nonsurgical intervention (HRadj: 0.35, 95% CI 0.33–0.38; p < 0.01). Surgery with chemoradiotherapy (HRadj: 0.69, 95% CI 0.60–0.7; p < 0.01) and surgery with chemotherapy (HRadj: 0.72, 95% CI 0.62–0.83; p < 0.01) but not surgery with radiotherapy (HRadj: 0.97, 95% CI 0.73–1.29; p = 0.87) was associated with a lower risk of mortality compared with surgery alone (Fig. 1). Compared with the T1 stage at diagnosis, patients with T2 (HRadj: 1.36, 95% CI 1.12–1.65; p < 0.01), T3 (HRadj: 1.66, 95% CI 1.39–1.99; p < 0.01), and T4 (HRadj: 1.71, 95% CI 1.38–2.11; p < 0.01) had a higher risk of mortality. Compared with M0 status at diagnosis, M1 (HRadj: 2.07, 95% CI 1.69–2.54; p < 0.01) had a higher mortality risk. Compared with the N0 stage at diagnosis, N1 (HRadj: 1.61, 95% CI 1.43–1.81, p < 0.01) had a higher mortality risk.
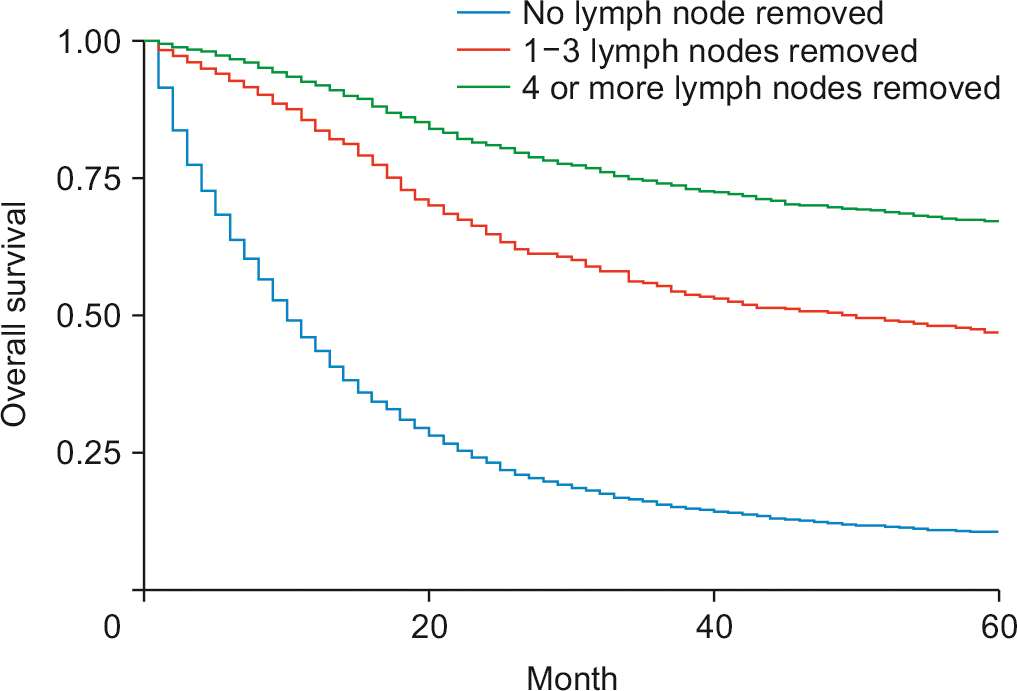
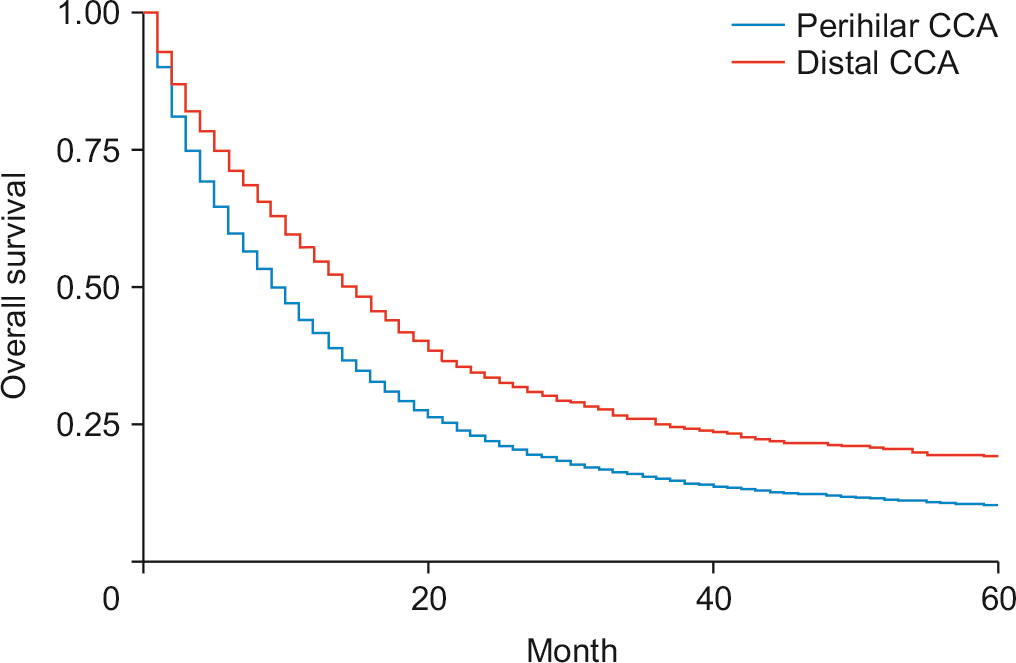
Surgical removal of one to three regional lymph nodes reduced the risk of death by 52.3% (HRadj: 0.57, 95% CI 0.47–0.69; p < 0.01) (Fig. 2), while excision of four or more regional lymph nodes reduced the risk of death by 58% (HRadj: 0.42, 95% CI 0.36–0.51; p < 0.01). Compared to patients without surgery, patients who underwent local tumor destruction (photodynamic therapy, electrocautery, fulguration, cryosurgery, laser, percutaneous ethanol injection-PEI, heat-radiofrequency ablation) did not have a significant improvement in survival (HRadj: 1.04, 95% CI 0.46–2.33; p = 0.91). Patients are undergoing bile duct resection (HRadj: 0.82, 95% CI 0.72–0.94; p < 0.01), simple or extended lobectomy (HRadj: 0.85, 95% CI 0.75–0.95; p = 0.009), and hepatectomy (HRadj: 0.80, 95% CI 0.72–0.88; p < 0.01) had significant improvement in survival outcomes compared to patients without surgery. These interventions were independent of whether patients received adjuvant therapy with chemoradiation. In the present study, 79.5% (n = 9,590) of cases were defined as pCCA, while 24% (n = 3,045) were distal cholangiocarcinoma’s (dCCA’s). Less than 1% is undefined. Patients with dCCA had a 17% higher survival than pCCA (HRadj: 0.83, 95% CI 0.74–0.92; p < 0.01) (Fig. 3). pCCA (HRadj: 0.55, 95% CI: 0.53–0.57; p < 0.01) and dCCA (HRadj: 0.76, 95% CI 0.51–0.57; p < 0.01) improved survival outcomes compared to any lymph node dissection.
Go to : 
The present study reports a comprehensive survival analysis based on various factors for eCCA patients. Previous survival studies in CCA have often primarily focused on specific subsets of these patients and comparisons between eCCA and iCCA [4,14]. Because almost all biliary CCA before 2000 were classified as iCCA, it is challenging to distinguish population-based data in eCCA from the SEER. A significant improvement in classification occurred in 2000 with changes in the ICD-O code [7]; therefore, the earliest year included in this review is 2000. The risk factors for iCCA and eCCA are similar, including parasitic infections, biliary tract disorders such as bile duct cysts or primary sclerosing cholangitis (PSC), and exposure to Thorotrast, a radiographic contrast agent used, in the US, until the 1950s [15,16]. The incidence of cholangiocarcinoma is high in Southeast Asia. There are no recent US data regarding survival analyses of eCCA, significantly when differentiating between therapeutic interventions [17-19].
We reported a 4% reduction in mortality in females compared to males without significance (HRadj: 0.96, 95% CI 0.86–1.10; p = 0.58). It can be hypothesized that males have a greater awareness of eCCA than females, due to its higher incidence compared to females, resulting in similar outcomes [20]. In addition, PSC, a known risk factor for CCA, has a higher incidence in males than females [21]. Although there was no comparison with eCCA, a previous meta-analysis for hilar CCA found no significant association between sex and overall survival, consistent with our analysis. However, the funnel plot for sex demonstrated significant asymmetry for included studies, indicating potential publication bias [22]. The median age at diagnosis in this study was 71 years, and nearly 80% of patients were 60 years or older; this distribution was in line with previous incidence studies [23]. There was no difference in survival based on their age groups. Although there was no significant difference in survival based on age group data, survival in eCCA correlates with age at diagnosis, with younger patients more likely to receive adjuvant therapy than older patients [24].
Although an increase in incidence among the Asian and Pacific Islander ethnic groups has been previously reported, they did not show a significant increase in mortality risk in our results compared with whites. Several factors may play a role, including genetic factors. Previous studies have reported that Asian patient have better survival for iCCA and hepatocellular carcinoma [25]. Several genes have predictive value for CCA. However, current literature linking these genes to Asian or Pacific Islanders is lacking [26]. Additionally, compared to Caucasians and blacks, Asians or Pacific Islanders are more likely to receive surgery for similar conditions [25,27]. The present study reported a significant decrease in mortality from 2013 (32%), compared to 2000–2006, compared to previous studies [20]. This may be explained by advances in diagnostic sensitivity and improved resection techniques [28,29].
The current standard of care for resectable eCCA is six months of adjuvant chemotherapy [30,31]. Our study revealed significantly increased survival in all groups receiving surgical resection regardless of adjuvant therapies. The most significant advantage in overall survival was in patients who received chemotherapy or chemoradiotherapy and surgery (Fig. 1). This information is consistent with the results of the BILCAP trial, which showed an improvement in overall survival in CCA treated with chemotherapy. This is consistent with data suggesting that radiation can use with adjuvant chemotherapy [31]. Recently, liver transplantation combined with neoadjuvant chemotherapy has shown promising results with improved disease-free survival [32].
Tumor staging has been recognized as a reasonable predictor of survival [22,33]. Previous analyses comparing cancer stage with overall survival in eCCA focused solely on postoperative patients [22]. The present study suggests that T2, T3 and T4 have increased mortality risk compared to T1. The present study reported increased mortality in all patients with metastatic disease when diagnosed with M1 (HRadj: 2.07, 95% CI 1.69–2.54; p < 0.01). Studies in similar cancers have reported improved survival outcomes in patients with lymph node resection [34]. Our study revealed that excision of lymph node dissection increases survival in all surgical patients, and lymph node dissection increases survival. The presence of lymph node metastasis has previously been shown to be significant prognostic factor in similar patients. Our data suggest that lymph node resection is beneficial in most patients [35].Due to previous misclassifications, Data on the survival of pCCA and dCCA are limited in the present literature. Prior literature reports high but nonsignificant survival effects in these two categories [36]; however, the study was very small-scale. We report significantly increased survival in dCCA compared with pCCA. We also report that lymph node dissection is equally beneficial for both subgroups. Further prospective multicenter studies would help elaborate on this finding.
This study was limited by the information collected for the SEER database. No reports of comorbid conditions were found for patients in our study. This information is useful for stratification, such as PSC or parasitic infections, which may confound the data. The survival dates in the SEER registry are listed by the month and the year, which provides approximate survival times, especially in low survival conditions, such as eCCA. Additionally, no data were available on patients undergoing the Mayo protocol for orthotopic liver transplantation. The database does not have data on pancreatoduodenectomy that may affect survival outcomes. We included only patients with a microscopically confirmed diagnosis, and only 12.54% had surgical intervention. The interventions may have been diagnostic, for example, endoscopic retrograde cholangiopancreatography, percutaneous transhepatic cholangiography, or cholangioscopy guided biopsies [37]. However, this information is not included in the SEER database to protect patient privacy. Despite the introduction of the ICD-0-3 system in 2000, there is still a risk of underestimation of eCCA (C24.0) due to the misclassification of the Klatskin tumor.
Our study reported no differences in survival based on sex or race. Surgery with chemoradiotherapy remains the treatment modality with a demonstrated increase in 5-year survival. Black or American Indian/Alaska Native patients did not differ significantly in mortality compared with whites, Asians, or Pacific Islanders. Lymph node resection during surgery is associated with significantly better outcomes. dCCA has better outcomes than pCCA; lymph node dissection is equally beneficial for pCCA and dCCA. This new information regarding eCCA survival-based sociodemographics and therapeutics approaches will positively influence treatment guidelines, resource planning, and management of this disease. With recent studies supporting the addition of immunotherapy to standard chemotherapy regimens, our data will be essential for monitoring changes in differential survival as new therapies are adopted. Further considerations after this study may focus on genetic factors prevalent in Asian and Pacific Islander patients. Because these patients appear to have a selective advantage compared to the general population, genetics may play a role and should be the topic of further study. Additionally, differential survival based on underlying conditions, such as parasitic infection, would be helpful for the study. If these patients have a better overall survival rate, this may change the guidelines for surgical resection.
Go to : 
Notes
AUTHOR CONTRIBUTIONS
Conceptualization: HA. Data curation: HA, PP. Methodology: HA, JZ, PP. Visualization: HA, JZ. Writing - original draft: JZ, PP, BT, RK. Writing - review & editing: All authors.
Go to : 
REFERENCES
1. Zhang H, Yang T, Wu M, Shen F. 2016; Intrahepatic cholangiocarcinoma: epidemiology, risk factors, diagnosis and surgical management. Cancer Lett. 379:198–205. DOI: 10.1016/j.canlet.2015.09.008. PMID: 26409434.

2. Ali H, Tedder B, Waqar SH, Mohamed R, Cate EL, Ali E. 2022; Changing incidence and survival of intrahepatic cholangiocarcinoma based on Surveillance, Epidemiology, and End Results Database (2000-2017). Ann Hepatobiliary Pancreat Surg. 26:235–243. DOI: 10.14701/ahbps.21-173. PMID: 35811455. PMCID: PMC9428430.

3. Blechacz B, Komuta M, Roskams T, Gores GJ. 2011; Clinical diagnosis and staging of cholangiocarcinoma. Nat Rev Gastroenterol Hepatol. 8:512–522. DOI: 10.1038/nrgastro.2011.131. PMID: 21808282. PMCID: PMC3331791.

4. Liao P, Cao L, Chen H, Pang SZ. 2021; Analysis of metastasis and survival between extrahepatic and intrahepatic cholangiocarcinoma: a large population-based study. Medicine (Baltimore). 100:e25635. DOI: 10.1097/MD.0000000000025635. PMID: 33879742. PMCID: PMC8078350.
5. Marcano-Bonilla L, Mohamed EA, Mounajjed T, Roberts LR. 2016; Biliary tract cancers: epidemiology, molecular pathogenesis and genetic risk associations. Chin Clin Oncol. 5:61. DOI: 10.21037/cco.2016.10.09. PMID: 27829275.

6. Forner A, Vidili G, Rengo M, Bujanda L, Ponz-Sarvisé M, Lamarca A. 2019; Clinical presentation, diagnosis and staging of cholangiocarcinoma. Liver Int. 39 Suppl 1:98–107. DOI: 10.1111/liv.14086. PMID: 30831002.

7. Welzel TM, McGlynn KA, Hsing AW, O'Brien TR, Pfeiffer RM. 2006; Impact of classification of hilar cholangiocarcinomas (Klatskin tumors) on the incidence of intra- and extrahepatic cholangiocarcinoma in the United States. J Natl Cancer Inst. 98:873–875. DOI: 10.1093/jnci/djj234. PMID: 16788161.

8. Surveillance Research Program, NCIs Division of Cancer Control and Population Sciences. SEER Registries [Internet]. Washington, D.C.: National Cancer Institute;2021. cited 2021 Sep 29. Available from: https://seer.cancer.gov/.
9. Surveillance Research Program, National Cancer Institute. SEER*Stat software version 8.3.9.2 [Internet]. Washington, D.C.: National Cancer Institute;2021. cited 2021 Sep 29. Available from: https://seer.cancer.gov/seerstat/.
10. Brar G, Greten TF, Graubard BI, McNeel TS, Petrick JL, McGlynn KA, et al. 2020; Hepatocellular carcinoma survival by etiology: a SEER-medicare database analysis. Hepatol Commun. 4:1541–1551. DOI: 10.1002/hep4.1564. PMID: 33024922. PMCID: PMC7527688.

11. Ali H, Pamarthy R, Vallabhaneni M, Sarfraz S, Ali H, Rafique H. Pancreatic cancer incidence trends in the United States from 2000-2017: analysis of Surveillance, Epidemiology and End Results (SEER) database. F1000Res. 2021; 10:529. DOI: 10.12688/f1000research.54390.1. PMID: 34527218. PMCID: PMC8411275.

12. Mao K, Liu J, Sun J, Zhang J, Chen J, Pawlik TM, et al. 2016; Patterns and prognostic value of lymph node dissection for resected perihilar cholangiocarcinoma. J Gastroenterol Hepatol. 31:417–426. DOI: 10.1111/jgh.13072. PMID: 26250532. PMCID: PMC4732906.

13. Zhang Y, Wu Z, Wang X, Li C, Chang J, Jiang W, et al. 2020; Development and external validation of a nomogram for predicting the effect of tumor size on survival of patients with perihilar cholangiocarcinoma. BMC Cancer. 20:1044. DOI: 10.1186/s12885-020-07501-0. PMID: 33126868. PMCID: PMC7596930.

14. Liu WW, Tu JF, Ying XH, Chen ZJ, Wang YB. 2022; Postoperative survival of extrahepatic and intrahepatic cholangiocarcinoma after surgery: a population-based cohort. BMJ Open. 12:e049789. DOI: 10.1136/bmjopen-2021-049789. PMID: 35414539. PMCID: PMC9006842.

15. Clements O, Eliahoo J, Kim JU, Taylor-Robinson SD, Khan SA. 2020; Risk factors for intrahepatic and extrahepatic cholangiocarcinoma: a systematic review and meta-analysis. J Hepatol. 72:95–103. DOI: 10.1016/j.jhep.2019.09.007. PMID: 31536748.

16. Tyson GL, El-Serag HB. 2011; Risk factors for cholangiocarcinoma. Hepatology. 54:173–184. DOI: 10.1002/hep.24351. PMID: 21488076. PMCID: PMC3125451.

17. Shaib Y, El-Serag HB. 2004; The epidemiology of cholangiocarcinoma. Semin Liver Dis. 24:115–125. DOI: 10.1055/s-2004-828889. PMID: 15192785.

18. ipa B Sr, Pairojkul C. 2008; Cholangiocarcinoma: lessons from Thailand. Curr Opin Gastroenterol. 24:349–356. DOI: 10.1097/MOG.0b013e3282fbf9b3. PMID: 18408464. PMCID: PMC4130346.

19. Lim JH. 2011; Liver flukes: the malady neglected. Korean J Radiol. 12:269–279. DOI: 10.3348/kjr.2011.12.3.269. PMID: 21603286. PMCID: PMC3088844.

20. Gad MM, Saad AM, Faisaluddin M, Gaman MA, Ruhban IA, Jazieh KA, et al. 2020; Epidemiology of cholangiocarcinoma; United States incidence and mortality trends. Clin Res Hepatol Gastroenterol. 44:885–893. DOI: 10.1016/j.clinre.2020.03.024. PMID: 32359831.

21. Molodecky NA, Kareemi H, Parab R, Barkema HW, Quan H, Myers RP, et al. 2011; Incidence of primary sclerosing cholangitis: a systematic review and meta-analysis. Hepatology. 53:1590–1599. DOI: 10.1002/hep.24247. PMID: 21351115.

22. Bird NTE, McKenna A, Dodd J, Poston G, Jones R, Malik H. 2018; Meta-analysis of prognostic factors for overall survival in patients with resected hilar cholangiocarcinoma. Br J Surg. 105:1408–1416. DOI: 10.1002/bjs.10921. PMID: 29999515.

23. Jang JY, Kim SW, Park DJ, Ahn YJ, Yoon YS, Choi MG, et al. 2005; Actual long-term outcome of extrahepatic bile duct cancer after surgical resection. Ann Surg. 241:77–84. DOI: 10.1097/01.sla.0000150166.94732.88. PMID: 15621994. PMCID: PMC1356849.

24. Hoehn RS, Wima K, Ertel AE, Meier A, Ahmad SA, Shah SA, et al. 2015; Adjuvant chemotherapy and radiation therapy is associated with improved survival for patients with extrahepatic cholangiocarcinoma. Ann Surg Oncol. 22 Suppl 3:S1133–S1139. DOI: 10.1245/s10434-015-4599-8. PMID: 25976862.

25. Ren F, Zhang J, Gao Z, Zhu H, Chen X, Liu W, et al. 2018; Racial disparities in the survival time of patients with hepatocellular carcinoma and intrahepatic cholangiocarcinoma between Chinese patients and patients of other racial groups: a population-based study from 2004 to 2013. Oncol Lett. 16:7102–7116. DOI: 10.3892/ol.2018.9550. PMID: 30546445. PMCID: PMC6256729.

26. Wang F, Liu Y, Zhang H. 2013; Loss of MTSS1 expression is an independent prognostic factor for Hilar cholangiocarcinoma. Pathol Oncol Res. 19:815–820. DOI: 10.1007/s12253-013-9649-6. PMID: 23852458.

27. Stewart SL, Kwong SL, Bowlus CL, Nguyen TT, Maxwell AE, Bastani R, et al. Racial/ethnic disparities in hepatocellular carcinoma treatment and survival in California, 1988-2012. World J Gastroenterol. 2016; 22:8584–8595. DOI: 10.3748/wjg.v22.i38.8584. PMID: 27784971. PMCID: PMC5064040.

28. Barr Fritcher EG, Voss JS, Brankley SM, Campion MB, Jenkins SM, Keeney ME, et al. 2015; An optimized set of fluorescence in situ hybridization probes for detection of pancreatobiliary tract cancer in cytology brush samples. Gastroenterology. 149:1813–1824.e1. DOI: 10.1053/j.gastro.2015.08.046. PMID: 26327129.

29. Ebata T, Mizuno T, Yokoyama Y, Igami T, Sugawara G, Nagino M. 2018; Surgical resection for Bismuth type IV perihilar cholangiocarcinoma. Br J Surg. 105:829–838. DOI: 10.1002/bjs.10556. PMID: 28488733.

30. Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. 2010; Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 362:1273–1281. DOI: 10.1056/NEJMoa0908721. PMID: 20375404.

31. Primrose JN, Fox RP, Palmer DH, Malik HZ, Prasad R, Mirza D, et al. 2019; Capecitabine compared with observation in resected biliary tract cancer (BILCAP): a randomised, controlled, multicentre, phase 3 study. Lancet Oncol. 20:663–673. Erratum in: Lancet Oncol 2019;20:e242.

32. Darwish Murad S, Heimbach JK, Gores GJ, Rosen CB, Benson JT, Kim WR. 2013; Excellent quality of life after liver transplantation for patients with perihilar cholangiocarcinoma who have undergone neoadjuvant chemoradiation. Liver Transpl. 19:521–528. DOI: 10.1002/lt.23630. PMID: 23447435.

33. Buettner S, Galjart B, van Vugt JLA, Bagante F, Alexandrescu S, Marques HP, et al. 2017; Performance of prognostic scores and staging systems in predicting long-term survival outcomes after surgery for intrahepatic cholangiocarcinoma. J Surg Oncol. 116:1085–1095. DOI: 10.1002/jso.24759. PMID: 28703880.

34. Liu ZP, Zhang QY, Chen WY, Huang YY, Zhang YQ, Gong Y, et al. 2022; Evaluation of four lymph node classifications for the prediction of survival in hilar cholangiocarcinoma. J Gastrointest Surg. 26:1030–1040. DOI: 10.1007/s11605-021-05211-x. PMID: 34973138. PMCID: PMC9085675.

35. Kim HJ, Kim CY, Hur YH, Koh YS, Kim JC, Kim HJ, et al. 2014; Prognostic factors for survival after curative resection of distal cholangiocarcinoma: perineural invasion and lymphovascular invasion. Surg Today. 44:1879–1886. DOI: 10.1007/s00595-014-0846-z. PMID: 24535697.

36. Waseem D, Tushar P. 2017; Intrahepatic, perihilar and distal cholangiocarcinoma: management and outcomes. Ann Hepatol. 16:133–139. DOI: 10.5604/16652681.1226927. PMID: 28051802. PMCID: PMC5630455.

37. Valero V 3rd, Cosgrove D, Herman JM, Pawlik TM. 2012; Management of perihilar cholangiocarcinoma in the era of multimodal therapy. Expert Rev Gastroenterol Hepatol. 6:481–495. DOI: 10.1586/egh.12.20. PMID: 22928900. PMCID: PMC3538366.

Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download