Coronavirus disease 2019 (COVID-19) pandemic poses increased risk in elderly individuals.
12 Globally, various types of COVID-19 vaccines have been introduced, which were given in different schedules and combinations.
34 However, little is known about vaccine effectiveness (VE) for different combinations of vaccines in elderly population during the omicron era. We conducted a national cohort study to assess VE of heterologous COVID-19 vaccine schedule against polymerase chain reaction (PCR)- or rapid antigen test (RAT)-confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) critical infection and death among elderly population aged 60 years and older in Korea.
From January to August 2022, omicron variant circulated in Korea. The primary vaccination using ChAdOx1 (AstraZeneca), Ad26.COV2.S (Janssen), BNT162b2 (Pfizer), and mRNA-1273 (Moderna) vaccines were initiated since early 2021. Booster vaccination with Pfizer and Moderna vaccines were recommended since October 2021 (3rd dose) and February 2022 (4th dose). The booster coverage rate was 70% (33,634,493/47,879,487) for 3-dose and 34% (7,511,472/21,962,768) for 4-dose by October 23, 2022 (denominator, as total eligible population). As of October 20, 2022, type of vaccines were 67% BNT162b2, 33% mRNA-1273 for 3-dose booster; 87% BNT162b2, 23% mRNA-1273 for 4-dose booster vaccination.
During the COVID-19 pandemic, all Korean residents had free access to PCR, which are registered centrally. Since January 2022, PCR testing was prioritized to those aged 60 years and above, those with COVID-19 suggestive symptoms, those who were epidemiologically-linked to COVID-19 cases, and screening for high-risk groups; while those who do not belong to a PCR priority group had free access to RAT.
We analyzed a linked dataset comprised of the National Immunization Registry, which holds records of all vaccines administered; and the National Notifiable Disease Surveillance System, which contains records of laboratory-confirmed COVID-19 in Korea. All individuals aged 60 years or older who reported laboratory-confirmed COVID-19 between January and August 2022 were eligible for the study. We excluded: 1) COVID-19 diagnosis before monitoring date; 2) reinfections; 3) undocumented vaccination status; and 4) persons who received Ad26.COV2.S, NVX-CoV2373, or 3-doses of ChAdOx1. Cases of critical infection were defined as persons with a positive SARS-CoV-2 test requiring noninvasive or invasive mechanical ventilation, extracorporeal membrane oxygenation, or continuous renal replacement therapy. Mortality case was defined as death occurring within 28 days of the sample collection date.
The adjusted relative risk (RR) comparing risk of critical infection or death between vaccinated and unvaccinated persons and its associated 95% CI were derived using logistic regression,
5 adjusting for potential confounders (age, sex, diagnosed month, residence, health status).
VE was estimated as 1-RR and expressed as a percentage. Vaccine types were classified as: viral vector (v), AstraZeneca; and mRNA (m), Pfizer and Moderna. The sequence of vaccine administration was described as: two dose primary series followed by one or two dose boosters as follows: i.e., v-v, first two doses with viral-vector vaccine; v-m first dose with viral vector, followed by mRNA vaccines. Statistical analyses were conducted using R v.4.02 (R Core Team, Vienna, Austria).
This study was conducted as a legally mandated public health investigation under the authority of the Korean Infectious Diseases Control and Prevention Act. The study was approved by the Korea Disease Control and Prevention Agency Institutional Review Board (IRB No. 2021-12-03-PE-A).
A total of 3,466,930 SARS-CoV-2 infections in persons aged ≥ 60 years were identified (
Supplementary Fig. 1 and
Table 1). The highest VE against death was shown in 4 doses of homologous mRNA primed (m-m-m-m) individuals (VE = 96.1%, 95% CI, 95.4–96.7%), followed by heterologous 2-dose viral vector primed + 2-dose mRNA (v-v-m-m) vaccines (V = 95.1%, 95% CI, 94.5–95.6%) (
Fig. 1). Heterologous 1-dose viral vector + 3-dose mRNA (v-m-m-m) vaccines had relatively lower VE of 90.8% (95% CI, 79.4–95.9%). Heterologous 1-dose viral vector + 2-dose mRNA (v-m-m) had lower VE against death (VE = 89.3%, 95% CI, 86.8–91.4%) compared to homologous 3-dose mRNA (m-m-m; VE = 94.0%, 95% CI, 93.7–94.2%) or heterologous 2-dose viral vector + 1-dose mRNA (v-v-m; VE = 94.7%, 95% CI, 94.4–95.0%).
Fig. 1
Effectiveness of different combination of coronavirus disease 2019 mRNA vaccine in elderly population during omicron circulation against (A) critical infection, and (B) death.
VE = vaccine effectiveness.
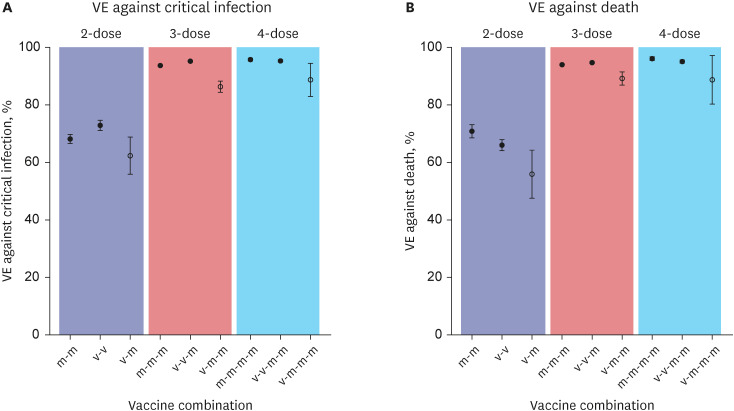

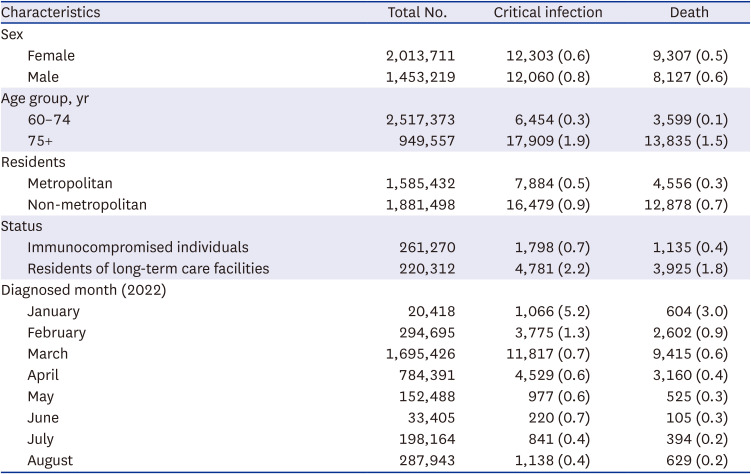
Table 1
Baseline characteristics of individuals with of critical infection or death from severe acute respiratory syndrome coronavirus 2
|
Characteristics |
Total No. |
Critical infection |
Death |
|
Sex |
|
|
|
|
Female |
2,013,711 |
12,303 (0.6) |
9,307 (0.5) |
|
Male |
1,453,219 |
12,060 (0.8) |
8,127 (0.6) |
|
Age group, yr |
|
|
|
|
60–74 |
2,517,373 |
6,454 (0.3) |
3,599 (0.1) |
|
75+ |
949,557 |
17,909 (1.9) |
13,835 (1.5) |
|
Residents |
|
|
|
|
Metropolitan |
1,585,432 |
7,884 (0.5) |
4,556 (0.3) |
|
Non-metropolitan |
1,881,498 |
16,479 (0.9) |
12,878 (0.7) |
|
Status |
|
|
|
|
Immunocompromised individuals |
261,270 |
1,798 (0.7) |
1,135 (0.4) |
|
Residents of long-term care facilities |
220,312 |
4,781 (2.2) |
3,925 (1.8) |
|
Diagnosed month (2022) |
|
|
|
|
January |
20,418 |
1,066 (5.2) |
604 (3.0) |
|
February |
294,695 |
3,775 (1.3) |
2,602 (0.9) |
|
March |
1,695,426 |
11,817 (0.7) |
9,415 (0.6) |
|
April |
784,391 |
4,529 (0.6) |
3,160 (0.4) |
|
May |
152,488 |
977 (0.6) |
525 (0.3) |
|
June |
33,405 |
220 (0.7) |
105 (0.3) |
|
July |
198,164 |
841 (0.4) |
394 (0.2) |
|
August |
287,943 |
1,138 (0.4) |
629 (0.2) |

The VE of four dose mRNA vaccines (m-m-m-m) and two dose viral vector primed + two dose mRNA booster vaccines (v-v-m-m) appear to be the most effective in preventing critical SARS-CoV-2 infection and death. This finding agrees with an immunogenicity study which showed a heterologous vector/mRNA boosting inducing strong humoral and cellular immune responses.
6 A meta-analysis showed that heterologous and homologous regimens work comparably well in preventing COVID-19 infections, even against different variants.
7 Our results imply that booster doses will continue to be recommended, especially in elderly population.
A heterologous primary series with the first dose of viral vector vaccine followed by the second dose with mRNA vaccines and booster doses (v-m, v-m-m, v-m-m-m) were associated with lower VE compared to two-dose viral vector vaccine followed by booster doses. Our findings on the lower protection of mixed-primary vaccination are not consistent with studies showing strong immune responses after BNT162b2 vaccination in persons who received one dose of ChAdOx1.
8 A plausible reason could be the difference in circulating strain; since January 2022, the predominant circulating strain in Korea was omicron variant that posed lower VE compared to its predecessors.
9 Our result highlights the importance of primary series vaccination (the first and the second doses) with same vaccine type in the era of omicron subvariant predominance.
This study has several limitations. First, there was a change in omicron subvariants from BA.1, BA.2 to BA.4/5 over time in Korea, but we adjusted for temporal trends in the analyses. Second, protection from the booster doses might be overestimated if unvaccinated people were more likely than vaccinated people to get tested. Third, although the surveillance data we used have large sample sizes that encompasses national population, it does not contain clinical details on underlying medical conditions or other factors, that allow for better control of effect modifiers. Lastly, the likelihood of receiving vaccines in healthier individuals may have affected in overall estimation of VE in our study, therefore, the result requires careful interpretation.
In conclusion, our findings provide supportive evidence for a sustained protection after using a heterologous booster of the mRNA vaccine in addition to the viral vector-primed individuals, especially for older people. Our analysis also indicated that the primary vaccine series with the same vaccine type has better protection against critical infection and death compared to heterologous primary series, however, the booster doses enhance level of protection in elderly population.
Ethics Statement
This study was conducted as a legally mandated public health investigation under the authority of the Korean Infectious Diseases Control and Prevention Act (No. 12,444 and No. 13,392). This study was approved by the Institutional Board Review of Korea Disease Control and Prevention Agency and the requirement for informed consent was waived (IRB No. 2021-12-03-PE-A).






 PDF
PDF Citation
Citation Print
Print



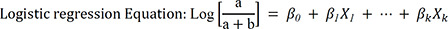
 XML Download
XML Download