Abstract
Remarkable advances in neonatal care have significantly improved the survival of extremely low birth weight infants in recent years. However, intraventricular hemorrhage (IVH) continues to be a major complication in preterm infants, leading to a high incidence of cerebral palsy and cognitive impairment. IVH is primarily caused by disruption of the fragile vascular network of the subependymal germinal matrix, and subsequent ventricular dilatation adversely affects the developing infant brain. Based on recent research, periventricular white matter injury is caused not only by ischemia and morphological distortion due to ventricular dilatation but also by free iron and inflammatory cytokines derived from hematoma and its lysates. The current guidelines for the treatment of posthemorrhagic hydrocephalus (PHH) in preterm infants do not provide strong recommendations, but initiating treatment intervention based on ultrasound measurement values before the appearance of clinical symptoms of PHH has been proposed. Moreover, in the past decade, therapeutic interventions that actively remove hematomas and lysates have been introduced. The era is moving beyond cerebrospinal fluid shunt toward therapeutic goals aimed at improving neurodevelopmental outcomes.
Although survival rates for extremely low birth weight (ELBW) infants have remarkably improved in recent years owing to technological and scientific advances in neonatal care, intraventricular hemorrhage (IVH) continues to be a major complication of prematurity that can affect long-term neurodevelopmental outcomes [1,7,20,30,38,44]. Severe types of IVH (grade 3 and 4 according to Volpe classification) continue to occur in up to 15% of ELBW infants, and more than half of these infants develop post-hemorrhagic ventricular dilatation (PHVD) resulting in periventricular white matter injury (WMI) [1,38,52].
While prevention of IVH has remained an important issue for neonatologists, pediatric neurosurgeons have focused only on the treatment of post-hemorrhagic hydrocephalus (PHH) for the last three decades. Specifically, the goal has been to successfully place a permanent ventriculoperitoneal (VP) shunt after temporary cerebrospinal fluid (CSF) management (ventricular access device [VAD] or ventriculosubgaleal shunt [VSg] shunt), and the main concerns have been infection and shunt failure. From now on, however, pediatric neurosurgeons should focus their attention and efforts on reducing the incidence of WMI and improving long-term neurodevelopmental functional outcomes in ELBW infants [14,21,42,66]. In this paper, the recently clarified mechanism of brain damage caused by IVH and PHVD and new treatment approaches for PHH are briefly summarized.
The pathogenesis of IVH in preterm infants is complex and multifactorial. IVH is primarily caused by destabilizing changes in cerebral blood flow (CBF) inflicting to the microvasculature of the germinal matrix (GM) [8,12,46].
Embryologically, the GM is the source of both neurons and glial cells, and it is active mainly between 8 and 28 weeks of gestation. The GM initially produces neurons and subsequently glial cells, which migrate to populate the cerebral cortex. Involution of the GM toward the caudothalamic groove begins late in the second trimester and is nearly completed by 32 weeks of gestation [31,50].
Anatomically, the GM is a highly vascularized region of the developing brain located underneath the lateral ventricles. The GM has been referred to as an “immature vascular rete” containing primitive vessels that cannot be classified as arterioles, venules, or capillaries [26,33]. The GM microvasculature is fragile and this fragility is derived from the histological feature of a single layer of surrounding endothelial cells and a scarcity of pericytes. Furthermore, the GM microvasculature lacks the basement membrane deposition, tight junctions, and glial end-foot investiture, all of which physiologically constitute the blood-brain barrier [31,50]. These anatomical features make it more prone to injury and bleeding.
On the other hand, in preterm infants with immature cardiopulmonary systems, vital signs are considerably unstable during the first few days of life, and complications such as respiratory distress syndrome can also occur. Therefore, hypotension, hypoxia, and hypercapnia are common [46]. As cerebral autoregulation is also immature, CBF fluctuation can cause repeated ischemia-reperfusion events, and a sudden increase in CBF can lead to excessive strain on fragile vessels in the GM, resulting in hemorrhage [17,31,46]. The hemorrhage can easily disrupt the ependymal lining and extend to the lateral ventricle, which is clinically recognized as IVH.
IVH is graded based on the extent of intraventricular hematoma and the presence of ventricular enlargement, and both Papile and Volpe classifications are widely recognized [41,60,61]. IVH grade 4 is known to be caused by venous drainage obstruction and bleeding into surrounding tissues after venous infarction and is not an extension of the original bleeding. IVH grade 3 and 4 are serious and followed by hydrocephalus, often requiring multiple neurosurgical interventions and inevitably resulting in poor neurodevelopmental outcomes [7,37,44].
The prevalence rate of each IVH grade has been reported in several large cohort studies. According to a study that included 9575 preterm infants (gestation age, ≤28 weeks; weight, ≤1500 g) registered in the nationwide registry of the USA, the incidence rate of grade 1 hemorrhage was 10%, grade 2 was 6%, grade 3 was 7%, and grade 4 was 9%. Overall, 16% of preterm infants developed severe IVH [52].
The pathophysiology of PHVD that develops after IVH is also complex. In addition to hemorrhage-related simple obstruction in the ventricular system, adhesive arachnoiditis, fibrosis in the basal cistern, mechanisms of reabsorption and inflammation-mediated imbalance in the production of CSF have been implicated [33]. Neuroendoscopic findings have frequently revealed obstruction of the Sylvian aqueduct (Fig. 1). Furthermore, PHVD has been attributed to fibrosis of the arachnoid granulations, meningeal fibrosis, and subependymal gliosis, which add to impaired CSF resorption [46]. Generally, PHH is defined when PHVD becomes clinically symptomatic.
The pathophysiology of periventricular WMI after IVH in preterm infants involves a complex of various adverse factors. The easily understood pathology is the damage caused by a dilated ventricle. The progressive accumulation of CSF changes the shape of the lateral ventricles from slit-like to balloon-like, and ultimately, the cerebral mantle becomes paper thin (Fig. 2). The highly dilated ventricles distort the brain and increase intracranial pressure subsequently. The developing brain in infants can be compromised not only by morphological distortion but also by ischemic injury due to reduced cerebral perfusion pressure [8,66].
In addition, the adverse effects of the hematoma itself and its lysate also need to be fully understood and have received little attention until recently [8,66]. Previous studies have reported that free iron and inflammatory cytokines are involved in WMI [47,48,53]. Recently, more detailed studies have revealed that the presence of IVH causes axonal degeneration and damages oligodendroglial progenitor cells (OPCs), resulting in reduced myelination of white matter [8]. Embryologically, at 23–28 weeks of gestation, the human cerebral cortex is populated mostly by early and late OPCs, which are pre-myelinating cells. Subsequently, at around 30–32 weeks of gestation, differentiation of OPCs is most active, which is characterized by a three-fold increase in myelinating OPCs and a marked increase in myelinated axons [5,6,8]. IVH occurs precisely during this period. Blood accumulates in the ventricles, resulting in clot lysis and subsequent release of hemoglobin, iron, thrombin, and complement [8]. Among the products of clot lysis, thrombin and iron have been widely studied and seem to be the key inducers of brain injury. These blood products induce oxidative stress, glutamate excitotoxicity, and inflammation, which are the main pathological reactions that result in damage to OPCs. IVH triggers extensive inflammation around the ventricles, damages axons, and induces apoptosis and maturational arrest of OPCs, leading to reduced myelination of white matter and ultimately causing WMI [8].
Neurodevelopmental impairments in ELBW infants were common in the 1990s; however, since 2000, recent advances in treatment have reliably improved neurodevelopmental outcomes [10,24]. Modern advanced neonatal intensive care occasionally results in favorable neurological development, even in ELBW infants [30,58]. However, when IVH, subsequent hydrocephalus, and periventricular WMI occur in preterm infants, the neurological prognosis is inevitably worsened.
There are optimistic reports that IVH has little effect on developmental disorders in preterm infants [13,40], but in actual clinical practice, most affected infants develop neurological disorders as they grow up [1,11,25,38]. An updated meta-analysis, published in 2022, comparing 3-year neurodevelopmental outcomes in preterm infants with IVH with those in preterm infants without IVH reported that the adjusted risk for moderate to severe neurodevelopmental disability was indicated by an odds ratio (OR) of 1.35 for IVH grade 1 and 2 and 4.26 for IVH grade 3 and 4. IVH grade 3 and 4 had significantly higher risks of cerebral palsy (OR, 4.98) and motor (OR, 2.7), cognitive (OR, 2.3), and visual (OR, 5.42) impairement [44].
McClugage et al. [37] reviewed all cases of preterm infants who developed IVH (grade 3 and 4) between 2003 and 2014 at their institution and reported detailed neurofunctional outcomes at 2 years of age. This report is considered valuable as it clearly describes the actual neurodevelopmental outcomes. Approximately 16% of the patients died, 88% had cerebral palsy or developmental delay, 48% were non-verbal, 55% were non-ambulatory, 33% had epilepsy, and 41% had visual impairment. Specifically, in patients with IVH grade 3, 74% had cerebral palsy or developmental delay, 32% had a non-verbal disability, and 37% were unable to walk. Particularly in patients with IVH grade 4, 97% had cerebral palsy or developmental delay, 59% were non-verbal, and 68% were non-ambulatory. This suggests that the neurodevelopmental outcomes are considerably poor when the grade of IVH increases.
Further, in recent years, attention has also been focused on the risk of psychiatric disorders in adolescent survivors. The occurrence of IVH has been shown to increase the risk of adolescent psychiatric disorders such as attention deficit hyperactivity disorder, poor social skills, anxiety disorders, and depression. Among adolescent survivors from the Neonatal Brain Hemorrhage Study cohort, the incidence rates of major depressive disorder (13% vs. 5.2%) and obsessive-compulsive neurosis (11.6% vs. 1.4%) were higher in those who were born preterm and developed IVH than in those who were born preterm and did not develop IVH [23,27,39,54,65].
These clinical data are important in setting treatment goals for IVH. We must recognize the importance of rehabilitation treatment, including social-psychological approaches from infancy to adolescence, in addition to neurosurgical treatment during the neonatal period [8].
The evidence-based guidelines published in 2014 have failed to identify sufficient data to support strong recommendations in the neurosurgical interventions for PHH in preterm infants [36]. Therefore, most pediatric medical centers have been using conventional methods of treating PHH based on their own concepts [22,63]. The lack of strong evidence has led to considerable variability in two major treatment decision considerations : the timing of treatment and the type of intervention.
Regarding the timing of treatment, the conventional idea is to consider therapeutic intervention at the onset of clinical signs such as increased head circumference, full fontanel, setting sun eyes, apnea, and bradycardia. This treatment concept is apparently a “too late” intervention.
As the infant brain, especially the preterm brain, is highly compliant, low intracranial pressure is sufficient to induce progressive ventricular dilatation in which the cortical mantle is compressed before the head circumference is affected. Moreover, changes in fontanelle pressure cannot occur unless the ventricles are considerably dilated [21]. Prolonged untreated severe ventricular dilatation leads to irreversible damage to the periventricular white matter, with consequent poor neurodevelopmental outcomes.
In recent years, intervention criteria based on ventricular measurements obtained using ultrasonography have been actively introduced. Intervention should be initiated based on standardized ventricular measurements before the appearance of clinical signs of hydrocephalus. Conventionally, it has been considered necessary to initiate treatment when the ventricular index (VI) has crossed the 97th percentiles +4 mm line [18,19,28,59]. However, this index proposed by Levene [32] only exists from the 27th week of gestation. Currently, interventions for IVH are often considered even in patients with gestational age <27 weeks; therefore, VI alone is not a sufficient indicator of ventricular dilation. More importantly, this index might be a “somewhat late” criterion for therapeutic intervention. Therefore, besides VI, measurements such as anterior horn width (AHW), third ventricular width (TVW), and thalamo-occipital dimension (TOD) provide additional reference ranges (Fig. 3). The combination of these three measurements (AHW >4 mm, TVW >3 mm, and TOD >26 mm) has been used as an alternative diagnostic index to detect PHVD [20,21].
In 2020, the collaborate study by prominent several experts in this field classified PHVD into three categories according to VI, AHW, and TOD values—the green zone : VI ≤97th percentiles and AHW ≤6 mm; the yellow zone : VI >97th percentiles and AHW >6 mm and/or TOD >25 mm; the red zone : VI >97th percentiles +4 mm and AHW >10 mm and/or TOD >25 mm [21]. Further, they proposed therapeutic intervention at the yellow zone stage (Fig. 4). This clinical approach is extremely practical, and this severity classification will undoubtedly become a standard criterion in the near future. Changing indications for interventions will lead to a trend toward earlier treatment attempts [18,19,43].
Regarding the type of intervention, the current standard treatment includes using a VAD or VSg shunt for temporary CSF management after repeated lumbar punctures [22,36,63]. The result of an international randomized controlled trial involving acetazolamide and furosemide indicated that diuretic drug treatment was associated with a high rate of permanent shunt placement and increased neurological morbidity and thus could not be recommended [29]. Ventricular punctures can cause secondary bleeding, risk of intracranial infection, and unexpected porencephaly [69]. Therefore, this method is rarely adopted.
CSF drainage by intermittent puncture with VAD placement and VSg shunt are the two most common treatments for PHH [36,63,64]. Cases requiring permanent VP shunt placement have been reported to range from 69% to 90% for VAD treatment and 60% to 86% for VSg shunt placement [15,22,34,51,62,63]. These results indicate no significant difference between treatments. Furthermore, a meta-analysis comparing the two treatment groups found no significant differences in the incidence of infection, obstruction, VP shunt dependence, subsequent shunt infection, mortality, and long-term disability [45,63,64]. Currently, pediatric medical centers choose between VAD and VSg shunt placement that are more familiar at their institution.
Indicators of ventricular enlargement are intended only for deciding the treatment of PHVD. As mentioned in the section on the mechanism of WMI, brain damage and poor neurodevelopmental outcomes are caused not only by PHVD but also by hematoma and its lysates, which have extremely detrimental effects on the immature brain [8,66].
In the early 2000s, the famous DRainage, Irrigation, and Fibrinolytic Therapy (DRIFT) trial conducted by Whitelaw et al. [70] was devised to actively dissolve and drain intraventricular hematomas. They injected tissue plasminogen activator (tPA), a fibrinolytic agent, into the lateral ventricle, and the therapeutic effect was verified in detail. At the time, this clinical trial was considered groundbreaking, but there were also concerns about its risks. Although the phase 1 trial reported a significant reduction in the proportion of cases of VP shunt surgery (26%) and good neurodevelopmental outcomes (normal, 42%; single disorder, 37%) at 12 months postoperatively [70], in the prospective multicenter randomized control study, the incidence of VP shunt surgery and death was not reduced in the DRIFT group as compared with the standard treatment group (44% vs. 50%); moreover, a high incidence of secondary IVH (35%) was reported [67].
Of note, subsequent follow-up studies revealed favorable results for neurodevelopmental outcomes, particularly cognitive function, at 2 and 10 years [35,68]. The DRIFT trial, designed to maintain low intracranial pressure, alleviate brain distortion, and reduce the increased levels of free iron and cytokines caused by IVH, was the first clinical trial involving intervention for PHH in preterm infants that demonstrated sustained cognitive improvement [35,68]. However, secondary IVH develops at a high rate, and it is impossible to perform fibrinolytic therapy with the same regimen. A new fibrinolytic therapy using urokinase instead of tPA has been reported, and future development is expected [43].
Recently, neuroendoscopic lavage (NEL) has been reported to be another promising treatment modality [9,16,49,57]. The greatest advantage of NEL is direct visualization of the progress of hematoma removal and control of bleeding sites. Residual hematoma and floating degradation products can be removed by irrigation. In the first report, the average body weight at birth was 1036 g (500–3460 g), and the average weight at surgery was 1475 g (750–3645 g) [49]. Regarding the severity of IVH, two patients had IVH grade 2, 12 patients had IVH grade 3, and three patients had IVH grade 4. Eleven of 19 (58%) infants required a shunt insertion later compared with 100% of infants who were treated conventionally. The number of patients studied was increased to 45, and neurocognitive results 2 years after treatment were reported. Although 26 of 44 patients (59%) required permanent shunt placement, 30% of patients revealed a fairly normal neurocognitive development, and 78% were able to walk independently or with minimal support [9].
Undoubtedly, NEL is the most effective method for removing hematoma but should be carefully performed and requires neuroendoscopic expertise and an advanced surgical team. NEL might be highly invasive and unsafe in the case of extremely small infants. Therefore, in my opinion, NEL should be adopted only in cases where the infant’s weight is at least 1500 g [42]. Currently, the TROPHY (treatment of post-hemorrhagic hydrocephalus registry study) registry has been established to collect international multicenter prospective data on the surgical management of neonatal PHH, and the results of its large-data clinical studies are awaited [55,56].
In the past, the main goal of treatment for IVH and subsequent PHH was only to reduce mortality as the target patient was a fragile premature infant. The current standard treatment goal is to perform permanent VP shunt placement safely after temporary CSF management. However, pediatric neurosurgeons have experienced numerous troublesome complications related to VP shunts in preterm infants. In particular, shunt infection is devastating to the brain, and isolated ventricle is a peculiar complication that is difficult to treat. Therefore, next, a therapeutic strategy to avoid permanent VP shunt is desired [14,42,43]. “No shunt is the best shunt.”
Furthermore, it is important to consider ways to improve neurological functions in premature infants with IVH. To that end, a thorough understanding of the mechanisms of WMI caused by IVH and subsequent PHVD, as well as therapeutic guidelines for safe and effective early interventions for actively removing hematomas, are essential. On the other hand, the results of innovative regenerative treatments that promote myelination and neurological recovery, such as mesenchymal stem cell transplantation therapy reported from Korea, are eagerly awaited [2-4]. Stem cell therapy might become a promising treatment for moderate or severe IVH in preterm infants [8].
Treatment of IVH in preterm infants remains an insurmountable barrier. A favorable neurodevelopmental outcome cannot be achieved with a neurosurgical approach alone. Further progress is eagerly awaited in collaboration with multidisciplinary specialists, including neonatologists and rehabilitation physicians.
ACKNOWLEDGMENTS
I would like to express my sincere gratitude to Andrew Whitelaw (Professor Emeritus, Bristol University) for his valuable advice and convey my deepest respect for his outstanding and excellent work over the years.
References
1. Adams-Chapman I, Hansen NI, Stoll BJ, Higgins R; NICHD Research Network. Neurodevelopmental outcome of extremely low birth weight infants with posthemorrhagic hydrocephalus requiring shunt insertion. Pediatrics. 121:e1167–e1177. 2008.

2. Ahn SY, Chang YS, Park WS. Mesenchymal stem cells transplantation for neuroprotection in preterm infants with severe intraventricular hemorrhage. Korean J Pediatr. 57:251–256. 2014.

3. Ahn SY, Chang YS, Sung SI, Park WS. Mesenchymal stem cells for severe intraventricular hemorrhage in preterm infants: phase I doseescalation clinical trial. Stem Cells Transl Med. 7:847–856. 2018.

4. Ahn SY, Sung DK, Kim YE, Sung S, Chang YS, Park WS. Brain-derived neurotropic factor mediates neuroprotection of mesenchymal stem cellderived extracellular vesicles against severe intraventricular hemorrhage in newborn rats. Stem Cells Transl Med. 10:374–384. 2021.

5. Back SA, Gan X, Li Y, Rosenberg PA, Volpe JJ. Maturation-dependent vulnerability of oligodendrocytes to oxidative stress-induced death caused by glutathione depletion. J Neurosci. 18:6241–6253. 1998.

6. Back SA, Luo NL, Borenstein NS, Levine JM, Volpe JJ, Kinney HC. Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J Neurosci. 21:1302–1312. 2001.

7. Badhiwala JH, Hong CJ, Nassiri F, Hong BY, Riva-Cambrin J, Kulkarni AV. Treatment of posthemorrhagic ventricular dilation in preterm infants: a systematic review and meta-analysis of outcomes and complications. J Neurosurg Pediatr. 16:545–555. 2015.

8. Ballabh P, de Vries LS. White matter injury in infants with intraventricular haemorrhage: mechanisms and therapies. Nat Rev Neurol. 17:199–214. 2021.

9. Behrens P, Tietze A, Walch E, Bittigau P, Bührer C, Schulz M, et al. Neurodevelopmental outcome at 2 years after neuroendoscopic lavage in neonates with posthemorrhagic hydrocephalus. J Neurosurg Pediatr. 26:495–503. 2020.

10. Bell EF, Hintz SR, Hansen NI, Bann CM, Wyckoff MH, DeMauro SB, et al. Mortality, in-hospital morbidity, care practices, and 2-year outcomes for extremely preterm infants in the US, 2013-2018. JAMA. 327:248–263. 2022.

11. Bolisetty S, Dhawan A, Abdel-Latif M, Bajuk B, Stack J, Lui K, et al. Intraventricular hemorrhage and neurodevelopmental outcomes in extreme preterm infants. Pediatrics. 133:55–62. 2014.

12. Brouwer AJ, Groenendaal F, Benders MJ, de Vries LS. Early and late complications of germinal matrix-intraventricular haemorrhage in the preterm infant: what is new? Neonatology. 106:296–303. 2014.

13. Brouwer AJ, van Stam C, Uniken Venema M, Koopman C, Groenendaal F, de Vries LS. Cognitive and neurological outcome at the age of 5-8 years of preterm infants with post-hemorrhagic ventricular dilatation requiring neurosurgical intervention. Neonatology. 101:210–216. 2012.

14. Chari A, Mallucci C, Whitelaw A, Aquilina K. Intraventricular haemorrhage and posthaemorrhagic ventricular dilatation: moving beyond CSF diversion. Childs Nerv Syst. 37:3375–3383. 2021.

15. Christian EA, Melamed EF, Peck E, Krieger MD, McComb JG. Surgical management of hydrocephalus secondary to intraventricular hemorrhage in the preterm infant. J Neurosurg Pediatr. 17:278–284. 2016.

16. d’Arcangues C, Schulz M, Bührer C, Thome U, Krause M, Thomale UW. Extended experience with neuroendoscopic lavage for posthemorrhagic hydrocephalus in neonates. World Neurosurg. 116:e217–e224. 2018.

17. Deger J, Goethe EA, LoPresti MA, Lam S. Intraventricular hemorrhage in premature infants: a historical review. World Neurosurg. 153:21–25. 2021.

18. de Vries LS, Groenendaal F, Liem KD, Heep A, Brouwer AJ, van ‘t Verlaat E, et al. Treatment thresholds for intervention in posthaemorrhagic ventricular dilation: a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 104:F70–F75. 2019.

19. de Vries LS, Liem KD, van Dijk K, Smit BJ, Sie L, Rademaker KJ, et al. Early versus late treatment of posthaemorrhagic ventricular dilatation: results of a retrospective study from five neonatal intensive care units in the Netherlands. Acta Paediatr. 91:212–217. 2002.

20. Dorner RA, Burton VJ, Allen MC, Robinson S, Soares BP. Preterm neuroimaging and neurodevelopmental outcome: a focus on intraventricular hemorrhage, post-hemorrhagic hydrocephalus, and associated brain injury. J Perinatol. 38:1431–1443. 2018.

21. El-Dib M, Limbrick DD Jr, Inder T, Whitelaw A, Kulkarni AV, Warf B, et al. Management of post-hemorrhagic ventricular dilatation in the infant born preterm. J Pediatr. 226:16–27.e3. 2020.
22. Fountain DM, Chari A, Allen D, James G. Comparison of the use of ventricular access devices and ventriculosubgaleal shunts in posthaemorrhagic hydrocephalus: systematic review and meta-analysis. Childs Nerv Syst. 32:259–267. 2016.

23. Franz AP, Bolat GU, Bolat H, Matijasevich A, Santos IS, Silveira RC, et al. Attention-deficit/hyperactivity disorder and very preterm/very low birth weight: a meta-analysis. Pediatrics. 141:e20171645. 2018.

24. Hintz SR, Kendrick DE, Vohr BR, Poole WK, Higgins RD; National Institute of Child Health and Human Development Neonatal Research Network. Changes in neurodevelopmental outcomes at 18 to 22 months’ corrected age among infants of less than 25 weeks’ gestational age born in 1993-1999. Pediatrics. 115:1645–1651. 2005.

25. Hollebrandse NL, Spittle AJ, Burnett AC, Anderson PJ, Roberts G, Doyle LW, et al. School-age outcomes following intraventricular haemorrhage in infants born extremely preterm. Arch Dis Child Fetal Neonatal Ed. 106:4–8. 2021.

26. Inder TE, Perlman JM, Volpe JJ : Preterm Intraventricular Hemorrhage/Posthemorrhagic Hydrocephalus in Volpe JJ, Inder TE, Barra BT, de Vries LS, du Plessis AJ, Neil JJ, et al. (eds) : Volpe’s Neurology of the Newborn (Sixth Edition). Philadelphia : Elsevier, 2018, pp637-698.
27. Indredavik MS, Skranes JS, Vik T, Heyerdahl S, Romundstad P, Myhr GE, et al. Low-birth-weight adolescents: psychiatric symptoms and cerebral MRI abnormalities. Pediatr Neurol. 33:259–266. 2005.

28. International PHVD Drug Trial Group. International randomised controlled trial of acetazolamide and furosemide in posthaemorrhagic ventricular dilatation in infancy. Lancet. 352:433–440. 1998.
29. Kennedy CR, Ayers S, Campbell MJ, Elbourne D, Hope P, Johnson A. Randomized, controlled trial of acetazolamide and furosemide in posthemorrhagic ventricular dilation in infancy: follow-up at 1 year. Pediatrics. 108:597–607. 2001.

30. Kusuda S, Fujimura M, Sakuma I, Aotani H, Kabe K, Itani Y, et al. Morbidity and mortality of infants with very low birth weight in Japan: center variation. Pediatrics. 18:e1130–e1138. 2006.

31. Langley EA, Blake SM, Coe KL. Recent review of germinal matrix hemorrhage-intraventricular hemorrhage in preterm infants. Neonatal Netw. 41:100–106. 2022.
32. Levene MI. Measurement of the growth of the lateral ventricles in preterm infants with real-time ultrasound. Arch Dis Child. 56:900–904. 1981.

33. Limbrick DD Jr, de Vries LS. New insights into the management of posthemorrhagic hydrocephalus. Semin Perinatol. 46:151597. 2022.

34. Limbrick DD Jr, Mathur A, Johnston JM, Munro R, Sagar J, Inder T, et al. Neurosurgical treatment of progressive posthemorrhagic ventricular dilation in preterm infants: a 10-year single-institution study. J Neurosurg Pediatr. 6:224–230. 2010.

35. Luyt K, Jary SL, Lea CL, Young GJ, Odd DE, Miller HE, et al. Drainage, irrigation and fibrinolytic therapy (DRIFT) for posthaemorrhagic ventricular dilatation: 10-year follow-up of a randomised controlled trial. Arch Dis Child Fetal Neonatal Ed. 105:466–473. 2020.

36. Mazzola CA, Choudhri AF, Auguste KI, Limbrick DD Jr, Rogido M, Mitchell L, et al. Pediatric hydrocephalus: systematic literature review and evidence-based guidelines. Part 2: management of posthemorrhagic hydrocephalus in premature infants. J Neurosurg Pediatr 14 Suppl. 1:8–23. 2014.

37. McClugage SG, Laskay NMB, Donahue BN, Arynchyna A, Zimmerman K, Aban IB, et al. Functional outcomes at 2 years of age following treatment for posthemorrhagic hydrocephalus of prematurity: what do we know at the time of consult? J Neurosurg Pediatr. 14:1–9. 2020.

38. Murphy BP, Inder TE, Rooks V, Taylor GA, Anderson NJ, Mogridge N, et al. Posthaemorrhagic ventricular dilatation in the premature infant: natural history and predictors of outcome. Arch Dis Child Fetal Neonatal Ed. 87:F37–F41. 2002.

39. Nosarti C, Giouroukou E, Micali N, Rifkin L, Morris RG, Murray RM. Impaired executive functioning in young adults born very preterm. J Int Neuropsychol Soc. 13:571–581. 2007.

40. O’Shea TM, Allred EN, Kuban KC, Hirtz D, Specter B, Durfee S, et al. Intraventricular hemorrhage and developmental outcomes at 24 months of age in extremely preterm infants. J Child Neurol. 27:22–29. 2012.

41. Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 92:529–534. 1978.

42. Park YS. Treatment strategies and challenges to avoid cerebrospinal fluid shunting for pediatric hydrocephalus. Neurol Med Chir (Tokyo). 62:416–430. 2022.

43. Park YS, Kotani Y, Kim TK, Yokota H, Sugimoto T, Nakagawa I, et al. Efficacy and safety of intraventricular fibrinolytic therapy for postintraventricular hemorrhagic hydrocephalus in extreme low birth weight infants: a preliminary clinical study. Childs Nerv Syst. 37:69–79. 2021.

44. Rees P, Callan C, Chadda KR, Vaal M, Diviney J, Sabti S, et al. Preterm brain injury and neurodevelopmental outcomes: a meta-analysis. Pediatrics. 150:e2022057442. 2022.

45. Riva-Cambrin J, Shannon CN, Holubkov R, Whitehead WE, Kulkarni AV, Drake J, et al. Center effect and other factors influencing temporization and shunting of cerebrospinal fluid in preterm infants with intraventricular hemorrhage. J Neurosurg Pediatr. 9:473–481. 2012.

46. Robinson S. Neonatal posthemorrhagic hydrocephalus from prematurity: pathophysiology and current treatment concepts. J Neurosurg Pediatr. 9:242–258. 2012.

47. Savman K, Nilsson UA, Blennow M, Kjellmer I, Whitelaw A. Non-protein-bound iron is elevated in cerebrospinal fluid from preterm infants with posthemorrhagic ventricular dilatation. Pediatr Res. 49:208–212. 2001.

48. Sävman K, Blennow M, Hagberg H, Tarkowski E, Thoresen M, Whitelaw A. Cytokine response in cerebrospinal fluid from preterm infants with posthaemorrhagic ventricular dilatation. Acta Paediatr. 91:1357–1363. 2002.

49. Schulz M, Bührer C, Pohl-Schickinger A, Haberl H, Thomale UW. Neuroendoscopic lavage for the treatment of intraventricular hemorrhage and hydrocephalus in neonates. J Neurosurg Pediatr. 13:626–635. 2014.

50. Shooman D, Portess H, Sparrow O. A review of the current treatment methods for posthaemorrhagic hydrocephalus of infants. Cerebrospinal Fluid Res. 6:1. 2009.

51. Spader HS, Hertzler DA, Kestle JR, Riva-Cambrin J. Risk factors for infection and the effect of an institutional shunt protocol on the incidence of ventricular access device infections in preterm infants. J Neurosurg Pediatr. 15:156–160. 2015.

52. Stoll BJ, Hansen NI, Bell EF, Walsh MC, Carlo WA, Shankaran S, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA. 314:1039–1051. 2015.

53. Szpecht D, Wiak K, Braszak A, Szymankiewicz M, Gadzinowski J. Role of selected cytokines in the etiopathogenesis of intraventricular hemorrhage in preterm newborns. Childs Nerv Syst. 32:2097–2103. 2016.

54. Taylor HG, Minich N, Bangert B, Filipek PA, Hack M. Long-term neuropsychological outcomes of very low birth weight: associations with early risks for periventricular brain insults. J Int Neuropsychol Soc. 10:987–1004. 2004.

55. Thomale UW, Auer C, Spennato P, Schaumann A, Behrens P, Gorelyshev S, et al. TROPHY registry - status report. Childs Nerv Syst. 37:3549–3554. 2021.

56. Thomale UW, Cinalli G, Kulkarni AV, Al-Hakim S, Roth J, Schaumann A, et al. TROPHY registry study design: a prospective, international multicenter study for the surgical treatment of posthemorrhagic hydrocephalus in neonates. Childs Nerv Syst. 35:613–619. 2019.

57. Tirado-Caballero J, Rivero-Garvia M, Arteaga-Romero F, Herreria-Franco J, Lozano-Gonzalez Á, Marquez-Rivas J. Neuroendoscopic lavage for the management of posthemorrhagic hydrocephalus in preterm infants: safety, effectivity, and lessons learned. J Neurosurg Pediatr. 26:237–246. 2020.

58. Valcamonico A, Accorsi P, Sanzeni C, Martelli P, La Boria P, Cavazza A, et al. Mid- and long-term outcome of extremely low birth weight (ELBW) infants: an analysis of prognostic factors. J Matern Fetal Neonatal Med. 20:465–471. 2007.

59. Ventriculomegaly Trial Group. Randomised trial of early tapping in neonatal posthaemorrhagic ventricular dilatation. Arch Dis Child. 65(1 Spec No):3–10. 1990.
60. Volpe JJ. Intraventricular hemorrhage in the premature infant--current concepts. Part I. Ann Neurol. 25:3–11. 1989.

61. Volpe JJ. Intraventricular hemorrhage in the premature infant--current concepts. Part II. Ann Neurol. 25:109–116. 1989.

62. Wang JY, Amin AG, Jallo GI, Ahn ES. Ventricular reservoir versus ventriculosubgaleal shunt for posthemorrhagic hydrocephalus in preterm infants: infection risks and ventriculoperitoneal shunt rate. J Neurosurg Pediatr. 14:447–454. 2014.

63. Wellons JC, Shannon CN, Kulkarni AV, Simon TD, Riva-Cambrin J, Whitehead WE, et al. A multicenter retrospective comparison of conversion from temporary to permanent cerebrospinal fluid diversion in very low birth weight infants with posthemorrhagic hydrocephalus. J Neurosurg Pediatr. 4:50–55. 2009.

64. Wellons JC 3rd, Shannon CN, Holubkov R, Riva-Cambrin J, Kulkarni AV, Limbrick DD Jr, et al. Shunting outcomes in posthemorrhagic hydrocephalus: results of a Hydrocephalus Clinical Research Network prospective cohort study. J Neurosurg Pediatr. 20:19–29. 2017.

65. Whitaker AH, Feldman JF, Lorenz JM, McNicholas F, Fisher PW, Shen S, et al. Neonatal head ultrasound abnormalities in preterm infants and adolescent psychiatric disorders. Arch Gen Psychiatry. 68:742–752. 2011.

66. Whitelaw A, Aquilina K. Management of posthaemorrhagic ventricular dilatation. Arch Dis Child Fetal Neonatal Ed. 97:F229–3. 2012.

67. Whitelaw A, Evans D, Carter M, Thoresen M, Wroblewska J, Mandera M, et al. Randomized clinical trial of prevention of hydrocephalus after intraventricular hemorrhage in preterm infants: brain-washing versus tapping fluid. Pediatrics. 119:e1071–e1078. 2007.

68. Whitelaw A, Jary S, Kmita G, Wroblewska J, Musialik-Swietlinska E, Mandera M, et al. Randomized trial of drainage, irrigation and fibrinolytic therapy for premature infants with posthemorrhagic ventricular dilatation: developmental outcome at 2 years. Pediatrics. 125:e852–e858. 2010.

Fig. 1.
Typical views of neuroendoscopic surgery for post hemorrhagic hydrocephalus. A : Numerous hemosiderin deposits on the ventricular wall. B : Obstruction of the Sylvian aqueduct (arrow).
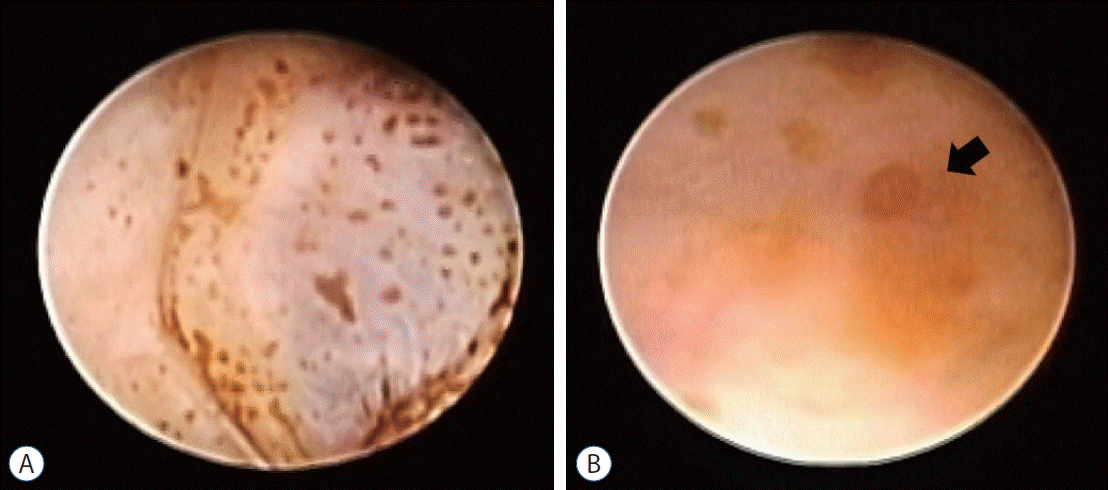
Fig. 2.
A case of progressive ventricular dilatation after intraventricular hemorrhage (IVH). A female infant was born at 23 weeks 3 days of gestation and weighed 487 g. A : IVH occurred on day 4 of birth. B : Ultrasonography on postnatal day 14. Severe ventricular dilation developed during just 10 days. C : On day 20, the cerebral mantle became paper thin. The progressive accumulation of cerebrospinal fluid changed the shape of the lateral ventricles to balloon-like.
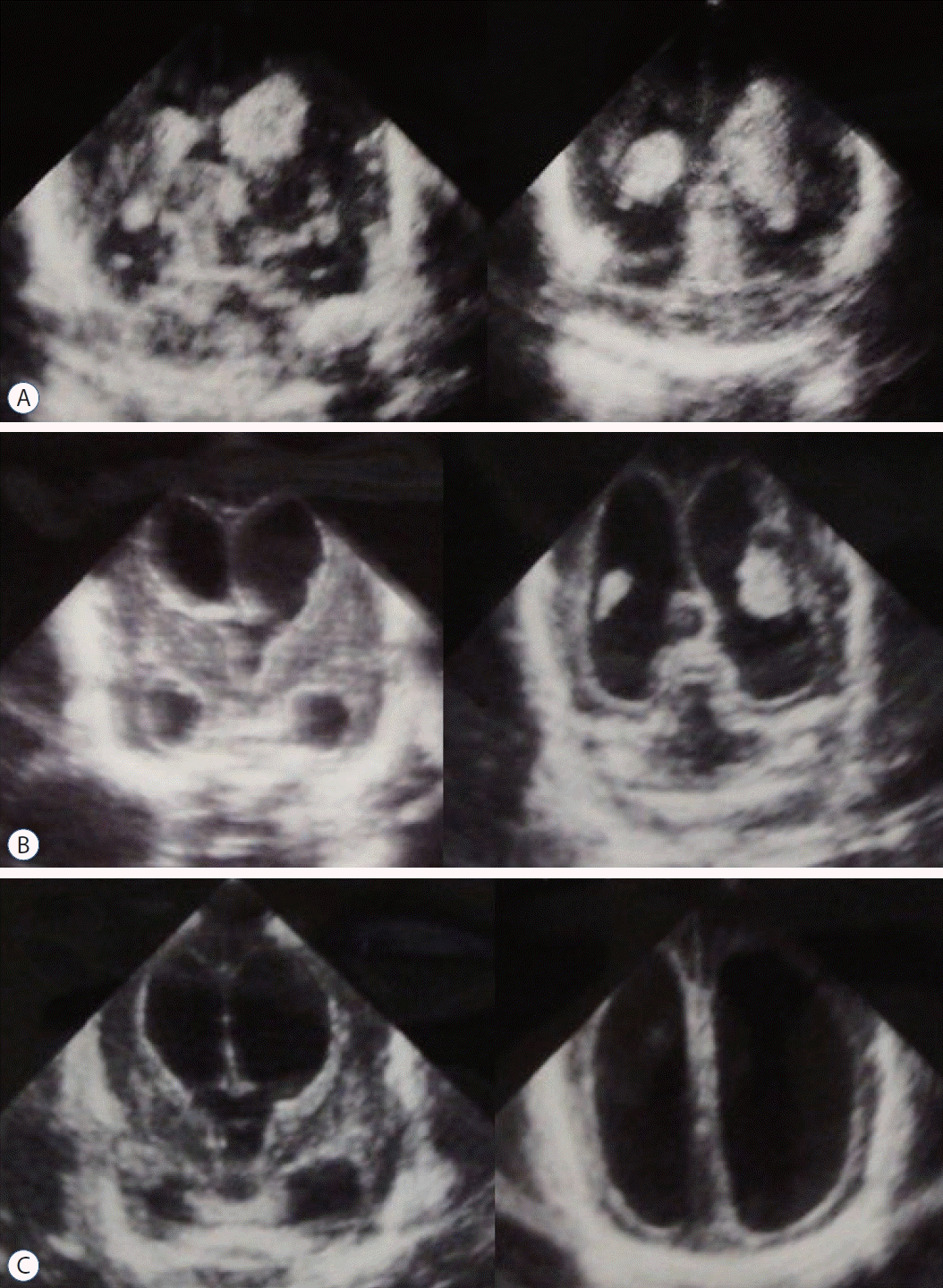
Fig. 3.
Various ventricular measurements on ultrasonography. Ventricular index (VI) is the distance between the lateral wall of the anterior horn of the lateral ventricle and the falx cerebri in the coronal view at the level of the foramen of Monro, just anterior to the choroid plexus in the third ventricle. Anterior horn width (AHW) is the oblique width of the anterior horn of the lateral ventricle at the widest point in the coronal plane. Thalamo-occipital distance (TOD) is the distance between the external walls of the thalamus and lateral border of the occipital horn of the lateral ventricle in parasagittal plane. A : Coronal view. B : Sagittal view.
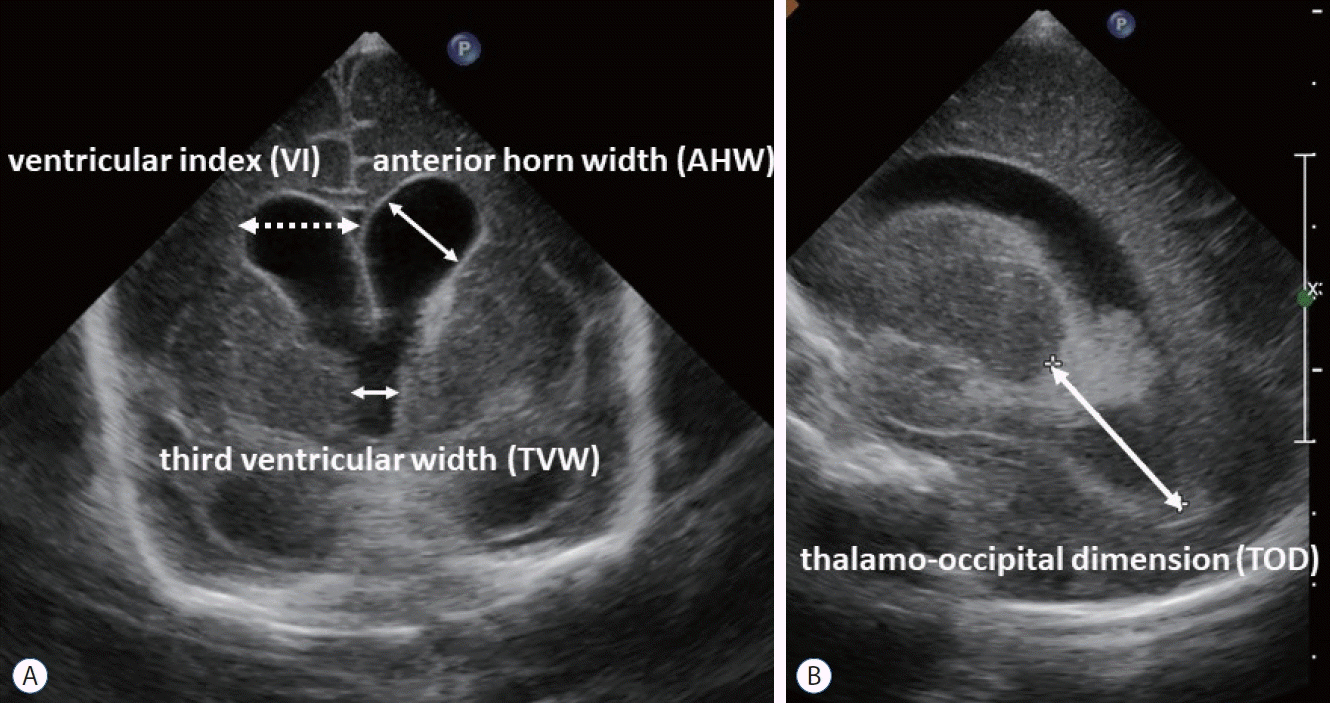
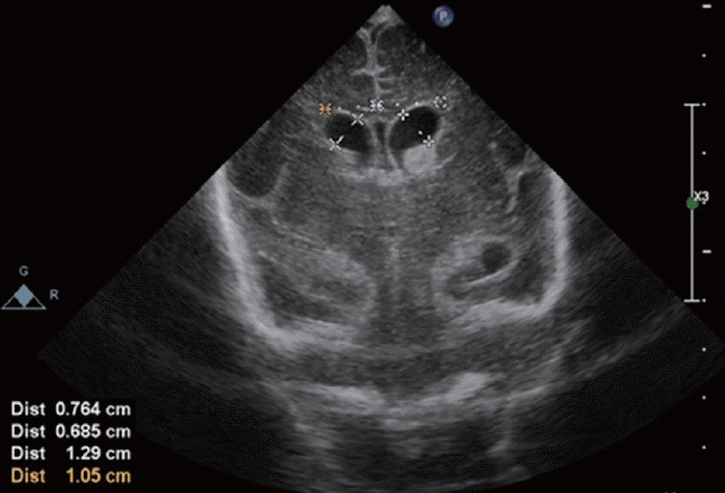
Fig. 4.
A male infant was born at 23 weeks 4 days of gestation and weighed 501 g. Intraventricular hemorrhage occurred on day 3 of birth. Ultrasonography on postnatal day 8 revealed a ventricular index : 10.5 mm (right) and 12.9 mm (left), anterior horn width : 6.85 mm (right) and 7.64 mm (left). Neuroendoscopic surgery at this stage is dangerous and invasive. Other treatments need to be initiated to obtain favorable neurofunctional outcomes.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download