Abstract
Recent updates in genomic-integrated glioma classification have caused confusion in current clinical practice, as management protocols and health insurance systems are based on evidence from previous diagnostic classifications. The Korean Brain Tumor Society conducted an electronic questionnaire for society members, asking for their ideas on risk group categorization and preferred treatment for each individual diagnosis listed in the new World Health Organization (WHO) classification of gliomas. Additionally, the current off-label drug use (OLDU) protocols for glioma management approved by the Health Insurance Review and Assessment Service (HIRA) in Korea were investigated. A total of 24 responses were collected from 20 major institutes in Korea. A consensus was reached on the dichotomic definition of risk groups for glioma prognosis, using age, performance status, and extent of resection. In selecting management protocols, there was general consistency in decisions according to the WHO grade and the risk group, regardless of the individual diagnosis. As of December 2022, there were 22 OLDU protocols available for the management of gliomas in Korea. The consensus and available options described in this report will be temporarily helpful until there is an accumulation of evidence for effective management under the new classification system for gliomas.
Glioma is a type of neoplasia for which genetic diagnosis has pioneered disease classification among all cancers. Recently, the new 2021 World Health Organization (WHO) Classification of Tumors of the Central Nervous System (CNS) has been updated to contain the definitive list of glioma diagnoses based on genetic signatures [32]. However, the treatment protocol for glioma has not yet changed and is based on past diagnoses, causing confusion in clinical settings where the new genomic-integrated diagnostic system is being implemented rapidly.
In most cases, first-line glioma treatment is performed according to standard management protocols covered by the National Health Insurance in Korea. However, there is an unmet need for gliomas that new or existing anticancer drugs can be prescribed alone or in combination under government approval, based on the latest clinical trial results. The Health Insurance Review and Assessment Service (HIRA) has officially allowed off-label drug use (OLDU) for cancer drugs in Korea through a prior review process submitted by hospitals after receiving Institutional Review Board approval.
To reduce confusion resulting from the mismatch between the novel diagnosis system and existing treatment policies in gliomas, the Korean Brain Tumor Society (KBTS), one of the subspecialty societies affiliated with the Korean Neurosurgical Society, has summarized consensus and available treatment options on glioma management that can be applied immediately in the clinical field in Korea. Since its foundation in 1991, about 630 members of neurosurgeons who have a special interest in neuro-oncology are registered with KBTS.
To perform the consensus survey, we used Google Form, a web-based survey system, and emailed it to all members of the KBTS. Respondents were able to complete the questionnaire online using the link provided between 23 October and 23 November 2022 (Supplementary Material 1). The questionnaire was divided into three sections : 1) defining risk groups in glioma management; 2) management plans for newly diagnosed gliomas according to the new WHO classification; and 3) OLDU protocols for glioma management approved by each institution. We collected respondents' emails and affiliated institution information to avoid duplication, and we analyzed all responses descriptively and quantitatively where appropriate.
In total, we received responses from 24 neuro-oncology experts from 20 major institutions in Korea. We also reported a total of 22 OLDU protocols for glioma management, which are listed in Table 1 and Supplementary Table 1 for detailed information in Korean.
It has been widely accepted that age, performance status, and extent of resection are the most important clinical factors for defining glioma prognosis and used in guidelines for glioma management proposed by multiple societies such as Society for Neuro-Oncology (SNO), European Association of Neuro-Oncology (EANO), Korean Society for Neuro-Oncology (KSNO), and National Comprehensive Cancer Network (NCCN) [11,13-15,21,22,31]. Therefore, questions were asked about the detailed criteria for defining the high-risk group of gliomas with respect to age, performance status, and extent of resection.
When we conducted the survey to determine the most appropriate age to define a high-risk group in the prognosis of gliomas, the most common response for high-grade gliomas was over 70 years old (8/24, 33.3%), while for low-grade gliomas it was over 40 years old (9/24, 37.5%). The criterion of 40 years of age, which defines the risk group for low-grade glioma, is widely accepted by many other neuro-oncology societies [15,29,31]. However, for high-grade gliomas, the high-risk group is defined within the age range of 65 to 70 years according to other neuro-oncology societies [12,22,29,31].
The high-risk group definitions for performance status in gliomas were predominantly answered with a Karnofsky Performance Score (KPS) of less than 70 for both high- and low-grade gliomas (12/24, 50.0%). In terms of the performance status criterion that defines the high-risk group of gliomas, the SNO and EANO use a KPS <70 as a cut-off, while KSNO and NCCN use a KPS <60 [12,22,29].
Defining the high-risk group for glioma based on extent of resection is a complex issue with varying opinions. In the survey, the most frequent response for contrast-enhancing tumors was that residual lesions of 5 mL or more and 1 mL or more after surgery with contrast enhancement should be defined as high-risk groups in equal numbers (9/24, 37.5%), respectively. Therefore, a measurable enhancing residual lesion was used for defining high-risk group that could encompass all of these response results. Other responses indicated that the high-risk group should include cases where a conceptual supratotal resection is not performed. In the case of tumors without contrast enhancement, the dominant response was that the high-risk group should be defined by residuals with more than 50% of T2/flair lesions (12/24, 50.0%) or more than 5 mL of remaining T2/flair lesions after surgery (10/24, 41.7%).
Combining these findings, we can provide practical recommendations for defining high-risk groups in glioma management, as summarized in Table 2. When any of the three factors (age, performance status, extent of resection) meet the high-risk criteria, the patient should be categorized as high-risk and managed accordingly.
In the survey, respondents were asked for their opinions on general management strategies for each glioma diagnosis listed in the WHO 2021 classification, based on risk group, without specifying a treatment protocol. One of the major changes in the new classification system is that neoplasms are now graded within tumor types in a manner similar to other non-CNS cancers, rather than in an entity-specific manner [19]. Despite the change in the classification system, there was still a tendency among respondents to determine management strategies based on the WHO grade in most cases (Fig. 1). This suggests that the previous way of thinking about glioma management based on WHO grades may still be prevalent among clinicians, despite the new classification system. It highlights the need for continued education and updates in glioma management guidelines to reflect the changes in the new WHO classification system.
For gliomas classified as WHO grade 4, concomitant chemoradiotherapy (CCRT) is generally preferred as primary treatment following surgery, regardless of diagnosis or risk group. Understandably, CCRT in this setting means a standard protocol used in glioblastoma (GBM) using temozolomide [24]. Furthermore, the majority of respondents allowed modifications to the CCRT protocol, such as incorporating hypofractionation of radiotherapy (RT) for the high-risk group [7]. The application of CCRT protocol to diffuse midline glioma, H3 K27-altered was recommended in the KSNO guideline [34]. The EANO guideline also recommends CCRT as a reasonable treatment option for diffuse hemispheric glioma, H3.3 G34-mutant [29]. There is controversy surrounding the use of CCRT in astrocytoma, isocitrate dehydrogenase (IDH)-mutant grade 4, as there is no validated evidence to suggest that it specifically benefits this type of glioma. The results of the CATNON study, which did not distinguish between grade 3 and 4 astrocytomas, showed that CCRT is not significantly superior to RT alone for IDH-mutant astrocytomas [27]. However, SNO consensus recommends treating astrocytoma, IDH-mutant grade 4 in a manner similar to the treatment strategy for IDH-wildtype GBM [20]. Other available OLDU option we can apply to newly diagnosed grade 4 glioma after surgery in Korea is adding lomustine (CCNU) to the CCRT followed by adjuvant temozolomide (OLDU#1, Table 1 and Supplementary Table 1) which was confirmed its effectiveness for O6-methylguanine-DNA methyltransferase (MGMT) gene promoter methylated GBMs [6].
In the management of WHO grade 3 gliomas, the majority of responders agreed that RT alone is the primary treatment choice after surgery in most circumstances, except for oligodendrogliomas with IDH mutation and 1p/19q codeletion (ODG). For ODG, the standard therapy is RT followed by chemotherapy (CT) using the procarbazine, lomustine, and vincristine (PCV) regimen or vice versa, regardless of the risk groups [16,27]. The potential benefit of substituting temozolomide for PCV or CCRT for RT in ODG treatment will be determined by the ongoing redesigned CODEL study (NCT00887146) in the future [8]. It is generally accepted that RT followed by CT is more beneficial compared to RT only in high-risk group of IDH-mutant lower-grade gliomas [2]. The current official CT option available in Korea after RT for lower-grade gliomas is the PCV regimen. Therefore, it is recommended to administer RT followed by PCV for the high-risk group of IDH-mutant astrocytomas [4]. Thanks to the final result of CATNON study, the preferred protocol for astrocytoma, IDH-mutant grade 3, especially for high-risk group, is RT followed by temozolomide [28]. However, the use of RT followed by temozolomide for WHO grade 3 gliomas is not yet officially approved in Korea. We have CCRT options available for high-risk group of astrocytoma, IDH-mutant grade 3 from OLDU in Korea. One option is CCRT with temozolomide followed by temozolomide and CCNU (OLDU#2) [9], and the other is CCRT with temozolomide followed by temozolomide only (OLDU#3), although the latter failed to show its efficacy in confirmation study [26,28].
The consensus of observation only after surgery could be reached for low-risk groups of the gliomas with WHO grade 2, except for ependymoma and atypical choroid plexus papilloma which about the same number of responders believe should be treated by adjuvant RT even after complete resection. However, for those high-risk group of gliomas with WHO grade 2, most of responders preferred to add adjuvant RT after surgery regardless of diagnosis. And serial CT (PCV regimen) after or before RT was also chosen for high-risk group of ODG as well as IDH-mutant astrocytomas, WHO grade 2 in particular [2,16,27].
There is generally no disagreement that WHO grade 1 gliomas in the low-risk group do not require additional treatment. However, the majority of respondents also preferred observation only, even in the high-risk group where there is residual tumor after surgery. This tendency was consistent across diagnoses, as long as the tumor is WHO grade 1.
There is currently no established or agreed-upon treatment protocol for gliomas with a vague WHO grade due to their rarity and lack of experience. In high-risk situations, most people in clinical practice tend to consider adding RT only. However, there is an OLDU option of temozolomide for pediatric patients with diffuse leptomeningeal glioneuronal tumors (OLDU#4) [1].
Available treatment options for recurrent gliomas within the scope permitted by regulations of daily clinical practice in Korea include surgery, RT (re-RT), temozolomide, bevacizumab (with or without irinotecan), PCV, and CCNU. Other options include participation in clinical trials or application of OLDU if indicated. The current available OLDU options for recurrent glioma management approved by HIRA (OLDU#5-#22) are listed in Table 1 and Supplementary Table 1. Among them, regorafenib, an oral multi-kinase inhibitor of angiogenic, stromal, and oncogenic receptor tyrosine kinases, for recurrent GBM (OLDU#5) showed superior outcome over CCNU [18]. However, its relatively high incidence of side effects makes it difficult to apply easily, and its effectiveness should be confirmed by the ongoing GBM AGILE study (NCT03970447). A combination of bevacizumab and CCNU is another option for recurrent GBM (OLDU#6) [25]. However, the confirmation study yielded negative results [33]. In addition, although the incidence is small, there are several available options (OLDU#17-#19) for BRAF-altered gliomas in Korea [10,23,30].
Radiosurgery is a controversial option for salvage therapy in glioma management. The evidence for the use of radiosurgery in recurrent GBM is limited to non-randomized retrospective institutional series, and should be interpreted with caution [3]. When we asked respondents if they would consider Gamma Knife radiosurgery as a treatment option for recurrent gliomas, 75% answered that they would consider it for appropriate cases, while 25% said they would never consider it as an option.
Recently, HIRA approved an OLDU of bevacizumab for radiation necrosis based on the accumulated evidence [5,17,35]. The approved indication is as follows : 1) patients who have been receiving radiation therapy or radiosurgery for primary or metastatic brain tumors for more than 6 months; 2) findings consistent with radiation necrosis on brain magnetic resonance imaging (MRI) (conventional and advanced MRI); 3) cases accompanied by progressive neurological symptoms due to radiation necrosis; and 4) when symptoms do not improve despite steroid treatment, or when steroid administration cannot be continued due to its side effects. If indicated, intravenous injection of bevacizumab 7.5 mg/kg can be administered every 3 weeks for four cycles, and an additional two cycles may be continued if there is an effect.
There is often a gap between the ideal recommendation for managing a disease and real-world clinical practice. Such differences arise due to disparities in timing of academic advancements in disease and drug knowledge, successful clinical trials based on novel knowledge, and institutional strategy for applying them to actual clinical practice. The consensus and available options described in this report will be temporarily helpful until evidence accumulates for effective management under the new classification system for gliomas.
Notes
Conflicts of interest
Chul-Kee Park has been editorial board of JKNS since November 2020. He was not involved in the review process of this original article. No potential conflict of interest relevant to this article was reported.
Supplementary materials
The online-only data supplement is available with this article at https://doi.org/10.3340/jkns.2023.0046.
Supplementary Table 1.
The current available off-label drug use options for recurrent glioma management approved by The Health Insurance Review and Assessment Service
Supplementary Material 1.
Online questionnaire forms for the consensus survey
References
1. Aguilera D, Castellino RC, Janss A, Schniederjan M, McNall R, MacDonald T, et al. Clinical responses of patients with diffuse leptomeningeal glioneuronal tumors to chemotherapy. Childs Nerv Syst. 34:329–334. 2018.

2. Buckner JC, Shaw EG, Pugh SL, Chakravarti A, Gilbert MR, Barger GR, et al. Radiation plus procarbazine, CCNU, and vincristine in low-grade glioma. N Engl J Med. 374:1344–1355. 2016.

4. Dono A, Ballester LY, Primdahl D, Esquenazi Y, Bhatia A. IDH-mutant low-grade glioma: advances in molecular diagnosis, management, and future directions. Curr Oncol Rep. 23:20. 2021.

5. Furuse M, Nonoguchi N, Kuroiwa T, Miyamoto S, Arakawa Y, Shinoda J, et al. A prospective, multicentre, single-arm clinical trial of bevacizumab for patients with surgically untreatable, symptomatic brain radiation necrosis†. Neurooncol Pract. 3:272–280. 2016.

6. Herrlinger U, Tzaridis T, Mack F, Steinbach JP, Schlegel U, Sabel M, et al. Lomustine-temozolomide combination therapy versus standard temozolomide therapy in patients with newly diagnosed glioblastoma with methylated MGMT promoter (CeTeG/NOA-09): a randomised, openlabel, phase 3 trial. Lancet. 393:678–688. 2019.

7. Hingorani M, Colley WP, Dixit S, Beavis AM. Hypofractionated radiotherapy for glioblastoma: strategy for poor-risk patients or hope for the future? Br J Radiol. 85:e770–e781. 2012.

8. Jaeckle KA, Ballman KV, van den Bent M, Giannini C, Galanis E, Brown PD, et al. CODEL: phase III study of RT, RT + TMZ, or TMZ for newly diagnosed 1p/19q codeleted oligodendroglioma. Analysis from the initial study design. Neuro Oncol. 23:457–467. 2021.

9. Jakacki RI, Cohen KJ, Buxton A, Krailo MD, Burger PC, Rosenblum MK, et al. Phase 2 study of concurrent radiotherapy and temozolomide followed by temozolomide and lomustine in the treatment of children with high-grade glioma: a report of the Children’s Oncology Group ACNS0423 study. Neuro Oncol. 18:1442–1450. 2016.

10. Kaley T, Touat M, Subbiah V, Hollebecque A, Rodon J, Lockhart AC, et al. BRAF inhibition in BRAFV600-mutant gliomas: results from the VEBASKET study. J Clin Oncol. 36:3477–3484. 2018.

11. Karschnia P, Young JS, Dono A, Häni L, Sciortino T, Bruno F, et al. Prognostic validation of a new classification system for extent of resection in glioblastoma: a report of the RANO resect group. Neuro Oncol. 2022; [Epub ahead of print].
12. Kim YZ, Kim CY, Lim DH. The overview of practical guidelines for gliomas by KSNO, NCCN, and EANO. Brain Tumor Res Treat. 10:83–93. 2022.

13. Kim YZ, Kim CY, Lim J, Sung KS, Lee J, Oh HJ, et al. The Korean Society for Neuro-Oncology (KSNO) guideline for glioblastomas: version 2018.01. Brain Tumor Res Treat. 7:1–9. 2019.

14. Kim YZ, Kim CY, Lim J, Sung KS, Lee J, Oh HJ, et al. The Korean Society for Neuro-Oncology (KSNO) guideline for WHO grade III cerebral gliomas in adults: version 2019.01. Brain Tumor Res Treat. 7:63–73. 2019.

15. Kim YZ, Kim CY, Wee CW, Roh TH, Hong JB, Oh HJ, et al. The Korean Society for Neuro-Oncology (KSNO) guideline for WHO grade II cerebral gliomas in adults: version 2019.01. Brain Tumor Res Treat. 7:74–84. 2019.

16. Lassman AB, Hoang-Xuan K, Polley MC, Brandes AA, Cairncross JG, Kros JM, et al. Joint final report of EORTC 26951 and RTOG 9402: phase III trials with procarbazine, lomustine, and vincristine chemotherapy for anaplastic oligodendroglial tumors. J Clin Oncol. 40:2539–2545. 2022.

17. Levin VA, Bidaut L, Hou P, Kumar AJ, Wefel JS, Bekele BN, et al. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys. 79:1487–1495. 2011.

18. Lombardi G, De Salvo GL, Brandes AA, Eoli M, Rudà R, Faedi M, et al. Regorafenib compared with lomustine in patients with relapsed glioblastoma (REGOMA): a multicentre, open-label, randomised, controlled, phase 2 trial. Lancet Oncol. 20:110–119. 2019.

19. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 23:1231–1251. 2021.

20. Miller JJ, Gonzalez Castro LN, McBrayer S, Weller M, Cloughesy T, Portnow J, et al. Isocitrate dehydrogenase (IDH) mutant gliomas: a Society for Neuro-Oncology (SNO) consensus review on diagnosis, management, and future directions. Neuro Oncol. 25:4–25. 2023.

21. Mohile NA, Messersmith H, Gatson NT, Hottinger AF, Lassman A, Morton J, et al. Therapy for diffuse astrocytic and oligodendroglial tumors in adults: ASCO-SNO guideline. J Clin Oncol. 40:403–426. 2022.

22. National Comprehensive Cancer Network : Central Nervous System Cancers (version 2.2022). Available at : https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1425.
23. Selt F, van Tilburg CM, Bison B, Sievers P, Harting I, Ecker J, et al. Response to trametinib treatment in progressive pediatric low-grade glioma patients. J Neurooncol. 149:499–510. 2020.

24. Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 352:987–996. 2005.

25. Taal W, Oosterkamp HM, Walenkamp AM, Dubbink HJ, Beerepoot LV, Hanse MC, et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): a randomised controlled phase 2 trial. Lancet Oncol. 15:943–953. 2014.

26. van den Bent MJ, Baumert B, Erridge SC, Vogelbaum MA, Nowak AK, Sanson M, et al. Interim results from the CATNON trial (EORTC study 26053-22054) of treatment with concurrent and adjuvant temozolomide for 1p/19q non-co-deleted anaplastic glioma: a phase 3, randomised, open-label intergroup study. Lancet. 390:1645–1653. 2017.

27. van den Bent MJ, Brandes AA, Taphoorn MJ, Kros JM, Kouwenhoven MC, Delattre JY, et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol. 31:344–350. 2013.

28. van den Bent MJ, Tesileanu CMS, Wick W, Sanson M, Brandes AA, Clement PM, et al. Adjuvant and concurrent temozolomide for 1p/19q non-co-deleted anaplastic glioma (CATNON; EORTC study 26053-22054): second interim analysis of a randomised, open-label, phase 3 study. Lancet Oncol. 22:813–823. 2021.

29. Weller M, van den Bent M, Preusser M, Le Rhun E, Tonn JC, Minniti G, et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol. 18:170–186. 2021.

30. Wen PY, Stein A, van den Bent M, De Greve J, Wick A, de Vos FYFL, et al. Dabrafenib plus trametinib in patients with BRAFV600E-mutant low-grade and high-grade glioma (ROAR): a multicentre, open-label, single-arm, phase 2, basket trial. Lancet Oncol. 23:53–64. 2022.

31. Wen PY, Weller M, Lee EQ, Alexander BM, Barnholtz-Sloan JS, Barthel FP, et al. Glioblastoma in adults: a Society for Neuro-Oncology (SNO) and European Society of Neuro-Oncology (EANO) consensus review on current management and future directions. Neuro Oncol. 22:1073–1113. 2020.

32. WHO Classification of Tumours Editorial Board : Central Nervous System Tumours. Lyon : International Agency for Research on Cancer, 2021.
33. Wick W, Gorlia T, Bendszus M, Taphoorn M, Sahm F, Harting I, et al. Lomustine and bevacizumab in progressive glioblastoma. N Engl J Med. 377:1954–1963. 2017.

Fig. 1.
Summary of the responses from an online survey on the preferred management plan for newly diagnosed gliomas according to the new World Health Organization (WHO) classification (numbers are shown in the color bar indicating the number of respondents). RT : radiotherapy, CT : chemotherapy, CCRT : concomitant chemoradiotherapy, IDH : isocitrate dehydrogenase.
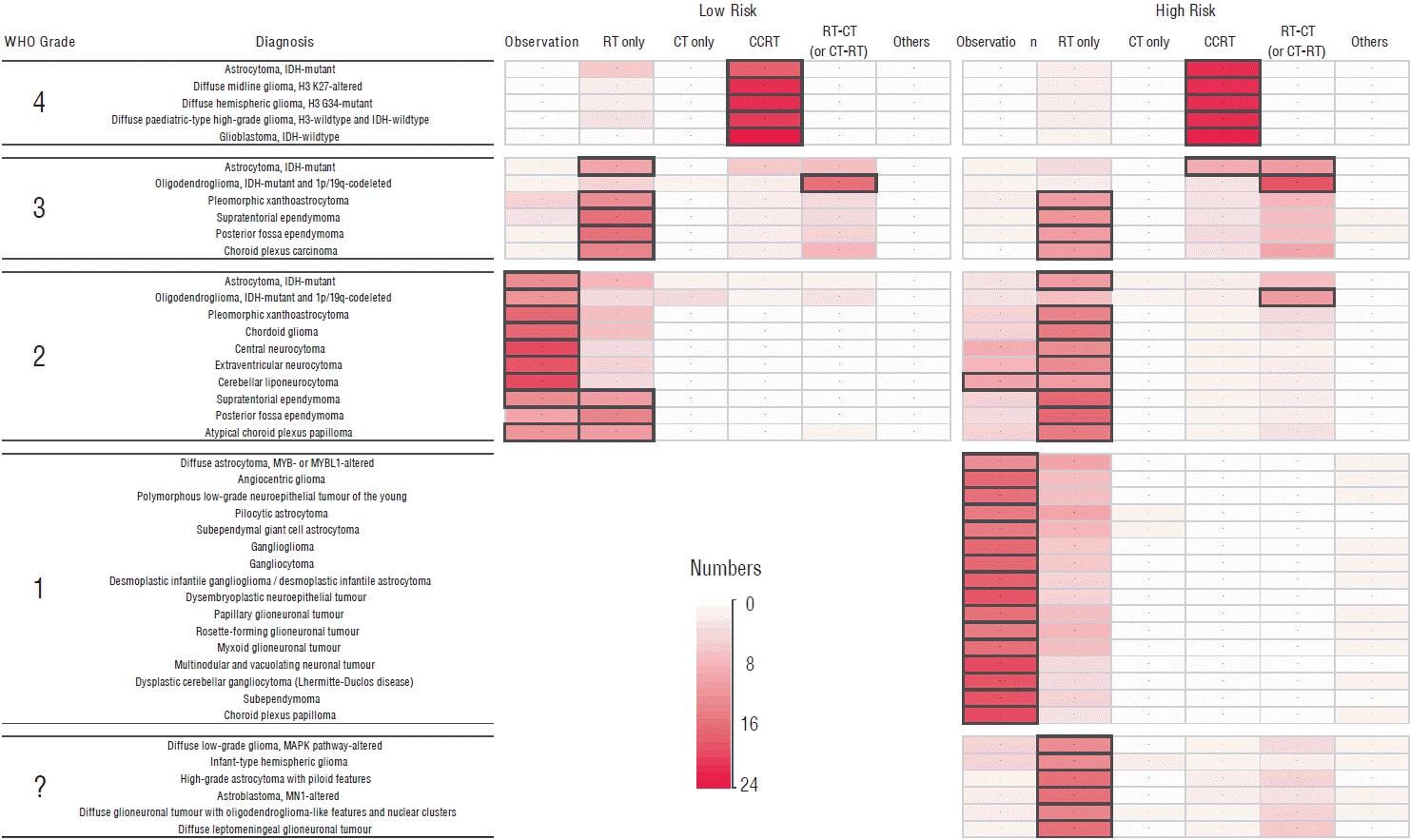
Table 1.
List of off-label drug use (OLDU) protocol for glioma management available in Korea (December 2022)
Table 2.
KBTS consensus of risk group definition in gliomas




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download