Abstract
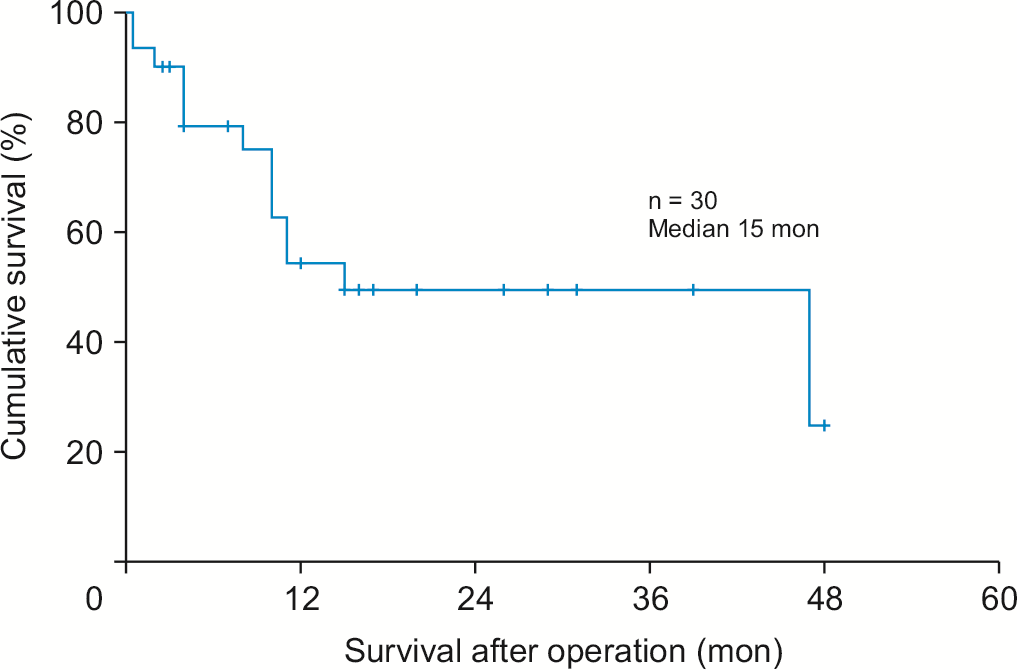
Pancreatic carcinosarcoma is a very rare malignancy with a poor prognosis. Because of these characteristics, a treatment strategy for it has not been established yet. The aim of this study was to establish a therapeutic strategy for pancreatic carcinosarcoma. We reviewed data of a 65-year-old female patient who was diagnosed with pancreatic carcinosarcoma through endoscopic ultrasound-guided fine needle aspiration biopsy before surgery. For literature review, we searched PubMed using terms of “Pancreatic” or “Pancreas” and “carcinosarcoma” or “carcinosarcomatous”. The patient received 11 cycles of neoadjuvant treatment with leucovorin, fluorouracil, irinotecan, oxaliplatin and pembrolizumab because the tumor was borderline resectable. She underwent stereotactic ablative body radiotherapy (SABR) with 35 Gy in 5 fractions, followed by robotic pylorus-preserving pancreaticoduodenectomy. After surgery, the patient received adjuvant chemotherapy in the same regimen as before surgery. She is alive without any recurrence. Among 48 patients within 33 available papers, the median survival time was 15 months. The survival rate of patients who received adjuvant chemotherapy tended to be higher than that of those who did not receive adjuvant chemotherapy, although the difference was not statistically significant (median survival, 47 vs. 15 months; p = 0.485). Three patients who received neoadjuvant chemotherapy had a survival period of 13–23.5 months. Surgery with lymphadenectomy, adjuvant therapy, and neoadjuvant therapy are thought to help improve survival outcomes. Modern treatment approaches for conventional pancreatic ductal adenocarcinoma could be applied to pancreatic carcinosarcoma.
Pancreatic carcinosarcoma is a very rare disease with a poor prognosis. Carcinosarcoma is a single tumor containing both epithelial and mesenchymal components. It usually occurs in the genitourinary system, head, neck, or breast, but rarely in the pancreas [1]. Due to the rarity of this condition, there are only case report-level studies. A recent study has analyzed 39 patients using the Surveillance, Epidemiology, and End Results (SEER) database [2], which is the largest study. Although the prognosis is generally poor, reported survival rates vary from 6 months to 15 months [2-7].
Studies reported so far did not consider a specific treatment strategy for pancreatic carcinosarcoma separately because most cases were diagnosed through pathological review after surgical resection [8]. Moreover, whether the treatment strategy applied to conventional pancreatic ductal adenocarcinoma (PDAC) can also be applied to pancreatic carcinosarcoma remains controversial [8-10]. Here, we report a case of a 65-year-old female patient. She underwent neoadjuvant chemotherapy after she was diagnosed with carcinosarcoma through endoscopic ultrasound-guided fine-needle aspiration biopsy (EUS-FNAB) before surgery. We also performed a literature review of reported cases to establish a therapeutic strategy for pancreatic carcinosarcoma.
We reviewed data of a 65-year-old female patient diagnosed with pancreatic carcinosarcoma and treated at Seoul National University Hospital. For literature review, terms of “Pancreatic” or “Pancreas” and “carcinosarcoma” or “carcinosarcomatous” were used for searching PubMed. Only papers with abstracts written in English were included. Papers were excluded if terms of carcinosarcoma and sarcomatoid carcinoma were used interchangeably because the former is often confused with the latter [11]. A flow chart of the literature search and papers included is described in Fig. 1. Continuous variables are reported as means (interquartile range, IQR) and categorical variables are reported as frequencies (percentile). Survival outcomes were calculated using the Kaplan–Meier method. The Institutional Review Board (IRB) of Seoul National University Hospital approved this study (IRB no. H-2207-145-1342).
A 65-year-old female patient with a history of a total hysterectomy for leiomyoma and a history of cholecystectomy for a gallbladder polyp visited our hospital with abdominal pain and weight loss. There were no special features in the review of systems or physical examination. Laboratory examination revealed high levels of carcinoembryonic antigen (75.7 ng/mL) and carbohydrate antigen 19-9 (238 U/mL). Other serum amylase, lipase, and liver function tests were within their normal ranges. Computed tomography (CT) imaging of the abdomen showed a 2.6 cm × 2.0 cm low attenuated mass with upstream pancreatic duct dilation and parenchymal atrophy, which invaded the superior mesenteric vein (SMV) and caused SMV deformity (Fig. 2). Fluorodeoxyglucose-positron emission tomography (FDG-PET) showed high uptake in the pancreatic head without metastasis elsewhere. EUS-FNAB was performed for diagnosis. High cellularity was observed with hematoxylin and eosin (H&E) staining (Fig. 3A). Hyperchromatic adenocarcinoma components were observed while forming irregular glands. Cells with poor differentiation but without forming glands were also observed in the surrounding stroma. When cells constituting the lesion were immunostained with markers, cytokeratin was strongly positive in the epithelial component and vimentin was strongly positive in the atypical cell component of the surrounding stroma (Fig. 3B, 3C). Based on these results, the patient was diagnosed with carcinosarcoma. As the tumor invaded the SMV, the patient was enrolled in a clinical study. She received 11 cycles of leucovorin, fluorouracil, irinotecan, oxaliplatin (FOLFIRINOX), and pembrolizumab. In the CT scan for re-staging, the tumor size decreased from the initial 2.6 cm to 1.8 cm. Hence, there was a radiologic response to treatment. In FDG-PET, the maximum standardized uptake value (SUVmax) decreased from 8.6 to 3.8. The tumor marker also reduced to a normal level, indicating a metabolic response. The patient underwent SABR with 35 Gy in 5 fractions, followed by robotic pylorus-preserving pancreaticoduodenectomy. At the time of surgery, the mass was abutted with the SMV. However, it detached well without additional vascular resection. No other organ invasion was observed. Postoperative pathological findings showed an adenocarcinoma component with vacuoles in the cytoplasm and formation of gland structures. The surrounding stroma showed fibrotic changes and contained a sarcomatous component composed of single cells with severe atypia (Fig. 4). It was labeled as grade 3 by the College of American Pathologists because of its high cellularity. The tumor size was 1.7 cm × 1.5 cm × 0.9 cm. There was no lymph node metastasis among 18 harvested lymph nodes (ypT1cN0). After surgery, the patient received adjuvant chemotherapy with the same protocol as neoadjuvant therapy. There has been no recurrence to date (seven months after surgery).
For literature review through PubMed, we were able to review reports on 48 patients within 33 available papers, including our case (Table 1) [3-10,12-35]. Most of these included papers were case reports. There were two case series. The study that reviewed the most patients in a single institution was the study by Li et al. [3] that analyzed nine patients. Except for cases where data for patient characteristics were unavailable (n = 2), of 46 patients, 26 (56.5%) were females with a median age of 62.5 years (IQR: 49.0–71.3 years). Tumor sites were the pancreatic head (69.6%) and body/tail (30.4%).
A total of 43 patients underwent surgery. Three patients, including our case, received neoadjuvant chemotherapy and 17 received adjuvant chemotherapy. The median survival time was 15 months (Fig. 5). Among patients with survival information, 23 of 30 patients had information about adjuvant chemotherapy. Radioactive seed implantation and traditional Chinese medical herbal treatments were not included as systemic adjuvant chemotherapies. Thirteen patients received adjuvant chemotherapy. The survival rate of patients who received adjuvant chemotherapy tended to be higher than that of those who did not receive adjuvant chemotherapy, although the difference was not statistically significant (median survival: 47 vs. 15 months, p = 0.485). There was no difference in disease-free survival (DFS) according to adjuvant chemotherapy (median DFS: 12 vs. 12 months, p = 0.656). Among patients with recurrence information, recurrence rate was 58.6% (17/29). The most common recurrence site was liver (64.7%). Three patients received neoadjuvant chemotherapy. The survival period after diagnosis considering the chemotherapy period was 13–23.5 months (Table 2).
Pancreatic carcinosarcoma is classified as a variant of undifferentiated carcinoma of PDAC according to the World Health Organization classification [9]. Carcinosarcoma usually occurs in the genitourinary system, head, neck, or breast, but rarely in the pancreas [1]. Pancreatic carcinosarcoma was first reported in 1951 [35]. Approximately 40 cases have been reported worldwide. Carcinosarcoma, as the name suggests, refers to cancer with both epithelial and mesenchymal components.
There are three main hypotheses regarding the development process of carcinosarcoma [3,4,8,36]. The collision theory states that two adjacent carcinomas and sarcomas arise independently, grow, and collide. The combination theory considers that early branching from a common precursor stem cell and cell clusters are mixed. The transformation theory states that a sarcomatous component occurs during carcinoma progression through the epithelial to mesenchymal transition [3,4,8,36]. While the exact pathophysiology of pancreatic carcinosarcoma remains unclear, recent studies using genetic analysis have supported the hypothesis that carcinosarcoma could have a monoclonal origin. Kim et al. [23] and Bai et al. [4] have demonstrated an identical mutation of KRAS gene and concordant strong nuclear immunoreactivity for P53 protein in both components. Okamura et al. [25] have revealed identical KRAS and TP53 mutations in both components. However, molecular studies by Khan et al. [9] showed no pathogenic mutations in neither component while Zhou et al. [14] demonstrated pathologic mutations only in osteosarcoma component, but not in adenocarcinoma or sarcoma component.
In our study, of 46 patients, 26 (56.5%) were females with a median age of 62.5 years (interquartile range, IQR: 49.0–71.3 years). Alhatem et al. [2] have recently analyzed 39 patients with pancreatic carcinosarcoma from 1973 to 2016 using the SEER database. In their study, the median age of patients was 67.4 years. There was a female dominance (53.8%), similar to our study. Tumor sites were dominant in pancreatic head (69.6 %) in our study whereas pancreatic head and body/tail ratios were equal (50% each) in the study of Alhatem et al. [2].
Shi et al. [21] have reported imaging features of pancreatic carcinosarcoma for diagnosis based on a literature review. Due to rapid growth of pancreatic carcinosarcoma, it is often accompanied by an internal cystic region and necrosis on CT images. Unlike other cystic tumors, pancreatic carcinosarcoma rarely has calcification within the tumor or septum. Therefore, it is important to differentiate it from benign cystic tumors so that the most effective treatment time will not be missed. They have also argued that pancreatic carcinosarcoma can be differentiated from PDAC because it has more vascularity and less common extrapancreatic perineural, vascular invasion, pancreatic parenchyma atrophy, and duct dilatation than PDAC. Nevertheless, including our case, some cases have revealed that carcinosarcoma shows similar imaging findings to conventional PDAC with educed enhancement on CT images [3,12,13]. This difference seems to originate from heterogeneity of the internal component. It is a refutation that it is difficult to make a definite distinction only with imaging findings.
Pancreatic carcinosarcoma can be diagnosed when both carcinomatous and sarcomatous components are visible in H&E staining. Divergent expression patterns on immunohistochemical examination with each marker can help us differentiate each component. The carcinoma component is stained with epithelial markers such as pancytokeratin or cytokeratin, but not with vimentin, which is a mesenchymal marker. The staining pattern is reversed in sarcoma components. Some studies have demonstrated positive expression of carcinoembryonic antigen and epithelial membrane antigen in the carcinomatous component [9,23,26,31]. Other markers such as cyclin D1 for the carcinomatous component or Discovered on GIST-1 (DOG-1), smooth muscle actin, S-100, CD10, p53, and desmin for the sarcomatous component are controversial, with several studies reporting conflicting results [4,5,9,11,13,15-17,22,23,26,31]. When each component is present at more than 30%, it can be diagnosed as carcinosarcoma [1,9].
Most studies to date have reported cases of carcinosarcoma confirmed after surgical resection. In one case [17], a 40-year-old female patient underwent pancreatic mass biopsy during a total abdominal hysterectomy with right salpingo-oophorectomy. It was the first case of pancreatic carcinosarcoma diagnosed before surgical resection. However, the tumor was unresectable. She received chemotherapy with gemcitabine and docetaxel without response and died 10 months after diagnosis due to cancer progression. Another case [12] was described in an abstract. The patient was diagnosed using endoscopic biopsy. However, description of the biopsy was omitted in the text. Therefore, the present case is the first reported case of pancreatic carcinosarcoma diagnosed before treatment through EUS-FNAB and immunostaining before surgical resection. In addition, it is the first pancreatic carcinosarcoma case with treatment decided according to results of biopsy.
Because pancreatic carcinosarcoma is a rare cancer, there are few papers about its prognosis. Literature review papers about it generally have only about 20 cases. In 2021, Alhatem et al. [2] analyzed 39 patients using the SEER database, which was the largest study to date. Most studies [2,5-7] showed a dismal survival outcome of approximately 6 months for pancreatic carcinosarcoma, although a few studies [3,4] showed a median survival of 14–15 months. In the present study, except when information on survival status or duration was not available (18/48), the median survival time was 15 months. Although this result was higher than those of previous reports, it was a dismal survival outcome compared to the median survival of 25–30 months for resectable conventional PDAC [37,38].
In case reports reviewed, all patients underwent surgery except for cases with autopsy or if the biopsy was performed during hysterectomy. In the analysis conducted by Alhatem et al. [2], 16 of 39 cases had distant metastasis at diagnosis. These 39 cases included 28 patients who underwent surgery, nine patients for whom surgery was not recommended, and two who did not receive surgery after surgery was recommended. The median survival of all patients was 6 months. The survival rate of patients who underwent surgery was significantly higher than that of patients who did not undergo surgery (median survival: 8 months vs. 2 months, p = 0.004). Among patients who underwent surgery, the survival rate of those who underwent lymphadenectomy was significantly higher than that of those who did not undergo lymphadenectomy (median survival: 8 months vs. 1.1 months; p < 0.001).
In this study, recurrence rate was 58.6%, with 64.7% of recurrences occurring in the liver. Only three papers dealt with histology of metastatic lesions. Shen et al. [24] did not perform examinations for recurrent liver lesions, but performed metastatic hepatic resection at the time of initial surgery, showing two separated adenocarcinoma and sarcoma components similar to those of the original pancreatic tumor. Kim et al. [23] observed with metastasis in both peritoneum and liver and showed metastatic adenocarcinoma from fluid cytology. Still et al. [15] showed metastatic sarcoma in a liver metastatic lesion. In the carcinosarcoma originated from organs other than pancreas, the majority component of metastasis is carcinomatous, showing a similar metastasis pattern [38-40]. Although Still et al. [15] explained that carcinoma was fully treated with adjuvant chemotherapy, the remnant sarcomatous component resulted in metastasis. Because metastatic components of above three described papers are different, the mechanism of disease progression remains unclear.
Among patients with survival information, 23 of 30 patients had information about adjuvant chemotherapy. Radioactive seed implantation and traditional Chinese medical herbal treatments were not included as systemic adjuvant chemotherapies. Thirteen patients received adjuvant chemotherapy. The survival rate of patients who received adjuvant chemotherapy tended to be higher than that of those who did not receive adjuvant chemotherapy. However, the difference was not statistically significant (median survival: 47 months vs. 15 months, p = 0.485). There was no significant difference in DFS according to adjuvant chemotherapy (median DFS: 12 months vs. 12 months, p = 0.656). Bai et al. [4] compared eight patients who underwent adjuvant chemotherapy with gemcitabine-based chemotherapy among 23 patients. In their study, the survival rate of patients who received adjuvant chemotherapy was significantly higher than that of patients who received only surgery (median survival: 46 months vs. 10 months, p = 0.034). Although there was no statistically significant difference in DFS, there was a trend toward higher survival rates in patients receiving adjuvant chemotherapy (p = 0.131).
In two previous case reports, neoadjuvant chemotherapy was administered. Still et al. [15] conducted six cycles of modified FOLFIRINOX and Lalonde et al. [8] conducted four cycles of FOLFIRINOX and concurrent chemoradiotherapy with capecitabine. In our case, the patient received eleven cycles of FOLFIRINOX and pembrolizumab. A partial response was observed in all three cases. The survival period after surgery was 7–15 months. The survival period after diagnosis considering the chemotherapy period was 13–23.5 months. Still et al. [15] reported that a moderate treatment response appeared in the carcinomatous portion. Conversely, Lalonde et al. [8] reported that a treatment response appeared in the sarcomatous component. In our case, we could not evaluate which component was more affected by treatment because EUS-FNAB confirmed only a part of the lesion with lesions scattered between fibrotic changes. However, there was a treatment response in all three neoadjuvant chemotherapy cases with FOLFIRINOX based regimen. The survival rate was higher than that of pancreatic adenocarcinoma in other studies. Although some have argued that chemotherapy for conventional PDAC may not be the best option for pancreatic carcinosarcoma owing to different components showing different sensitivities to chemotherapy [9,10], the above results indicate that chemotherapy treatment for conventional PDAC can also bring survival gain to pancreatic carcinosarcoma.
Pancreatic carcinosarcoma is a rare malignant tumor with a worse prognosis than conventional PDAC. It is a rapidly growing tumor. It can be suspected if rapid growth rate and necrosis accompany a cystic component through imaging examination. In case of doubt, it can be diagnosed using contrasting marker staining patterns through immunohistochemistry after biopsy. Although a treatment strategy has not yet been established, surgery with lymphadenectomy, adjuvant therapy, and neoadjuvant therapy might improve survival outcomes. Modern treatment approaches for conventional PDAC could be applied to pancreatic carcinosarcoma.
However, studies have been conducted only at the case report level so far. A customized approach to carcinosarcoma has not been developed yet. Since the number of patients diagnosed with carcinosarcoma before surgery will increase with the development of diagnostic technology, further research studies are necessary to establish a treatment strategy for pancreatic carcinosarcoma.
REFERENCES
1. Kimura T, Fujimoto D, Togawa T, Ishida M, Iida A, Sato Y, et al. 2020; Sarcomatoid carcinoma of the pancreas with rare long-term survival: a case report. World J Surg Oncol. 18:105. DOI: 10.1186/s12957-020-01879-8. PMID: 32450860. PMCID: PMC7249341.

2. Alhatem A, Quinn PL, Xia W, Chokshi RJ. 2021; Pancreatic carcinosarcoma clinical outcome analysis of the National Cancer Institute database. J Surg Res. 259:62–70. DOI: 10.1016/j.jss.2020.11.033. PMID: 33279845.

3. Li J, Wei T, Zhang J, Wei S, Chen Q, Chen BW, et al. 2020; Carcinosarcoma of the pancreas: comprehensive clinicopathological and molecular characterization. HPB (Oxford). 22:1590–1595. DOI: 10.1016/j.hpb.2020.01.017. PMID: 32081541.

4. Bai Q, Zhang X, Zhu X, Wang L, Huang D, Cai X, et al. 2016; Pancreatic carcinosarcoma with the same KRAS gene mutation in both carcinomatous and sarcomatous components: molecular evidence for monoclonal origin of the tumour. Histopathology. 69:393–405. DOI: 10.1111/his.12975. PMID: 27307095.

5. Zhu WY, Liu TG, Zhu H. 2012; Long-term recurrence-free survival in a patient with pancreatic carcinosarcoma: a case report with a literature review. Med Oncol. 29:140–143. DOI: 10.1007/s12032-010-9804-9. PMID: 21264541.

6. Gelos M, Behringer D, Philippou S, Mann B. 2008; Pancreatic carcinosarcoma. Case report of multimodal therapy and review of the literature. JOP. 9:50–55.
7. Jia Z, Zhang K, Huang R, Zhou X, Jiang L. 2017; Pancreatic carcinosarcoma with rare long-term survival: case report and review of the literature. Medicine (Baltimore). 96:e5966. DOI: 10.1097/MD.0000000000005966. PMID: 28121946. PMCID: PMC5287970.
8. Lalonde CS, Wang L, Quigley B, Patel P, Maithel SK, El-Rayes BF, et al. 2022; Neoadjuvant treatment of pancreatic carcinosarcoma: a case report and review of literature. Chin Clin Oncol. 11:8. DOI: 10.21037/cco-21-119. PMID: 35073708.

9. Khan J, Cheng L, House MG, Guo S. 2021; Carcinosarcoma, a rare malignant neoplasm of the pancreas. Curr Oncol. 28:5295–5303. DOI: 10.3390/curroncol28060442. PMID: 34940081. PMCID: PMC8699933.

10. Quinn PL, Ohioma D, Jones AMK, Ahlawat SK, Chokshi RJ. 2020; Treatment of rare and aggressive pancreatic carcinosarcoma. ACG Case Rep J. 7:e00379. Erratum in: ACG Case Rep J 2020;7:e00435. DOI: 10.14309/crj.0000000000000379. PMID: 32607379. PMCID: PMC7289284.

11. Zhou DK, Gao BQ, Zhang W, Qian XH, Ying LX, Wang WL. 2019; Sarcomatoid carcinoma of the pancreas: a case report. World J Clin Cases. 7:236–241. DOI: 10.12998/wjcc.v7.i2.236. PMID: 30705901. PMCID: PMC6354085.

12. Cheng L, Liu Y, Lou C, Cheng L. 2021; A case of pancreatic carcinosarcoma detected on PET/CT. Hell J Nucl Med. 24:262–264.
13. Liu Y, Hao H, Guo X, Xu J, Kang L, Zheng G, et al. 2019; Rare pancreatic carcinosarcoma in a patient with medical history of esophageal cancer: a case report and literature review. Medicine (Baltimore). 98:e15238. DOI: 10.1097/MD.0000000000015238. PMID: 31008956. PMCID: PMC6494381.
14. Zhou X, Li M, Wang P, Teng X, Sun L, Chen J, et al. 2018; Carcinosarcoma colliding osteosarcoma of the pancreas: a rare case report of multiple clonal originated pancreatic tumors. Int J Clin Exp Pathol. 11:1746–1753.
15. Still SA, Becerra CR, Clement-Kruzel SE, Cavaness KM. 2018; Locally advanced carcinosarcoma of the pancreas. Proc (Bayl Univ Med Cent). 31:210–212. DOI: 10.1080/08998280.2018.1444302. PMID: 29706823. PMCID: PMC5914398.

16. Ruess DA, Kayser C, Neubauer J, Fichtner-Feigl S, Hopt UT, Wittel UA. 2017; Carcinosarcoma of the pancreas: case report with comprehensive literature review. Pancreas. 46:1225–1233. DOI: 10.1097/MPA.0000000000000904. PMID: 28902796.
17. Salibay CJ, Rewerska J, Gupta S, Ree N. 2017; Primary carcinosarcoma of the pancreas with CD10-positive sarcoma component. J Investig Med High Impact Case Rep. 5:2324709617740906. DOI: 10.1177/2324709617740906. PMID: 29152519. PMCID: PMC5680943.

18. Mszyco S, Teng L, Annunziata J, Hartman MS. 2017; Pancreatic carcinosarcoma: a case report highlighting computed tomography characteristics. Curr Probl Diagn Radiol. 46:342–345. DOI: 10.1067/j.cpradiol.2017.01.007. PMID: 28318763.

19. Li BQ, Liu QF, Chang XY, Hu Y, Chen J, Guo JC. 2017; Pancreatic carcinosarcoma mimics malignant intraductal papillary mucinous neoplasm: a rare case report and literature review. Medicine (Baltimore). 96:e6961. DOI: 10.1097/MD.0000000000006961. PMID: 28591030. PMCID: PMC5466208.
20. Lee J, Hyun JJ, Lee HS. 2015; A rare cause of abdominal pain by pancreatic mass in a young female patient. Carcinosarcoma of the pancreas. Gastroenterology. 149:e3–e5. DOI: 10.1053/j.gastro.2014.12.051. PMID: 26123557.
21. Shi HY, Xie J, Miao F. 2015; Pancreatic carcinosarcoma: first literature report on computed tomography imaging. World J Gastroenterol. 21:1357–1361. DOI: 10.3748/wjg.v21.i4.1357. PMID: 25632213. PMCID: PMC4306184.

22. Oymaci E, Argon A, Coşkun A, Uçar AD, Carti E, Erkan N, et al. 2013; Pancreatic carcinosarcoma: case report of a rare type of pancreatic neoplasia. JOP. 14:212–215.
23. Kim HS, Joo SH, Yang DM, Lee SH, Choi SH, Lim SJ. 2011; Carcinosarcoma of the pancreas: a unique case with emphasis on metaplastic transformation and the presence of undifferentiated pleomorphic high-grade sarcoma. J Gastrointestin Liver Dis. 20:197–200.
24. Shen ZL, Wang S, Ye YJ, Wang YL, Sun KK, Yang XD, et al. 2010; Carcinosarcoma of pancreas with liver metastasis combined with gastrointestinal stromal tumour of the stomach: is there a good prognosis with the complete resection? Eur J Cancer Care (Engl). 19:118–123. DOI: 10.1111/j.1365-2354.2008.00977.x. PMID: 19486125.

25. Okamura J, Sekine S, Nara S, Ojima H, Shimada K, Kanai Y, et al. 2010; Intraductal carcinosarcoma with a heterologous mesenchymal component originating in intraductal papillary-mucinous carcinoma (IPMC) of the pancreas with both carcinoma and osteosarcoma cells arising from IPMC cells. J Clin Pathol. 63:266–269. DOI: 10.1136/jcp.2009.071613. PMID: 20203229.

26. Nakano T, Sonobe H, Usui T, Yamanaka K, Ishizuka T, Nishimura E, et al. 2008; Immunohistochemistry and K-ras sequence of pancreatic carcinosarcoma. Pathol Int. 58:672–677. DOI: 10.1111/j.1440-1827.2008.02289.x. PMID: 18801090.

27. Bloomston M, Chanona-Vilchis J, Ellison EC, Ramirez NC, Frankel WL. 2006; Carcinosarcoma of the pancreas arising in a mucinous cystic neoplasm. Am Surg. 72:351–355. DOI: 10.1177/000313480607200416. PMID: 16676863.

28. Chmiel B, Wodołazski A, Kozaczka A. 2005; [Carcinosarcoma of the pancreas--case report and literature review]. Wiad Lek. 58:243–246. Polish.
29. Barkatullah SA, Deziel DJ, Jakate SM, Kluskens L, Komanduri S. 2005; Pancreatic carcinosarcoma with unique triphasic histological pattern. Pancreas. 31:291–292. DOI: 10.1097/01.mpa.0000178283.96276.8a. PMID: 16163064.

30. Yamazaki K. 2003; A unique pancreatic ductal adenocarcinoma with carcinosarcomatous histology, immunohistochemical distribution of hCG-beta, and the elevation of serum alpha-feto-protein. J Submicrosc Cytol Pathol. 35:343–349.
31. Darvishian F, Sullivan J, Teichberg S, Basham K. 2002; Carcinosarcoma of the pancreas: a case report and review of the literature. Arch Pathol Lab Med. 126:1114–1117. DOI: 10.5858/2002-126-1114-COTP. PMID: 12204065.
32. Watanabe M, Miura H, Inoue H, Uzuki M, Noda Y, Fujita N, et al. 1997; Mixed osteoclastic/pleomorphic-type giant cell tumor of the pancreas with ductal adenocarcinoma: histochemical and immunohistochemical study with review of the literature. Pancreas. 15:201–208. DOI: 10.1097/00006676-199708000-00013. PMID: 9260206.

33. Millis JM, Chang B, Zinner MJ, Barsky SH. 1994; Malignant mixed tumor (carcinosarcoma) of the pancreas: a case report supporting organ-induced differentiation of malignancy. Surgery. 115:132–137.
34. Takahashi K, Wakayama M, Asaji A, Ando M, Shibuya K, Shibuya H, et al. 1987; [An autopsy case of a so-called carcinosarcoma of the pancreas]. Gan No Rinsho. 33:1481–1487. Japanese.
35. Van Damme J, Snoeks T. 1951; [Carcinosarcoma of the body of the pancreas]. Acta Gastroenterol Belg. 14:106–113. Belgian.
36. Almond LM, Warfield AT, Desai A, Gourevitch D, Ford SJ. 2017; Biphasic malignant tumours of the abdominal cavity. Int J Clin Oncol. 22:635–640. DOI: 10.1007/s10147-017-1153-7. PMID: 28656498.

37. Strobel O, Lorenz P, Hinz U, Gaida M, König AK, Hank T, et al. 2022; Actual five-year survival after upfront resection for pancreatic ductal adenocarcinoma: who beats the odds? Ann Surg. 275:962–971. DOI: 10.1097/SLA.0000000000004147. PMID: 32649469.

38. Singh R. 2014; Review literature on uterine carcinosarcoma. J Cancer Res Ther. 10:461–468. DOI: 10.4103/0973-1482.138197. PMID: 25313723.

39. Matsuo K, Takazawa Y, Ross MS, Elishaev E, Podzielinski I, Yunokawa M, et al. 2016; Significance of histologic pattern of carcinoma and sarcoma components on survival outcomes of uterine carcinosarcoma. Ann Oncol. 27:1257–1266. DOI: 10.1093/annonc/mdw161. PMID: 27052653.

40. Loh TL, Tomlinson J, Chin R, Eslick GD. 2014; Cutaneous carcinosarcoma with metastasis to the parotid gland. Case Rep Otolaryngol. 2014:173235. DOI: 10.1155/2014/173235. PMID: 25328737. PMCID: PMC4190691.

Fig. 2
Axial view of computed tomography demonstrating a 2.6-cm sized low attenuated mass in the head of the pancreas.
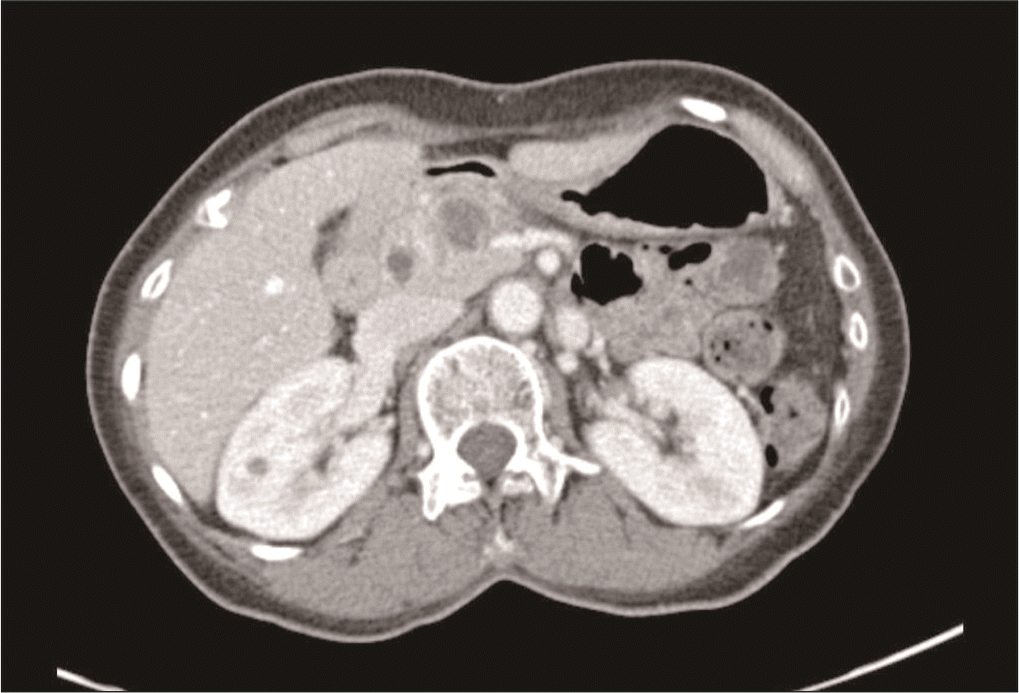
Fig. 3
Histopathologic slide of endoscopic ultrasound-guided fine-needle aspiration biopsy. (A) Hematoxylin and eosin staining, (B) cytokeratin immunostaining, and (C) vimentin immunostaining. Original magnification ×40.
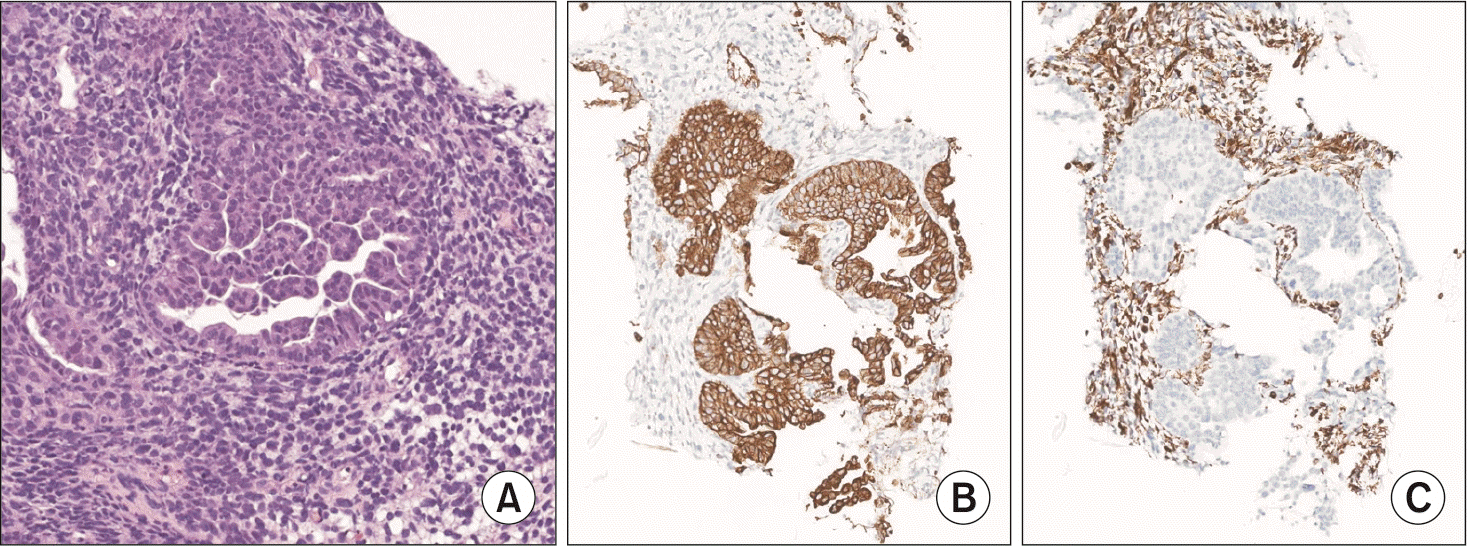
Fig. 4
Histopathologic slide after surgical resection (original magnification ×40). A, carcinoma component; B, sarcoma component.
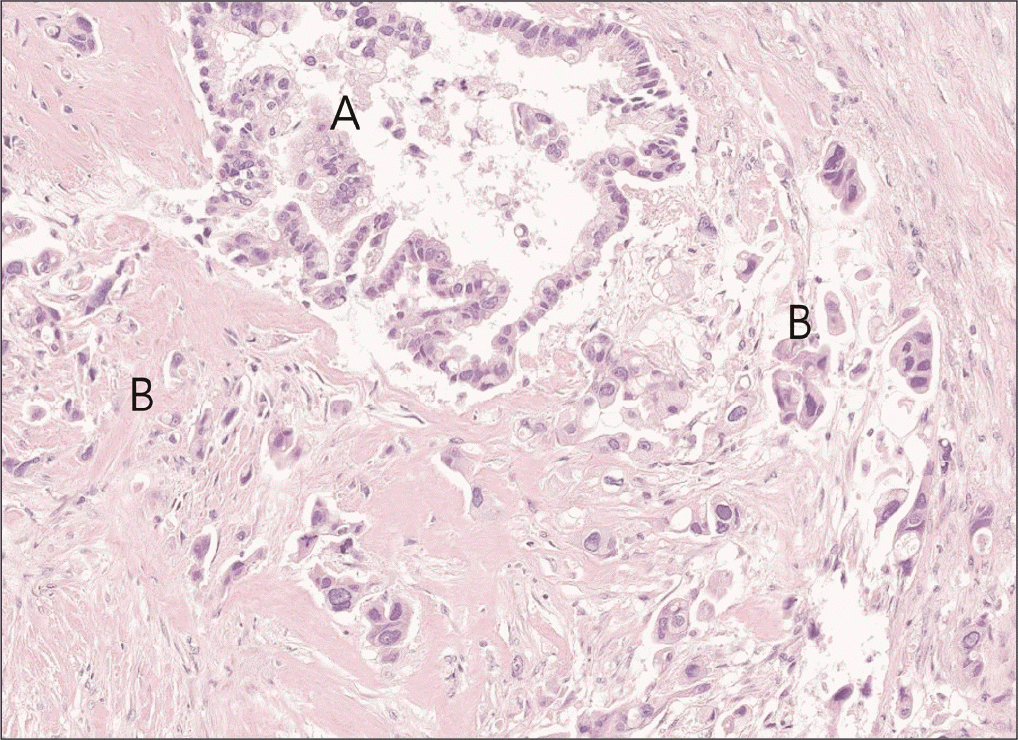
Table 1
Treatment of pancreatic carcinosarcoma cases of the literatures
| Reference | Age (yr) | Sex | Site | Initial imaging size (cm) | Biopsy method, diagnosis | Neoadjuvant treatment | Surgery | Adjuvant therapy | Status | Overall survival (mon) | Recurrence | DFS (mon) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||||
| Yes/No | Regimen | Yes/No | Site | |||||||||||
| Present study | 65 | F | H | 2.5 | EUS FNA; carcinosarcoma | 11 cycles of FOLFIRINOX, pembrolizumab | PPPD | Y | FOLFIRINOX, pembrolizumab | Alive | 7 | N | - | 7 |
| Cheng et al., 2021 [12] | 73 | F | B | 2.8 | - | - | Pancreatocaudal resection | - | - | - | - | - | - | - |
| Khan et al., 2021 [9] | 68 | M | H | 8.1 | EUS FNA; high grade dysplasia | - | PPPD | NA | Planned but follow up loss | - | 3 | - | - | 3 |
| Lalonde et al., 2022 [8] | 52 | F | H | 4.5 | EUS FNA; ADC | 4 cycles FOLFIRINOX, radiation with concurrent capecitabine | PD | - | - | Alive | 15 | N | - | 15 |
| Quinn et al., 2020 [10] | 42 | F | B, T | 11.9 | Transgastric FNA; ADC | - | STP, left partial adrenalectomy, left hemicolectomy | Y | 9 cycles gemcitabine & paclitaxel, 11 cycles FOLFOX | Alive | 16 | Y | Liver | 2 |
| Li et al., 2020 [3] | 60 | M | T | - | - | - | TP | N | - | 2 | Y | Liver | 1 | |
| 66 | M | H | - | - | - | PD | Y | Gemcitabine plus Nab-paclitaxel | - | 11 | Y | Liver | 3 | |
| 69 | M | H | - | - | - | PD | Y | mFOLFIRINOX | - | 19 | Y | LN | 13 | |
| 56 | F | H | - | - | - | TP | N | - | 39 | Y | Liver | 3 | ||
| 51 | F | H | - | - | - | PD | Y | Gemcitabine | - | 17 | Y | Liver | 10 | |
| 48 | F | T | - | - | - | TP | N | - | - | - | N | |||
| 67 | F | H | - | - | - | PD | N | - | - | 4 | Y | Liver | 3 | |
| 59 | M | H | - | - | - | PD | N | - | - | - | N | |||
| 49 | F | B | - | - | - | DP | N | - | - | - | N | |||
| Liu et al., 2019 [13] | 66 | M | H | 4.1 | - | - | Cholecystectomy with bile duct-jejunum Roux-en-Y anastomosis, radioactive seed implantation | N | Radioactive seed implantation at surgery | Alive | 12 | - | - | - |
| Zhou et al., 2018 [14] | 44 | F | H | 3 | - | - | PD | NA | - | Alive | 48 | - | - | - |
| Still et al., 2018 [15] | 59 | F | H | 2.9 | EUS FNA; ADC | 6 cycles modified FOLFIRINOX | PD | Y | 1 cycle gemcitabine & paclitaxel | Death | 10 | Y | Liver | - |
| Ruess et al., 2017 [16] | 73 | F | H | - | - | - | PPPD | N | - | Death | 4 | - | - | - |
| Salibay et al., 2017 [17] | 49 | F | B, T | - | Biopsy during hysterectomy: high grade spindle cell sarcoma & moderately differentiated adenocarcinoma | - | - | Y | Gemcitabine & docetaxel, followed by ifosfamide & doxorubicin | Death | 10 | - | - | - |
| Mszyco et al., 2017 [18] | 85 | M | H | 7.2 | EUS FNA; atypical spindle & myxoid type sarcoma | - | PD | N | - | Alive | 2.5 | Y | Liver, bone | 2.5 |
| Jia et al., 2017 [7] | 44 | F | H | 2.9 | - | - | PD | Y | 8 cycles gemcitabine & raltitrexed | Alive | 31 | N | - | 31 |
| Li et al., 2017 [19] | 60 | M | H | 8 | - | - | TP | N | - | Alive | 7 | N | - | 7 |
| Bai et al., 2016 [4] | 71 | M | H | 5 | - | - | PD + splenectomy | Y | - | Death | 11 | Y | - | 9 |
| 49 | M | H | 5 | - | - | PD | Y | - | Alive | 39 | N | - | 39 | |
| 74 | M | H | 8 | - | - | PD | N | - | Death | 10 | Y | - | 9 | |
| 38 | M | T | 16 | - | - | DP, gamma knife radiosurgery | Y | - | Alive | 26 | Y | Adrenal gland | 12 | |
| 67 | M | H | 3.5 | - | - | PD | Y | - | Death | 47 | N | - | 44 | |
| 60 | F | B, T | 7.5 | - | - | PD | N | - | Death | 15 | Y | - | 12 | |
| 75 | F | H | 4.5 | - | - | PD | N | Traditional chinese medical herbal treatment | Alive | 29 | N | - | 29 | |
| 59 | M | B, T | 5.5 | - | - | PD, splenectomy | Y | - | Alive | 17 | N | - | 17 | |
| Lee et al., 2015 [20] | 24 | F | T | 4.8 | - | - | DP | NA | - | - | - | - | - | - |
| Shi et al., 2015 [21] | 74 | F | T | 2.2 | - | - | DP | NA | - | - | - | - | - | - |
| Oymaci, 2013 [22] | 66 | M | H | 3 | ERCP with brushings; ADC | - | PD | NA | - | Death | 0.5 | - | - | - |
| Zhu et al., 2012 [5] | 53 | F | H | 4 | - | - | PD | Y | 5 cycles gemcitabine, doxorubicin, cisplatin | Alive | 20 | - | - | 20 |
| Kim et al., 2011 [23] | 48 | M | T | 3.5 | - | - | DP, colon segmental resection | Y | 3 cycles gemcitabine | Death | 4 | Y | Liver, peritoneum | 3 |
| Shen et al., 2010 [24] | 72 | F | H | 4 | - | - | PD, left heptaic lobe resection, gastric wedge resetion | N | - | Death | 2 | Y | Liver | 1.5 |
| Okamura et al., 2010 [25] | 64 | F | T | 3.5 | - | - | DP | NA | - | Alive | 12 | N | - | 12 |
| Gelos et al., 2008 [6] | 61 | F | H | - | - | - | PD | Y | Gemcitabine | Death | 11 | Y | - | 9 |
| Nakano et al., 2008 [26] | 82 | F | H | - | - | - | PD, colon partial resection | NA | - | Death | 0.5 | - | - | - |
| Bloomston et al., 2006 [27] | 67 | F | H | - | - | - | PPPD | NA | - | Death | 4 | Y | Liver, peritoneum | Unknown |
| Chmiel et al., 2005 [28] | 47 | M | H | - | - | - | PD | NA | - | - | - | - | - | - |
| Barkatullah et al., 2005 [29] | 67 | F | H | 2.1 | EUS FNA; poorly differentiated carcinoma with multinucleated giant cells | - | PPPD | NA | - | Death | 8 | - | - | - |
| Yamazaki, 2003 [30] | 90 | M | - | - | - | - | - | - | Autopsy | - | - | - | - | |
| Darvishian et al., 2002 [31] | 74 | M | H | - | ERCP with brushings; ADC | - | PD | N | - | Alive | 4 | - | - | - |
| Watanabe et al., 1997 [32] | 76 | M | H | - | - | - | - | - | - | - | 3 | - | - | - |
| Millis et al., 1994 [33] | - | - | H | - | - | - | PD | - | - | - | - | - | - | - |
| Takahashi et al., 1987 [34] | 48 | F | - | - | - | - | - | - | - | Autopsy | - | - | - | - |
| Van damme and Snoeks, 1951 [35] | - | - | B | - | - | - | - | - | - | - | - | - | - | - |
DFS, disease-free survival; F, female; M, male; H, head; B, body; T, tail; EUS FNA, endoscopic ultrasound guided fine needle aspiration; PPPD, pylorus preserving pancreaticoduodenectomy; ADC, adenocarcinoma; PD, pancreaticoduodenectomy; STP, subtotal pancreatectomy; TP, total pancreatectomy; LN, lymph node; DP, distal pancreatectomy; ERCP, endoscopic retrograde cholangiopancreaticography; -, not available.
Table 2
Neoadjuvant treatment of pancreatic carcinosarcoma cases of the literatures
| Reference | Age (yr) | Sex | Size (cm) | Biopsy method & diagnosis | Neoadjuvant treatment | Surgery | Adjuvant treatment | Partial response | Status | Survival after operation (mon) | Survival after diagnosis (mon) | Recurrence | DFS (mon) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||||||
| Yes/No | Regimen | Yes/No | Site | ||||||||||||
| Present study | 65 | F | 2.5 | EUS FNA; carcinosarcoma | 11 cycles of FOLFIRINOX, pembrolizumab | PPPD | Y | FOLFIRINOX, pembrolizumab | ypT1cN0 (0/18) | Alive | 7 | 16 | N | - | 7 |
| Lalonde et al., 2022 [8] | 52 | F | 4.5 | EUS FNA; ADC | 4 cycles FOLFIRINOX, radiation with concurrent capecitabine | PD | N | ypT2N0 (0/25) | Alive | 15 | 23.5 | N | - | 15 | |
| Still et al., 2018 [15] | 59 | F | 2.9 | EUS FNA; ADC | 6 cycles modified FOLFIRINOX | PD | Y | 1 cycle gemcitabine & paclitaxel | ypT3N1 (2/28) | Death | 10 | 13 | Y | Liver | - |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download