Abstract
While the standard management for posthemorrhagic hydrocephalus (PHH) has not been determined, many patients initially receive temporary treatment such as a ventricular drainage, a ventricular reservoir, or a ventriculosubgaleal shunt. Subsequently, approximately 15% of patients with PHH will require permanent cerebrospinal fluid diversion. Shunt placement is most commonly performed for PHH as permanent treatment. However, shunting still has high complication rates. Since the development of the neuroendoscopic technique has progressed, and indication has been expanded, endoscopic third ventriculostomy with or without choroid plexus cauterization has performed more frequently in recent years in patients with PHH. In this paper, the permanent treatment for PHH will be reviewed based on the latest evidence.
Intraventricular hemorrhage (IVH) has been decreasing due to remarkable advances in obstetric and neonatal medicine. However, IVH still continues to occur in approximately 15–20% of very low birth weight infants born weighing less than 1500 g [13]. IVH and subsequent posthemorrhagic hydrocephalus (PHH) cause periventricular white matter damage and a high rate of developmental delay in motor and language functions as well as cognitive functions [30]. In cases of severe IVH, the risk of developing PHH is approximately 40–50% and requires neurosurgical treatment [11,20]. Currently, there are no standard treatment guidelines for the management of PHH [22]. When to initiate therapeutic intervention and how to treat PHH is left to the discretion of each facility.
Most PHH in preterm infants is treated with temporary cerebrospinal fluid (CSF) management, followed by placement of a ventriculoperitoneal (VP) shunt to complete treatment. It is not feasible to perform VP shunt surgery as initial treatment for vulnerable infants, and temporary therapeutic devices such as ventricular reservoirs, ventriculo-subgaleal shunt, and ventricular drainage are placed [30,36]. The rate of need for permanent CSF diversions varies considerably among reports and temporary CSF management rarely cures PHH. VP shunt placement has been the standard treatment for several decades, but endoscopic third ventriculostomy (ETV) and choroid plexus cautery (CPC) have recently been indicated as alternatives in some cases [34,39].
Here, VP shunt surgery as a permanent surgical treatment for symptomatic PHH is primarily outlined, and the combination of ETV and CPC is also discussed.
Due to the vulnerability of preterm infants, VP shunt placement is not conducted as an initial treatment option as an intervention for PHH. VP shunt is inevitably chosen when temporary management to drain CSF is unsuccessful. It is important to note that there are some cases in which PHH becomes arrested hydrocephalus and no additional treatment is necessary. In rare cases, ventricular dilatation might improve with spontaneous resolution.
As for when exactly to convert to VP shunt placement, the size of the ventricles and degree of head circumference (HC) enlargement are indicators, not to mention if the clinical signs do not improve with temporary CSF management. And, as will be discussed later, the infant’s physical growth and the degree of clearance in hemorrhagic CSF are also important determinants. Full fontanelle, splaying sutures, irritability, apneas, bradycardia and sunset phenomenon are typical clinical signs of progressive hydrocephalus [20,37]. We must recognize that VP shunt surgery after the appearance of these clinical signs is somewhat too late to make a decision.
Quantitative measurement of the ventricles by transcranial ultrasonography is routinely performed. The most frequently used index is ventricular enlargement of 4 mm above the 97th percentile on the ventricular index. Another indicator is progressive HC enlargement. HC enlarges by approximately 1 mm per day between 26 weeks of gestation and 32 weeks, and about 0.7 mm per day between 32 and 40 weeks. A persistent increase of 2 mm per day is regarded as excessive [40]. An increase of 6 mm over 3 days is more likely to be real and an increase of 14 mm over 1 week is definitely excessive [40]. Before chronic intracranial hypertension is left untreated and various clinical symptoms appear, shunt placement should be considered based on objective judgment using measurements such as ventricular size and head circumference as indicators.
There is insufficient evidence to recommend a specific body weight or CSF parameters for the timing of shunt creation in premature infants with PHH, but the most important parameter is the body weight [31]. Even if PHH is progressive, VP shunt is done when infants have gained sufficient weight. Very preterm infants have an immature immune system, inadequate peritoneal absorption capacity, and fragile and thin skin, which leads to an extremely high incidence of shunt-related complications, including shunt infection [5,6,9,24,29,37,41]. Some reports recommend to until the infant weighs 2.5 kg [31,40], while others recommend surgery once the infant weighs 2 kg [29]. In addition, it has been reported that good neurologic functional outcome was achieved if surgery was performed when the weight reached 1.5 kg [4,32]. On the other hand, performing VP shunt placement at a weight of less than 2.0 kg is likely to result in shunt infection and ultimately worsen the neurological function. If a VP shunt is recklessly performed for a very small infant, ulceration of the skin at the valve site would be inevitable.
In a recent single-center retrospective study, permanent shunts were placed at an average gestational age of 43 weeks and an average weight of 2.9 kg [15]. Each children’s hospital has its own criteria for when to place a VP shunt, but it is common practice to wait until the infant has grown to a weight of 2.5 to 3.0 kg.
If CSF contains large amounts of blood and protein, it is very likely that the shunt system will not drain properly and will require early revision surgery. Lower CSF protein levels have been advocated. It is generally recommended that a shunt be inserted once CSF protein has decreased to less than 150–200 mg/dL, although some have suggested that less than 1000 mg/dL is acceptable [16,18]. Of course, the absence of signs of infection in the CSF is also an essential requirement.
A wide variety of shunt systems are now available to clinicians, but their impact on shunt failure rates is still unknown [24]. A comparative study of newly developed valves has not identified any differences in reoperation rates or incidence of over drainage between valve types [3].
Evidence-based guidelines also point to insufficient evidence to recommend the use of programmable versus nonprogrammable valves. Both programmable and non-programmable valves are possible treatments for PHH [1], but in actual clinical practice, small and low-profile shunt valves tend to be preferred because of the fragile scalp of infants.
Previous reports have shown that placement of a VP shunt in premature PHH infants is more likely to result in shunt revision, slit ventricle syndrome, loculated hydrocephalus, and shunt infection than in infants with hydrocephalus of other causes [30]. Based on reports from various centers, shunt revision rates ranged from 45.2% to 84.1%, and 1-year and 5-year survival rates ranged from 40% to 73.6% and 13.8% to 44.1%, respectively (Table 1) [5,6,9,24,29,37,41]. It is clear that children with PHH are more likely to undergo shunting earlier than other categories of patients in terms of weeks of gestation rather than weeks after birth [24]. It is widely known that very young age and low body weight at the time of initial shunt insertion are associated with higher rates of shunt failure [23,35].
Thus, younger age and lower weight at the time of initial shunt placement undoubtedly have a significant impact on shunt survival in PHH, which is attributed to the difficulty of the surgical technique and the fragility of the infant’s brain, intestinal tract, and skin. Fragile infants are vulnerable to any invasion.
In PHH infants undergoing shunt surgery, the condition of being post-IVH is also significantly associated with shunt survival. Excessive CSF protein levels contribute to shunt dysfunction and catheter occlusion [29]. This is also true for the treatment of PHH in adults.
Another troublesome problem associated with shunt creation in preterm infants is the complication of infection. The high incidence of shunt infections is likely due to an immature immune system and inherently weak antimicrobial ability [30]. Shunt infections have been reported in 7.1–22.4% of preterm infants (Table 1) [5,6,29,37,41].
For these susceptible infants, antibiotic-impregnated catheter is highly recommended [27,32], and every effort should be made to reduce shunt infections to near zero using strict infection prevention protocols [17]. New Hydrocephalus Clinical Research Network protocol advocated by Kestle et al. [17] in 2016 and the protocol of my department are presented in Table 2.
An isolated TFV is a peculiar complication of VP shunt placement for PHH and is caused by over drainage of the supraventricular shunt [25]. PHH occurring in preterm infants is known to be a complex combination of both communicating and non-communicating hydrocephalus factors, but the Sylvian aqueduct and foramen Luschka and Magendi may have membranous obstruction due to inflammatory scarring after IVH [28].
When the supratentorial CSF shunt is overactive, the occlusive mechanism of the Sylvian aqueduct becomes even more pronounced and combined with the obstruction of the fourth ventricular outlet, the flow path of CSF produced by the choroid plexus in the fourth ventricle is blocked and the fourth ventricle is isolated and enlarged.
Although TFV may result from other etiology, the frequency of TFV is higher in PPH in preterm infants. Pomeraniec et al. [28] reported that the frequency of TFV following VP shunt for PHH in preterm infants was 15.4%.
Treatment options include fourth ventriculostomy with posterior fossa craniotomy, placement of an additional shunt in the fourth ventricle, and endoscopic procedures (aqueductoplasty and/or stenting, cystoventricular stenting) [10]. Note, however, that even if the fourth ventricle is isolated and enlarged on computed tomography or magnetic resonance imaging imaging, it is often asymptomatic, and furthermore, because cerebellar atrophy is not rarely seen in preterm infants with PHH, the indication for treatment of TFV should be carefully considered.
PHH is thought to result from inflammation and fibrosis of the arachnoid granulation and subependymal layers of the ventricles, which impairs CSF circulation and absorption [8]. As mentioned previously, hydrocephalus after IVH in preterm infants presents a mechanism that combines both communicating and non-communicating pathologies. ETV creates a new channel for CSF circulation and has been shown to be effective in obstructive hydrocephalus, with high success rates reported primarily for sylvian aqueduct stenosis [12,19]. As shown in the success score of ETV, the success rate is known to be lower in patients with hydrocephalus due to post-infection, post-hemorrhage, or myelomeningocele [12,38], and in children younger than 1 year of age [2,12].
Although it is true that the development of neuroendoscopic techniques has made this procedure safer and simpler, resulting in a much wider range of indications, the indication for PHH in preterm infants should be carefully considered. Certainly, the option of ETV, which avoids various shunt-related complications, might be extremely attractive, but it is easy to understand that a high success rate is difficult to achieve given the pathogenesis of hydrocephalus.
There have been reports of increasing indications for ETV in PHH in recent years; however, because of its much higher initial failure rate compared to shunts, it tends to be the unavoidable choice in cases with co-morbidities such as necrotizing enterocolitis. It is an undeniable fact that ETV alone has not been reliably effective in preterm infants with PHH, and Elgamal et al. [14] reported that ETV was effective in children under 1 year of age with Sylvian aqueductal stenosis (success rate 77%) but not in preterm infants with PHH (success rate 14%).
Warf [38] found that the addition of CPC to ETV, primarily in Africa, may increase the success rate of ETV for post-infection hydrocephalus. Subsequently, they showed that ten infants with PHH underwent ETV/CPC and four (40%) required no further operation [39]. In a recent report of 50 consecutive cases from a single institution, the success rate of ETV/CPC for infants with PHH was only 22% [21]. The authors concluded that ETV/CPC may be justified as initial treatment for hydrocephalus because of its lower complication rate compared to shunt treatment. The disadvantage of prolonging the pathological condition of hydrocephalus and adversely affecting neurodevelopmental functional outcome is overlooked. Pediatric neurosurgeons need to fully consider whether this treatment should be preferred over shunt placement.
The success rate of ETV and risk factor in infants with PHH are indicated in Table 3 [7,19,21]. Several studies show that patient selection might increase the success rate. The scarring of the prepontine cistern [4,39], younger age [4], and symmetric IVH [21] appear to correlate well with treatment failure of ETV and ETV/CPC.
Although the benefits of VP shunt in terms of neurodevelopmental outcomes as well as morphological improvement of the brain have accumulated over the past several decades, no one has the knowledge and experience of the long-term consequences of permanently disrupting CSF production capacity by coagulating the choroid plexus. Currently, VP shunt placement should be considered as a priority unless there are special reasons such as necrotizing enterocolitis that make it impossible to place a catheter in the abdominal cavity or CSF infection.
In recent years, there has been a great deal of interest in how to treat PHH without CSF diversion surgery, and attention has focused on endoscopic ventricular lavage [33] and fibrinolytic therapy based on ventricular drainage management [26]. Given the non-negligible probability of troublesome problems in VP shunt surgery and subsequent management of PHH in preterm infants, the treatment concept of VP shunt avoidance is an important issue for us pediatric neurosurgeons to address. However, since not all children’s hospitals are able to implement the two aggressive treatment strategies described above, it is essential that VP shunt surgery and subsequent long-term management be carried out without trouble after appropriate temporary CSF management.
A female infant was born at 24 weeks 6 days of gestational and weighted 401 g. Her Apgar score was 1 point (1 minute) and 1 point (5 minutes). A grade IV IVH occurred on day 1 (Fig. 1A). Ventricular dilatation progressed (Fig. 1B) and ventricular drainage with peripherally inserted catheter underwent on 28 days after birth. VP shunt with a programmable valve was performed at the age of 4 months (Fig. 1C) because of the enlargement of her HC. Although shunt infection or revision are not required for 10 years, she has moderate disability. If surgical intervention such as endoscopic ventricular lavage [32] or fibrinolytic therapy [25] had been performed earlier, the prognosis might be better.
Although there is insufficient evidence of permanent treatment for PHH, my personal strategy is indicated in Fig. 2. When the baby reaches 2.5 kg and its CSF protein has decreased to less than 200 mg/dL, permanent treatment should be considered. If the baby has a history of meningitis or abdominal surgery, ETV with or without CPC is recommended. If ETV/CPC is ineffective, VP shunt should be subsequently performed. If the baby does not have any complications, VP shunt is recommended with antibiotic-impregnated shunt catheter.
Notes
References
1. Baird LC, Mazzola CA, Auguste KI, Klimo P Jr, Flannery AM; Pediatric Hydrocephalus Systematic Review and Evidence-Based Guidelines Task Force. Pediatric hydrocephalus: systematic literature review and evidence-based guidelines. Part 5: effect of valve type on cerebrospinal fluid shunt efficacy. J Neurosurg Pediatr. 14 Suppl 1:35–43. 2014.

2. Balthasar AJ, Kort H, Cornips EM, Beuls EA, Weber JW, Vles JS. Analysis of the success and failure of endoscopic third ventriculostomy in infants less than 1 year of age. Childs Nerv Syst. 23:151–155. 2007.

3. Beez T, Sarikaya-Seiwert S, Bellstädt L, Mühmer M, Steiger HJ. Role of ventriculoperitoneal shunt valve design in the treatment of pediatric hydrocephalus--a single center study of valve performance in the clinical setting. Childs Nerv Syst. 30:293–297. 2014.

4. Benzel EC, Reeves JD, Kesterson L, Hadden TA. Slit ventricle syndrome in children: clinical presentation and treatment. Acta Neurochir (Wien). 117:7–14. 1992.

5. Bir SC, Konar S, Maiti TK, Kalakoti P, Bollam P, Nanda A. Outcome of ventriculoperitoneal shunt and predictors of shunt revision in infants with posthemorrhagic hydrocephalus. Childs Nerv Syst. 32:1405–1414. 2016.

6. Bock HC, Feldmann J, Ludwig HC. Early surgical management and long-term surgical outcome for intraventricular hemorrhage-related posthemorrhagic hydrocephalus in shunt-treated premature infants. J Neurosurg Pediatr. 22:61–67. 2018.

7. Chamiraju P, Bhatia S, Sandberg DI, Ragheb J. Endoscopic third ventriculostomy and choroid plexus cauterization in posthemorrhagic hydrocephalus of prematurity. J Neurosurg Pediatr. 13:433–439. 2014.

8. Cherian S, Whitelaw A, Thoresen M, Love S. The pathogenesis of neonatal post-hemorrhagic hydrocephalus. Brain Pathol. 14:305–311. 2004.

9. Chittiboina P, Pasieka H, Sonig A, Bollam P, Notarianni C, Willis BK, et al. Posthemorrhagic hydrocephalus and shunts: what are the predictors of multiple revision surgeries? J Neurosurg Pediatr. 11:37–42. 2013.

10. Cinalli G, Spennato P, Savarese L, Ruggiero C, Aliberti F, Cuomo L, et al. Endoscopic aqueductoplasty and placement of a stent in the cerebral aqueduct in the management of isolated fourth ventricle in children. J Neurosurg. 104(1 Suppl):21–27. 2006.

11. Cizmeci MN, Groenendaal F, Liem KD, van Haastert IC, BenaventeFernández I, van Straaten HLM, et al. Randomized controlled early versus late ventricular intervention study in posthemorrhagic ventricular dilatation: outcome at 2 years. J Pediatr. 226:28–35.e3. 2020.
12. Drake JM; Canadian Pediatric Neurosurgery Study Group. Endoscopic third ventriculostomy in pediatric patients: the Canadian experience. Neurosurgery. 60:881–886. 2007.
13. du Plessis AJ. Cerebrovascular injury in premature infants: current understanding and challenges for future prevention. Clin Perinatol. 35:609–641. v, 2008.

14. Elgamal EA, El-Dawlatly AA, Murshid WR, El-Watidy SM, Jamjoom ZA. Endoscopic third ventriculostomy for hydrocephalus in children younger than 1 year of age. Childs Nerv Syst. 27:111–116. 2011.

15. Fulkerson DH, Vachhrajani S, Bohnstedt BN, Patel NB, Patel AJ, Fox BD, et al. Analysis of the risk of shunt failure or infection related to cerebrospinal fluid cell count, protein level, and glucose levels in lowbirth-weight premature infants with posthemorrhagic hydrocephalus. J Neurosurg Pediatr. 7:147–151. 2011.

16. Heep A, Engelskirchen R, Holschneider A, Groneck P. Primary intervention for posthemorrhagic hydrocephalus in very low birthweight infants by ventriculostomy. Childs Nerv Syst. 17:47–51. 2001.

17. Kestle JR, Holubkov R, Douglas Cochrane D, Kulkarni AV, Limbrick DD Jr, Luerssen TG, et al. A new Hydrocephalus Clinical Research Network protocol to reduce cerebrospinal fluid shunt infection. J Neurosurg Pediatr. 17:391–396. 2016.

18. Köksal V, Öktem S. Ventriculosubgaleal shunt procedure and its longterm outcomes in premature infants with post-hemorrhagic hydrocephalus. Childs Nerv Syst. 26:1505–1515. 2010.

19. Kulkarni AV, Riva-Cambrin J, Holubkov R, Browd SR, Cochrane DD, Drake JM, et al. Endoscopic third ventriculostomy in children: prospective, multicenter results from the Hydrocephalus Clinical Research Network. J Neurosurg Pediatr. 18:423–429. 2016.

20. Limbrick DD Jr, de Vries LS. New insights into the management of posthemorrhagic hydrocephalus. Semin Perinatol. 46:151597. 2022.

21. Lu VM, Wang S, Niazi TN, Ragheb J. Impact of intraventricular hemorrhage symmetry on endoscopic third ventriculostomy with choroid plexus cauterization for posthemorrhagic hydrocephalus: an institutional experience of 50 cases. J Neurosurg Pediatr. 31:245–251. 2022.

22. Mazzola CA, Choudhri AF, Auguste KI, Limbrick DD Jr, Rogido M, Mitchell L, et al. Pediatric hydrocephalus: systematic literature review and evidence-based guidelines. Part 2: management of posthemorrhagic hydrocephalus in premature infants. J Neurosurg Pediatr 14 Suppl. 1:8–23. 2014.

23. McGirt MJ, Leveque JC, Wellons JC 3rd, Villavicencio AT, Hopkins JS, Fuchs HE, et al. Cerebrospinal fluid shunt survival and etiology of failures: a seven-year institutional experience. Pediatr Neurosurg. 36:248–255. 2002.

24. Notarianni C, Vannemreddy P, Caldito G, Bollam P, Wylen E, Willis B, et al. Congenital hydrocephalus and ventriculoperitoneal shunts: influence of etiology and programmable shunts on revisions. J Neurosurg Pediatr. 4:547–552. 2009.

25. Oi S, Matsumoto S. Pathophysiology of aqueductal obstruction in isolated IV ventricle after shunting. Childs Nerv Syst. 2:282–286. 1986.

26. Park YS, Kotani Y, Kim TK, Yokota H, Sugimoto T, Nakagawa I, et al. Efficacy and safety of intraventricular fibrinolytic therapy for postintraventricular hemorrhagic hydrocephalus in extreme low birth weight infants: a preliminary clinical study. Childs Nerv Syst. 37:69–79. 2021.

27. Parker SL, Attenello FJ, Sciubba DM, Garces-Ambrossi GL, Ahn E, Weingart J, et al. Comparison of shunt infection incidence in high-risk subgroups receiving antibiotic-impregnated versus standard shunts. Childs Nerv Syst. 25:77–83. discussion 85. 2009.

28. Pomeraniec IJ, Ksendzovsky A, Ellis S, Roberts SE, Jane JA Jr. Frequency and long-term follow-up of trapped fourth ventricle following neonatal posthemorrhagic hydrocephalus. J Neurosurg Pediatr. 17:552–557. 2016.

29. Reinprecht A, Dietrich W, Berger A, Bavinzski G, Weninger M, Czech T. Posthemorrhagic hydrocephalus in preterm infants: long-term followup and shunt-related complications. Childs Nerv Syst. 17:663–669. 2001.

30. Robinson S. Neonatal posthemorrhagic hydrocephalus from prematurity: pathophysiology and current treatment concepts. J Neurosurg Pediatr. 9:242–258. 2012.

31. Valdez Sandoval P, Hernández Rosales P, Quiñones Hernández DG, Chavana Naranjo EA, García Navarro V. Intraventricular hemorrhage and posthemorrhagic hydrocephalus in preterm infants: diagnosis, classification, and treatment options. Childs Nerv Syst. 35:917–927. 2019.

32. Sciubba DM, Noggle JC, Carson BS, Jallo GI. Antibiotic-impregnated shunt catheters for the treatment of infantile hydrocephalus. Pediatr Neurosurg. 44:91–96. 2008.

33. Schulz M, Bührer C, Pohl-Schickinger A, Haberl H, Thomale UW. Neuroendoscopic lavage for the treatment of intraventricular hemorrhage and hydrocephalus in neonates. J Neurosurg Pediatr. 13:626–635. 2014.

34. Stone SS, Warf BC. Combined endoscopic third ventriculostomy and choroid plexus cauterization as primary treatment for infant hydrocephalus: a prospective North American series. J Neurosurg Pediatr. 14:439–446. 2014.

35. Tuli S, Drake J, Lawless J, Wigg M, Lamberti-Pasculli M. Risk factors for repeated cerebrospinal shunt failures in pediatric patients with hydrocephalus. J Neurosurg. 92:31–38. 2000.

36. Wang JY, Amin AG, Jallo GI, Ahn ES. Ventricular reservoir versus ventriculosubgaleal shunt for posthemorrhagic hydrocephalus in preterm infants: infection risks and ventriculoperitoneal shunt rate. J Neurosurg Pediatr. 14:447–454. 2014.

37. Wang JY, Jackson EM, Jallo GI, Ahn ES. Shunt revision requirements after posthemorrhagic hydrocephalus of prematurity: insight into the time course of shunt dependency. Childs Nerv Syst. 31:2123–2130. 2015.

38. Warf BC. Comparison of endoscopic third ventriculostomy alone and combined with choroid plexus cauterization in infants younger than 1 year of age: a prospective study in 550 African children. J Neurosurg. 103(6 Suppl):475–481. 2005.

39. Warf BC, Campbell JW, Riddle E. Initial experience with combined endoscopic third ventriculostomy and choroid plexus cauterization for post-hemorrhagic hydrocephalus of prematurity: the importance of prepontine cistern status and the predictive value of FIESTA MRI imaging. Childs Nerv Syst. 27:1063–1071. 2011.

Fig. 1.
Case presentation. A : Ultrasonography at the age of 1 day (left) and 28 days (right) demonstrating bilateral intraventricular hemorrhage developing to ventricular dilatation. B : T2-weighted magnetic resonance images at the age of 4 months before shunt operation showing enlargement of the ventricle. C : Lateral view of X-ray after shunt operation with a programmable valve.
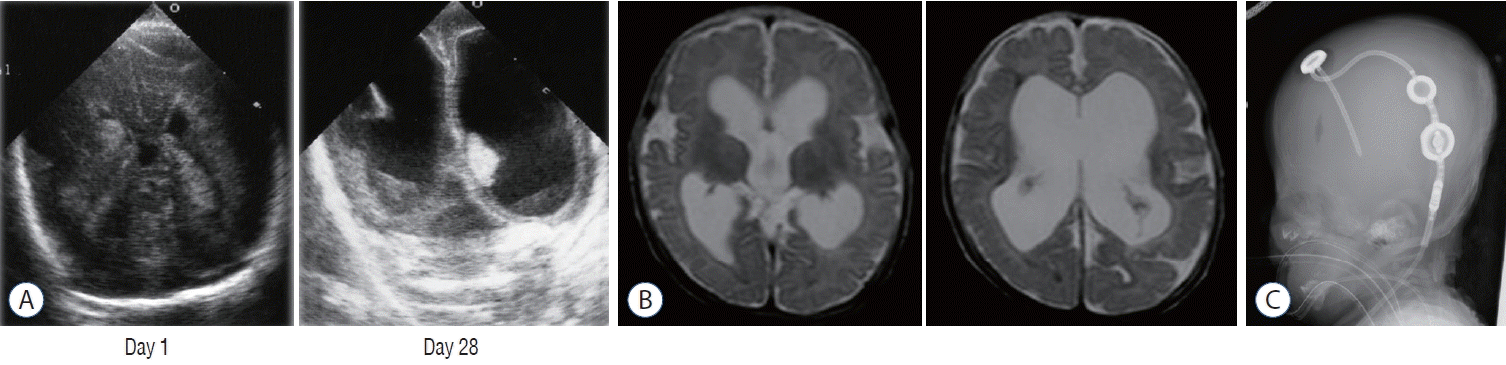
Fig. 2.
Strategy for the permanent treatment for posthemorrhagic hydrocephalus. CSF : cerebrospinal fluid, VP : ventriculo-peritoneal, ETV : endoscopic third ventriculostomy, CPC : choroid plexus cauterization.
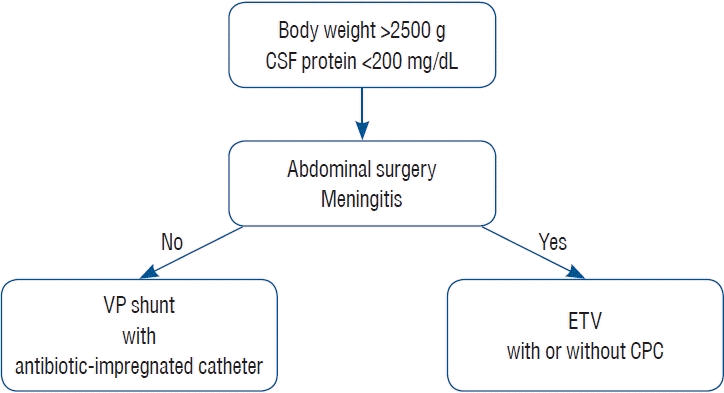
Table 1.
Ventriculo-peritoneal shunt in infants with post hemorrhagic hydrocephalus
| Study | Total |
Treatment before VP shunt |
VP shunt | Infection rate | Revision rate | Shunt survival rate | ||
|---|---|---|---|---|---|---|---|---|
| Drainage | Reservoir | Subgaleal shunt | ||||||
| Reinprecht et al. [29] (2001) | 76 | 76 | - | - | VP 32, VA 10 | 7.10% | 22/42 (45.2%) | NDA |
| Willis et al. [41] (2009) | 32 | - | 15 | - | 29 | 3/29 (10.3%) | 14/29 (48.3%) | NDA |
| Notarianni et al. [24] (2009) | 69 | - | - | - | 69 | NDA | 58/69 (84.1%) | 5 years : 13.8% |
| Chittiboina et al. [9] (2013) | 109 | - | 65 | - | 104 | NDA | 78/104 (71.6%) | NDA |
| Wang et al. [37] (2015) | 89 | - | 43 | 46 | 69 | 10/69 (14.5%) | 33 cases, 66 revisions | 1 year : 73.6%, 5 years : 44.1%, 10 years : 36.7% |
| Bir et al. [5] (2016) | 122 | - | - | - | 122 | 53/380 (17.2%) | 102/122 (84%) | 1 year : 40%, 5 years : 31.2% |
| Bock et al. [6] (2018) | 104 | 76 | - | - | 104 | 52/232 (22.4%) | 78/104 (75%) | NDA |
Table 2.
Infection prevention protocol
| New Hydrocephalus Clinical Research Network protocol [17] | Takatsuki protocol |
|---|---|
| - | Sign on operation room door to minimize traffic |
| Antibiotic received before incision (cefazolin 30 mg/kg) | Same |
| Antibiotic received one dose postoperatively (cefazolin 30 mg/kg) | 2–3 days |
| - | Hair clipped, not shaved |
| ChloraPrep applied to surgical field and not washed off | Povidone iodine |
| Wait 3 minutes to allow CholoraPrep to dry | Wait 3 minutes to allow Povidone iodine to dry |
| Hand scrub by all participants (antiseptic cream not allowed) | Same |
| Double gloving by all team members | Same |
| Ioban use | Same |
| AICs | Sometimes use AICs |
| Two (cranial and distal) for insertion procedures | Three (cranial and distal) for insertion procedures |
| One (cranial or distal) or both for revision procedures | One to three for revision procedures |
Table 3.
Endoscopic third ventriculostomy in infants with post hemorrhagic hydrocephalus
| Study | Total | Success rate | CPC | Risk factor |
|---|---|---|---|---|
| Warf et al. [39] (2011) | 10 | 40% | All with CPC | Scarring in the prepontine cistern |
| Chamiraju et al. [7] (2014) | 27 | 37% | 24 with CPC, 3 ETV only | Corrected age at operation ≤38 weeks; normal prepontine cistern |
| Lu et al. [21] (2022) | 50 | 36% at 6 months, 22% at 42 months | All with CPC | IVH symmetry |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download