Abstract
Post-hemorrhagic hydrocephalus (PHH) in preterm infant is common, life-threatening and the main cause of bad developmental outcomes. Ventriculoperitoneal (VP) shunt is used as the ultimate treatment for PHH. Low birth weight and low gestational age are the combination of worse prognostic factors while the single most important prognostic factor of VP shunting is age. Aggressive and early intervention have better effect in intraventricular hemorrhage and intracranial pressures control. It reduces infection rate and brain damage resulted in delayed shunt insertion. It is extremely important to let PHH infants get older and gain weight to have internal organs to be matured before undergoing VP shunt. As premature infants undergo shunt after further growth, shunt-related complications would be reduced. So temporary surgical intervention is critical for PHH infants to have them enough time until permanently shunted.
Intraventricular hemorrhage (IVH) is very common in preterm infants, and the infants with severe IVH develop posthemorrhagic hydrocephalus (PHH). It is important that proper care is given with the possibility of IVH at all times when preterm infants are born, since PHH leads to neurodevelopmental disorders and high mortality rates in the long term. Low gestational age (GA) and low body weight are important risk factors for IVH and also affect treatment outcomes of IVH and related PHH [32].
IVH is divided into four grades according to the amount of bleeding which is mainly based on the bedside cranial ultrasound of infants. Grade I IVH is the bleeding occurred only in germinal matrix or minimal part of the ventricle. Grade II IVH occurs inside the ventricle. Grade III IVH accumulates more blood in the ventricle, causing it to be enlarged. Grade IV IVH is the state that bleeding extends into the brain parenchyma near the ventricle and causes periventricular infarction. Grades I and II are common and usually have no serious complications. Grades III and especially IV are much more critical, which can lead to long term brain injury [14,26].
Treatments for IVH include temporary cerebrospinal fluid (CSF) diversions such as lumbar or ventricular punctures and external ventricular drainage (EVD), and permanent ventriculoperitoneal (VP) shunts. Infants with very low birth weight (VLBW), low GA, or high IVH grades usually require permanent VP shunts [32]. Depending on the current condition, such as the weight of infants, the insertion of VP shunt may be done immediately, or be delayed by temporary procedures [5].
There is no evidence-based consensus for the optimal initial treatment or subsequent management strategy. Clinical signs and symptoms, increasing head circumference, and imaging criteria (size of ventricle, density and/or volume of blood clot) are the traditional bases of decision-making. Relatively low body weight, large volume of IVH, high CSF protein levels, and comorbidities such as neonatal necrotizing enterocolitis, cannot bear permanent shunting. Child should undergo temporary management until permanent shunting can be applicable. The published rates of using permanent shunt after temporary management are variable between over 50% and 85% [15,20,31].
Premature infants with low immunity are at high risk of infection which can be occurred at any time during the period of temporary treatment and also after the shunt insertion. Two major causes of early shunt revision (<1 year) are shunt infection and blockage [18]. To reduce these complications, more sophisticated pre-shunt treatment is needed. Also, since early insertion of VP shunt is related with high failure rate and complications rate, it is better to delay inserting VP shunt by using effective temporary treatment until the infants become mature [40].
Major factors that affect IVH to become PHH are volume of intraventricular blood clot, and floating extracellular matrix produced from inflammatory reaction [24]. Removal of blood clot and extracellular matrix are important for restablishing CSF circulation and reducing intracranial pressure (ICP).
The infant who has grade III or IV IVH and fronto-occipital horn ratio ≥0.55 should be treated. The expected life expectancy of the infant should be more than 72 hours [38]. Less than 1800 g of body weight, extra-choroidal IVH, sepsis and/or CSF infection and unable to use abdomen are criteria for temporary managements. 1800 to 2000 g body weight is debating interval between temporary and permanent managements. The types of temporary management are listed below.
LP removes CSF from lower back by direct puncture of lumbar cistern by a needle. Repeated LP tend to remove only a small amount of CSF. In a systemic review, routine use of serial LP did not show a benefit over the reduction of disability, death, or permanent shunt [39]. LP may be useful only as an immediate removal of CSF for sampling or temporarily lower the increased ICP [8].
Ventricular tapping removes CSF by direct puncture of lateral ventricle by a needle. There is no evidence to support the beneficial effect of repeated CSF removal by direct ventricular tapping. Ventricular tapping did not reduce disability, death, or permanent shunt according to a review [39]. LP or ventricular tapping should only be used in limited conditions, such as raised ICP for a short period time until the insertion of a ventricular access device (VAD), ventriculo-subgaleal shunt (VSGS) or EVD. Repeated transcortical ventricle puncture can result in complications such as intracranial hemorrhage or cystic encephalomalacia.
A catheter that leads to the lateral ventricle and is attached to a reservoir implanted under the scalp can remove CSF directly from the lateral ventricle via fontanelle taping [39]. CSF should be removed 1 mL/min (10 mL/kg) with aseptic techniques to decrease ICP slowly. At least two of cranial ultrasonography a week should be performed to determine how much fluid should be removed. The aim is to reduce the ventricular index to the 97th percentile over 7–10 days [24]. Hydrocephalus Clinical Research Network (HCRN) reported the permanent shunt rate of 69% in a 147-patient retrospective non-randomized cohort who received VAD. Complications of the reservoir, such as CSF leak, infection, or possible skin breakdown, are reported up to 22% of cases [20,29]. Intermittent tapping of reservoir allows CSF to build up so that natural CSF absorption system can be challenged. Evidence-based review recommend VADs over EVD to reduce morbidity and mortality [25].
HCRN published the permanent shunt rate of 86% for those who received VSGS in a 147-patient retrospective non-randomized cohort. HCRN concluded that VAD appears to lead to a lower rate of permanent shunt, but this initial result was denoted by institutional bias. So, HCRN designed a standardized prospective study called SOPHH (Shunting Outcomes in Post-Hemorrhagic Hydrocephalus) to investigate the efficacy of VAD and VSGS [38]. Inclusion criteria were VLBW (<1500 g) premature infants with grade III or IV IVH. The primary outcome was the conversion of either a ventricular reservoir (VR) or VSGS to a permanent shunt within 6 months of the temporization procedure. Secondary outcomes for the purposes of the primary study were the rates of complications, including infection, CSF leak, death, or new intracranial hemorrhage. One hundred forty-five premature infants were enrolled from six centers, and 102 infants were included for outcome analysis. There is no difference in 180-day rate of conversion to CSF shunt (63.5% for VSGS and 74.0% for VAD; p=0.36, log-rank test) or infection rate (VSGS was 14% [5/36] and for VR was 17% [11/66; p=0.71]) [38]. VSGSs is a kind of closed system without repeated tapping and also challenge the natural CSF absorption system. Additionally, VSGS can reduce the daily CSF aspiration compared with VADs. However, this system can be used for an average of 37 days and then should be revised. Second VSGS functions for 32 days after the first revision. Pocket contraction and/or more than 3 months of VSGS will be an indication of revision of VSGS. If there are still extra-choroidal IVH, unusable abdomen, and a body weight of less than 1800 g, VSGS is indicated to be revised. VAD and VSGS are similar but physiologically different. The decision to choose VAS or VSGS is based on personal training and/or institutional policy [25].
EVD is one of the useful treatment options for PHH. By using EVD, the permanent shunt rate can be reduced to less than 50% of preterm infants of PHH [6,21,30]. Continuous controlled removal of CSF can reduce ventricle size and ICP as well as remove blood clot and protein materials. With EVD, repeated skin puncture to remove CSF are not necessary. Transcranial ultrasonography once or twice a week is still needed to monitor the ventricle’s status and the size of blood clots. The primary complication of EVD is an infection rate of 0% to 45%. The origin of EVD-related infections may be the inoculation of skin flora, contamination of the drainage system, or retrograde infection [6]. Antibiotic-impregnated EVD has a similar infection rate without increasing the risk of ventriculostomy-associated infection up to 2 months [9]. IVH is one of the risk factors of EVD-related infection. The others are short tunneling length, repeated EVD insertions, duration of EVD, violation of the EVD insertion and maintenance protocol, CSF leak at wound or catheter exit point, absence of intraoperative prophylactic antibiotics, and frequency of CSF sampling [22]. Extreme care shall be given to manage surgical wound intraoperatively and postoperatively [27]. Routine dressing change, repeated CSF sampling, manipulation of system and/or routine change of EVD should be avoided. EVD was a procedure of choice, along with a lot of research on its effects, especially in the early 10 years between 2000 and 2010 [6,11,17,30]. However, there has been constant interest in the use of VAD and VSGS beyond the EVD [7,12,37]. A systemic review published in 2015 showed that VSGS and VAD could be effective temporary procedures, as well as EVD [3]. A prospective cohort study published in 2017 found no evidence for differences of the conversion rate to a permanent VP shunt among the temporary methods [38]. Another systemic review found VSGS decreased the risk of infections by reducing the need for daily CSF aspiration and that VAD had lower morbidity and mortality compared with EVD [25].
The neuroendoscopic intervention technique offers several advantages over conventional temporary procedures such as serial LP, ventricular tapping, and EVD [19,41]. With the endoscope, it is possible to directly view inside the ventricle and determine the amount of remaining blood clots, even in the dependent position of the ventricle. Floating debris can also be removed by forceps. As hemosiderin from the accumulated old blood can cause iron-induced brain damage and adversely affects the neurodevelopment of infants [33,35], it is important to wash it thoroughly by looking at it directly by endoscope. The water jet unit that can control the inflow and outflow also allows the ventricle to be irrigated minimizing pressure-induced brain injury.
At the author’s institute, routine screening of IVH in newborns is performed through cranial ultrasonography and head circumference measurement. The indications for surgical intervention among IVH patients are continually monitored. Progressive ventricular dilatation on ultrasonography or brain magnetic resonance imaging, together with clinical sign such as vomiting, bradycardia, and bulging fontanelle are considered as indications of disturbed CSF dynamics [4]. If abnormalities in CSF dynamics are observed, patients are initially treated with temporary treatments as long as possible. Once they are deemed mature enough to tolerate surgical intervention, VP shunt insertion is performed.
Neuroendoscopic intervention is performed prior to the insertion of the EVD catheter. The hole made in Kocher’s point for EVD insertion is utilized for the endoscope insertion. Patients are placed under general anesthesia in a supine position with their head fixed on a gel pad. The peel-away catheter and endoscope are then introduced. Once the endoscopic unit reaches the ventricular anatomy, the color of the CSF and the presence of blood clots or other debris are observed. Clotted hematoma and debris that may adhere to the choroid plexus or ventricular wall are then removed by irrigation (passive inflow and active aspiration) and endoscopic forceps. The water jet flow is carefully controlled to maintain intracranial pressure and volume to minimize brain damage and reduce slit ventricle. The gap between the endoscope and peel-away catheter allows CSF to flow freely from the ventricles. The 3rd ventricle is entered to remove possible hematoma clots and ensure that the cerebral aqueduct is patent. Most patients receive an interventricular septostomy to approach the contralateral ventricle and prevent unilateral ventricle isolation. A small hole is created between the right and left ventricles using a monopolar coagulator, allowing CSF to pass through the hole. Irrigation is performed using 1500–3000 mL of 37°C lactate-free Ringer solution. The EVD catheter is then inserted, and the cortical tract is sealed with fibrin glue, gelatin sponge, and hemostatic patch. As a prophylactic antibiotic for interventions, ampicillin/sulbactam or cefotaxime (a 3rd generation cephalosporin) is used.
Thirty-nine patients received neuroendoscopic intervention in addition to EVD between April 2008 and June 2019. In contrast, between January 1998 and March 2008, 17 patients were treated by a conventional procedure such as serial LPs, ventricular tapping, and EVD. The outcomes were evaluated retrospectively. There were no significant differences in GA or weight at birth between the two groups, and both groups had mostly grade IV IVH. There was no difference in the rate of shunt insertion between the two groups (94% vs. 97%, p=0.23). However, there was a significant difference in the time interval from the first intervention to shunt insertion (median, 5+5 weeks vs. 12+2 weeks; p<0.05). The GA (median, 42+1 weeks vs. 50+4 weeks; p<0.05) and weight (median, 3035 g vs. 4780 g; p<0.05) at the time of shunt insertion were higher in the neuroendoscopic group. The incidence of infection (47% vs. 18%), shunt revision (63% vs. 37%), and other complications (multiloculated hydrocephalus, isolated 4th ventricle, and slit ventricle) was relatively low during the period of 1 year after shunt insertion from the first treatment. The group that underwent neuroendoscopic intervention had relatively favorable outcomes. As most of the hospital’s patients were at high-risk with grade IV IVH and extremely low birth weight (ELBW) or VLBW, the temporary procedure alone did not reduce the VP shunt insertion rate. 94% and 97% of patients in each group respectively had VP shunts inserted. However, the GA and weight at the time of shunt insertion were higher in patients who received neuroendoscopic intervention. The interval between the first intervention and shunt insertion also increased significantly, indicating that endoscopic intervention controlled IVH better than conventional procedures as a temporary treatment.
The treatment of premature infants with severe IVH is often aggressive in order to improve prognosis [2]. High grade IVH is associated with poor neurodevelopmental outcomes and even death [16], and ELBW infants are also at risk for worse outcomes, even in cases of low grade IVH [28]. Temporary treatment alone is often not sufficient for preterm infants with severe IVH, and VP shunt insertion is often necessary. However, it is recommended that shunt surgery be postponed until the patient has grown sufficiently [13], as GA and weight are important factors for success and are related to organ development [36]. Shunt surgery and general anesthesia are both more dangerous in immature conditions, and lower GA and birth weight are associated with higher rates of shunt failure and post-operative mortality [1]. Low birth weight infants with early VP shunt are more prone to have shunt infection and blockage which lead to shunt multiple revisions [10,34]. Therefore, the goal is to effectively control severe IVH through temporary treatment in order to minimize complications and enable the patient to grow before undergoing shunt surgery.
Slit ventricle syndrome is a potential complication of VP shunt insertion that occurs when there is excessive drainage of CSF from the ventricles. Infants are more susceptible to this complication due to their softer brain tissue and higher intracranial compliance, which can lead to overdrainage of the shunt. However, as infants grow and their brain tissue matures, they are better able to tolerate the pressure of the shunt, reducing the risk of slit ventricle syndrome. Delaying the insertion of a VP shunt can help to maintain a stable state by allowing time for the infant to grow and for the brain tissue to mature, reducing the risk of complications such as slit ventricle syndrome. The important thing is how long we can delay the insertion of VP shunt. The delay of shunt insertion means that the stable state was maintained well by the temporary procedure alone.
More than half of shunt-related complications occur within 2 years after shunt insertion, with about 70% occurring within 6 months [23]. Early revisions done between 6 months and 2 years are mainly due to shunt infection or occlusion, whereas late revisions performed a few years later are usually caused by tissue adhesion, calcification, or disconnection. Endoscopic intervention can inspect the status of the ventricles to determine if they are clean enough for VP shunt insertion. Additionally, infection during the period of temporary treatment is likely reduced, as potential infectious agents are well removed by endoscopic manipulation.
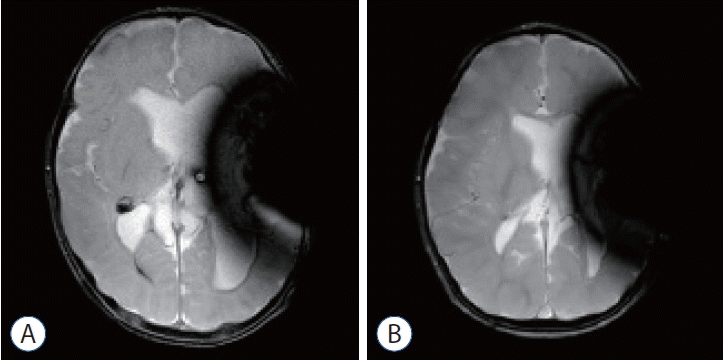
A 15-month-old female infant was delivered via C-section to a mother with hypothyroidism and was the first baby of twins. The infant was born extremely preterm with a gestational age of 27+2 weeks and had an ELBW of 980 g. While being treated in the neonatal intensive care unit of another hospital, the infant’s grade IV IVH worsened, and she was transferred to our hospital on the 62nd day of life. Upon hospitalization, encephalomalacia and post-hemorrhagic hydrocephalus were confirmed through imaging (Fig. 1). Endoscopic intervention with an EVD was performed every three weeks. After six interventions, the progression of encephalomalacia stopped, the hydrocephalus decreased significantly, and the ventricle size remained stable. However, since the IVH did not completely resolve, a VP shunt was inserted on the 158th day of life (GA 49+5 weeks, 4910 g). A reduction in ventricle size was observed on the post-operative neurosonogram (Fig. 2), and no specific complications were shown. The patient is currently receiving follow-up care from outpatient pediatric neurology, pediatric neurosurgery, and rehabilitation medicine. There were no shunt-related complications such as infection, blockage, or seizures during the 10-month followup after shunt insertion, and the patient is growing well.
Temporary surgical management options must be carefully selected and tailored to the condition of PHH infants. Neonatologists and pediatric neurosurgeons should discuss the infant’s condition and choose the best options available. The endoscopic approach allows for visualized manipulation, which helps maintain the ventricle in a better condition. Since the shunt is inserted when the ventricular state is relatively well preserved, the occurrence of shunt-related complications is also reduced.
Notes
References
1. Agarwal N, Shukla RM, Agarwal D, Gupta K, Luthra R, Gupta J, et al. Pediatric ventriculoperitoneal shunts and their complications: an analysis. J Indian Assoc Pediatr Surg. 22:155–157. 2017.

2. Alan N, Manjila S, Minich N, Bass N, Cohen AR, Walsh M, et al. Reduced ventricular shunt rate in very preterm infants with severe intraventricular hemorrhage: an institutional experience. J Neurosurg Pediatr. 10:357–364. 2012.

3. Badhiwala JH, Hong CJ, Nassiri F, Hong BY, Riva-Cambrin J, Kulkarni AV. Treatment of posthemorrhagic ventricular dilation in preterm infants: a systematic review and meta-analysis of outcomes and complications. J Neurosurg Pediatr. 16:545–555. 2015.

4. Bassan H, Benson CB, Limperopoulos C, Feldman HA, Ringer SA, Veracruz E, et al. Ultrasonographic features and severity scoring of periventricular hemorrhagic infarction in relation to risk factors and outcome. Pediatrics. 117:2111–2118. 2006.

5. Behjati S, Emami-Naeini P, Nejat F, El Khashab M. Incidence of hydrocephalus and the need to ventriculoperitoneal shunting in premature infants with intraventricular hemorrhage: risk factors and outcome. Childs Nerv Syst. 27:985–989. 2011.

6. Berger A, Weninger M, Reinprecht A, Haschke N, Kohlhauser C, Pollak A. Long-term experience with subcutaneously tunneled external ventricular drainage in preterm infants. Childs Nerv Syst. 16:103–109. discussion 110. 2000.

7. Bock HC, Feldmann J, Ludwig HC. Early surgical management and long-term surgical outcome for intraventricular hemorrhage-related posthemorrhagic hydrocephalus in shunt-treated premature infants. J Neurosurg Pediatr. 22:61–67. 2018.

8. Chaplin ER, Goldstein GW, Myerberg DZ, Hunt JV, Tooley WH. Posthemorrhagic hydrocephalus in the preterm infant. Pediatrics. 65:901–909. 1980.

9. Cheng YK, Liu CL. Antibiotic-impregnated external ventricular drainage for the management of post-hemorrhagic hydrocephalus in low birth weight premature infants following intraventricular hemorrhage. Childs Nerv Syst. 38:1567–1572. 2022.

10. Chittiboina P, Pasieka H, Sonig A, Bollam P, Notarianni C, Willis BK, et al. Posthemorrhagic hydrocephalus and shunts: what are the predictors of multiple revision surgeries? J Neurosurg Pediatr. 11:37–42. 2013.

11. Cornips E, Van Calenbergh F, Plets C, Devlieger H, Casaer P. Use of external drainage for posthemorrhagic hydrocephalus in very low birth weight premature infants. Childs Nerv Syst. 13:369–374. 1997.

12. Fountain DM, Chari A, Allen D, James G. Comparison of the use of ventricular access devices and ventriculosubgaleal shunts in posthaemorrhagic hydrocephalus: systematic review and meta-analysis. Childs Nerv Syst. 32:259–267. 2016.

13. Fulkerson DH, Sivaganesan A, Hill JD, Edwards JR, Shoja MM, Boaz JC, et al. Progression of cerebrospinal fluid cell count and differential over a treatment course of shunt infection clinical article. J Neurosurg Pediatr. 8:613–619. 2011.

14. Futagi Y, Toribe Y, Ogawa K, Suzuki Y. Neurodevelopmental outcome in children with intraventricular hemorrhage. Pediatr Neurol. 34:219–224. 2006.

15. Gaskill SJ, Marlin AE, Rivera S. The subcutaneous ventricular reservoir: an effective treatment for posthemorrhagic hydrocephalus. Childs Nerv Syst. 4:291–295. 1988.

16. Gilard V, Chadie A, Ferracci FX, Brasseur-Daudruy M, Proust F, Marret S, et al. Post hemorrhagic hydrocephalus and neurodevelopmental outcomes in a context of neonatal intraventricular hemorrhage: an institutional experience in 122 preterm children. BMC Pediatr. 18:288. 2018.

17. Harbaugh RE, Saunders RL, Edwards WH. External ventricular drainage for control of posthemorrhagic hydrocephalus in premature infants. J Neurosurg. 55:766–770. 1981.

18. Hasanain AA, Abdullah A, Alsawy MFM, Soliman MAR, Ghaleb AA, Elwy R, et al. Incidence of and causes for ventriculoperitoneal shunt failure in children younger than 2 years: a systematic review. J Neurol Surg A Cent Eur Neurosurg. 80:26–33. 2019.

19. Idris Z, Raj J, Abdullah JM. Early experience in endoscopic management of massive intraventricular hemorrhage with literature review. Asian J Neurosurg. 9:124–129. 2014.

20. Jian L, Hang-song S, Zheng-lang L, Li-sheng Y, Heng W, Nu Z. Implantation of ommaya reservoir in extremely low weight premature infants with posthemorrhagic hydrocephalus: a cautious option. Childs Nerv Syst. 28:1687–1691. 2012.

21. Kreusser KL, Tarby TJ, Taylor D, Kovnar E, Hill A, Conry JA, et al. Rapidly progressive posthemorrhagic hydrocephalus. Treatment with external ventricular drainage. Am J Dis Child. 138:633–637. 1984.
22. Kubilay Z, Amini S, Fauerbach LL, Archibald L, Friedman WA, Layon AJ. Decreasing ventricular infections through the use of a ventriculostomy placement bundle: experience at a single institution. J Neurosurg. 118:514–520. 2013.

23. Lee JY, Wang KC, Cho BK. Functioning periods and complications of 246 cerebrospinal fluid shunting procedures in 208 children. J Korean Med Sci. 10:275–280. 1995.

24. Limbrick DD Jr, de Vries LS. New insights into the management of posthemorrhagic hydrocephalus. Semin Perinatol. 46:151597. 2022.

25. Mazzola CA, Choudhri AF, Auguste KI, Limbrick DD Jr, Rogido M, Mitchell L, et al. Pediatric hydrocephalus: systematic literature review and evidence-based guidelines. Part 2: management of posthemorrhagic hydrocephalus in premature infants. J Neurosurg Pediatr 14 Suppl. 1:8–23. 2014.

26. Mukerji A, Shah V, Shah PS. Periventricular/intraventricular hemorrhage and neurodevelopmental outcomes: a meta-analysis. Pediatrics. 136:1132–1143. 2015.

27. Park YS, Kotani Y, Kim TK, Yokota H, Sugimoto T, Nakagawa I, et al. Efficacy and safety of intraventricular fibrinolytic therapy for postintraventricular hemorrhagic hydrocephalus in extreme low birth weight infants: a preliminary clinical study. Childs Nerv Syst. 37:69–79. 2021.

28. Patra K, Wilson-Costello D, Taylor HG, Mercuri-Minich N, Hack M. Grades i-ii intraventricular hemorrhage in extremely low birth weight infants: effects on neurodevelopment. J Pediatr. 149:169–173. 2006.

29. Peretta P, Ragazzi P, Carlino CF, Gaglini P, Cinalli G. The role of ommaya reservoir and endoscopic third ventriculostomy in the management of post-hemorrhagic hydrocephalus of prematurity. Childs Nerv Syst. 23:765–771. 2007.

30. Rhodes TT, Edwards WH, Saunders RL, Harbaugh RE, Little CL, Morgan LJ, et al. External ventricular drainage for initial treatment of neonatal posthemorrhagic hydrocephalus: surgical and neurodevelopmental outcome. Pediatr Neurosci. 13:255–262. 1987.

31. Rizvi SA, Wood M. Ventriculosubgaleal shunting for post-haemorrhagic hydrocephalus in premature neonates. Pediatr Neurosurg. 46:335–339. 2010.

32. Robinson S. Neonatal posthemorrhagic hydrocephalus from prematurity: pathophysiology and current treatment concepts. J Neurosurg Pediatr. 9:242–258. 2012.

33. Strahle J, Garton HJ, Maher CO, Muraszko KM, Keep RF, Xi G. Mechanisms of hydrocephalus after neonatal and adult intraventricular hemorrhage. Transl Stroke Res. 3(Suppl 1):25–38. 2012.

34. Taylor AG, Peter JC. Advantages of delayed vp shunting in post-haemorrhagic hydrocephalus seen in low-birth-weight infants. Childs Nerv Syst. 17:328–333. 2001.

35. Türkmen İnanır N, Eren F, Akgöz S, Eren B, Çetin S, Gündoğmuş UN, et al. The importance of hemosiderin deposition in the infant brain: an autopsy study. Hippokratia. 19:164–171. 2015.
36. Vinchon M, Rekate H, Kulkarni AV. Pediatric hydrocephalus outcomes: a review. Fluids Barriers CNS. 9:18. 2012.

37. Wang JY, Amin AG, Jallo GI, Ahn ES. Ventricular reservoir versus ventriculosubgaleal shunt for posthemorrhagic hydrocephalus in preterm infants: infection risks and ventriculoperitoneal shunt rate. J Neurosurg Pediatr. 14:447–454. 2014.

38. Wellons JC 3rd, Shannon CN, Holubkov R, Riva-Cambrin J, Kulkarni AV, Limbrick DD Jr, et al. Shunting outcomes in posthemorrhagic hydrocephalus: results of a hydrocephalus clinical research network prospective cohort study. J Neurosurg Pediatr. 20:19–29. 2017.

39. Whitelaw A, Lee-Kelland R. Repeated lumbar or ventricular punctures in newborns with intraventricular haemorrhage. Cochrane Database Syst Rev. 4:CD000216. 2017.

Fig. 1.
Neurosonography at the time when the patient was transferred to our hospital. Left side dominant encephalomalacia and posthemorrhagic hydrocephalus were seen. a : Right ventricle in midline sagittal view. b : More dilated left ventricle in midline sagittal view. C and D : asymmetric size of ventricles in coronal views.
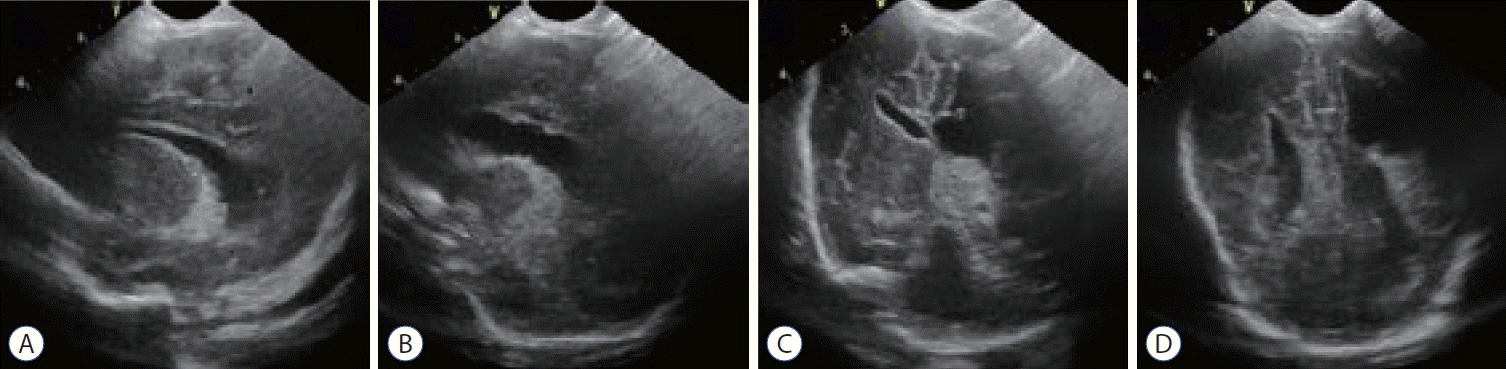
Fig. 2.
Noncontrast brain magnetic resonance imaging T2 weighted image (dark signals on left side of brain are artifacts from programmable valve). a : The day of ventriculoperitoneal (VP) shunt insertion. Destructed left hemisphere and marked hydrocephalus were seen. b : after 10 months from the VP shunt insertion. No significant other abnormalities (such as hemorrhage, mass, hydrocephalus, ischemic lesion) were seen. The size of the ventricle was decreased.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download