INTRODUCTION
ULTRASONOGRAPHIC DIAGNOSIS OF GM-IVH
Techniques in cranial ultrasound
Timing of cranial ultrasound
Grading of GM-IVH
Table 1.
Cranial ultrasound findings of GM-IVH
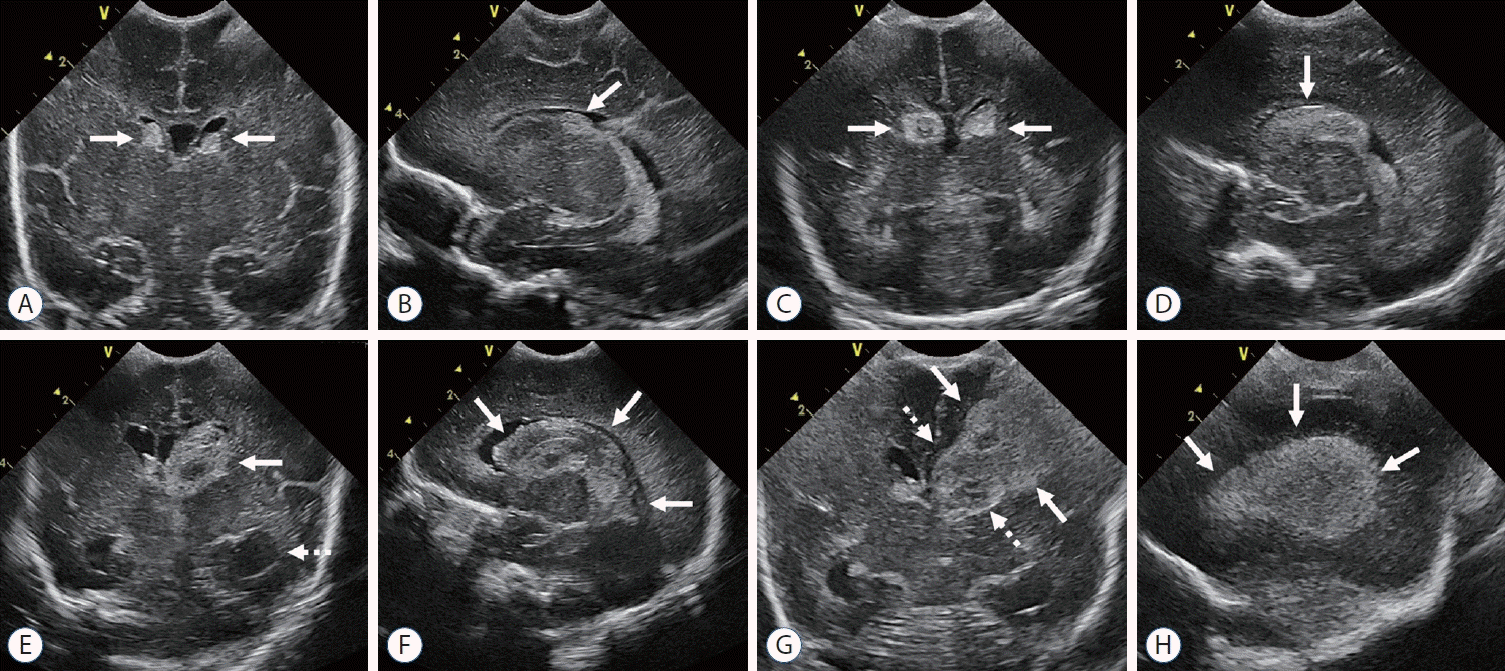 | Fig. 1.Cranial ultrasound grading of germinal matrix and intraventricular hemorrhage (GM-IVH). A and B : Grade 1 GM-IVH. Coronal scan and parasagittal scans show echogenic globular hemorrhage (arrows) at the bilateral caudothalamic groove, a typical location of GMH. C and D : Grade 2 GM-IVH. Bilateral IVH (arrows) extending anteriorly to the caudothalamic groove occupy more than 10% of ventricle without ventricular dilatation. E and F : Grade 3 GM-IVH. Coronal scan shows left IVH (arrow) with ventricular dilatation (dashed arrow). Parasagittal scan shows IVH (arrows) occupying more than 50% of ventricle. G and H : Grade 4 GM-IVH. Coronal and left parasagittal scans show fan-shaped echogenic periventricular hemorrhagic infarction (arrows) with ipsilateral grade III IVH (dashed arrows). |
 | Fig. 2.Cystic degeneration of grade II germinal matrix and intraventricular hemorrhage (GM-IVH) in preterm infant (gestational age gestational age 26+2 weeks). Coronal (A) and right parasagittal (B) scans of cranial ultrasound show heterogeneous hematoma (arrow) at caudothalamic groove with cystic degeneration (arrow). C and D : Magnetic resonance imaging at term-equivalent age, 4 months later. Axial T2-weighted image (C) shows cysts with low signal intensity rim (white arrows) at bilateral caudothalamic groove and low signal intensity along bilateral choroid plexus (black arrows) indicate previous hemorrhage. Susceptibility-weighted imaging (D) shows linear dark signal intensity (black arrows) along wall of bilateral occipital horns of lateral ventricles, indicating minimal IVH, not detectable at cranial ultrasound. |
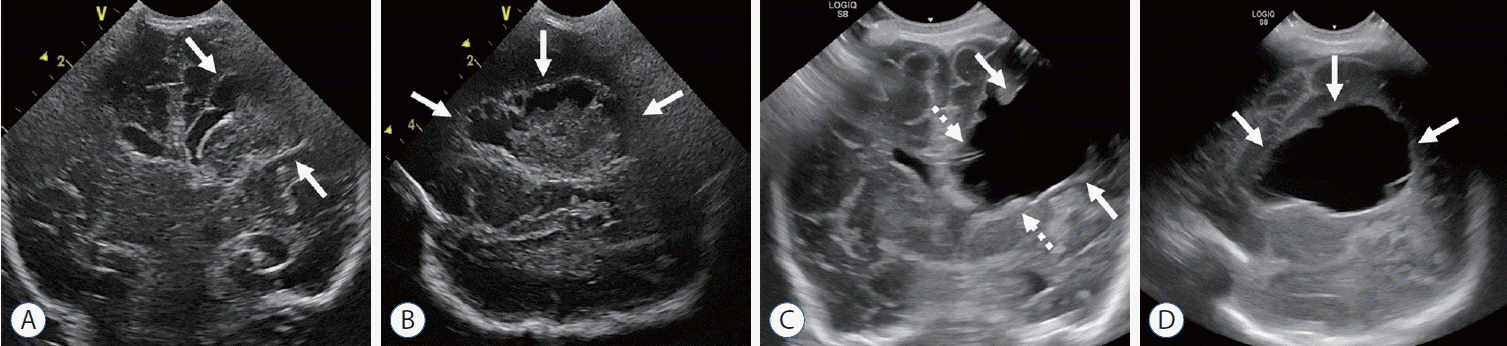 | Fig. 3.Porencephalic cyst after periventricular hemorrhagic infarction (PVHI). Coronal (A) and left parasagittal (B) scans at 10 weeks of age show focal cystic change (arrows) of PVHI. Follow up coronal (C) and left parasagittal (D) scans at 4 months of age show large porencephalic cyst (arrows) at the site of PVHI with dilatation of left lateral ventricle (dashed arrows). |
MAGNETIC RESONANCE IMAGING OF GM-IVH
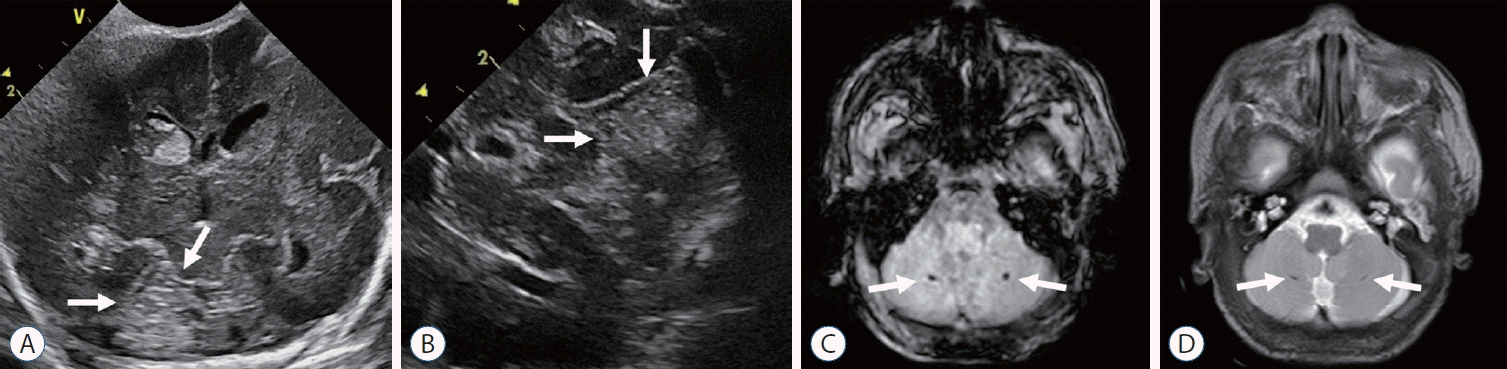 | Fig. 4.Cerebellar hemorrhage identified on cranial ultrasound (A and B) and brain magnetic resonance imaging (C and D) in preterm infants with germinal matrix and intraventricular hemorrhage. Coronal (A) and trans-mastoid (B) scans show intraparenchymal hemorrhage (arrows) in the right cerebellar hemisphere. Axial T2-weighted image (C) and susceptibility-weighted imaging (D) at term-equivalent age of other preterm infant show punctate microhemorrhages (arrows) in cerebellum, not detectable at cranial ultrasound. |
POSTHEMORRHAGIC VENTRICULAR DILATATION
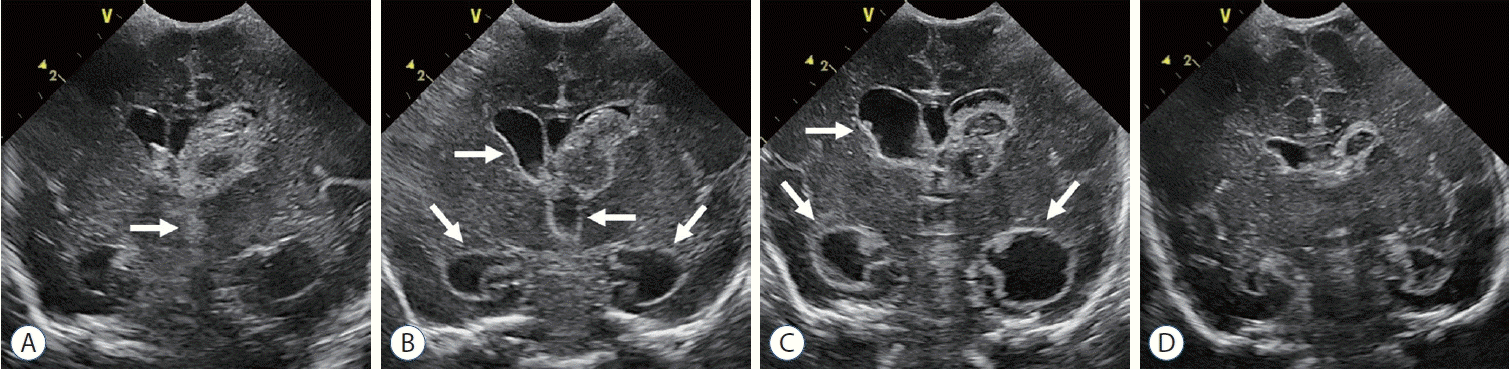 | Fig. 5.The natural history of posthemorrhagic ventricular dilation in premature infant (gestational age 29+1 weeks). A : Coronal scan on day 2 shows right grade III and left grade IV germinal matrix and intraventricular hemorrhage (GM-IVH). Echogenic hemorrhage in third ventricle (arrow) and both lateral ventricles. B : Echogenic ependymal lining (arrows) and ventricular dilatation begins 1 week after GM-IVH. C : Coronal scan on month 1 shows progression of ventricular dilatation (arrows). D : Coronal scan on month 2 shows spontaneous improvement in ventricular dilatation without treatment. |
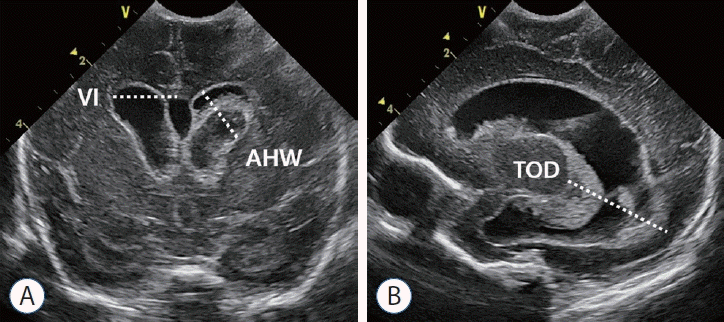
Ventricle measurements
Role of cranial ultrasound in PHVD
 | Fig. 7.Response of posthemorrhagic ventricular dilatation to ventricular drainage in premature infant. Serial cranial ultrasound images before (A) and after (B) drainage device insertion show change in degree of ventricular dilatation (bidirectional arrows) after procedure. C and D : Coronal scans show the drainage device (arrow). |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download