This article has been
cited by other articles in ScienceCentral.
Abstract
Phosphatase and tensin homolog (PTEN) hamartoma tumor syndrome (PHTS) is a hereditary disorder caused by germline inactivating mutations in the PTEN tumor suppressor gene. As a type of PHTS, Cowden syndrome is associated with abnormalities of the thyroid, breast, uterus, and gastrointestinal tract. A 52-year-old-woman visited the outpatient clinic of our endocrinology clinic with multiple thyroid nodules and Hashimoto's thyroiditis. Computed tomography imaging revealed a multinodular mass measuring up to 3.5 cm in the left thyroid lobe, causing laryngotracheal airway displacement. The total thyroidectomy specimen revealed multiple follicular adenomas and adenomatous nodules with lymphocytic thyroiditis and lipomatous metaplasia in the background. The patient was suspected of PTHS based on her thyroid pathology, family history, and numerous hamartomatous lesions of the breast, uterus, and skin. Her diagnosis was confirmed through molecular testing. This case demonstrates that pathologists must be well acquainted with thyroid pathology in PHTS.
Keywords: PTEN, Hamartoma tumor syndrome, Thyroid pathology, Cowden
Phosphatase and tensin homolog (
PTEN) hamartoma tumor syndrome (PHTS) is a hereditary disorder caused by mutations in the
PTEN tumor suppressor gene located on 10q23.3 [
1]. It is a rare, autosomal dominant spectrum of disease, causing hamartomatous overgrowth of tissues in the thyroid, gastrointestinal tract, and skin. Patients with this disease are at increased risk of thyroid, breast, endometrial, renal, and possibly colorectal cancers [
2-
4]. PHTS includes Cowden syndrome (CS), Bannayan-RileyRuvalcaba syndrome (BRRS),
PTEN-related Proteus syndrome (PS), and Proteus-like syndrome. CS is the most common PTHS, usually presenting in young to middle-aged adults. Thyroid pathologic findings in patients with CS are distinctive and characteristic [
1,
5,
6]. Recognition of CS by a pathologist is critical for cancer screenings and genetic counseling.
We encountered a patient who presented with unusual thyroid pathology, suspicious of CS. We aim to demonstrate that thyroid pathology can be a clue for diagnosing PHTS, including clinical and immunophenotypical features and molecular testing results. Our observations will aid pathologists and clinicians in properly diagnosing PHTS.
CASE REPORT
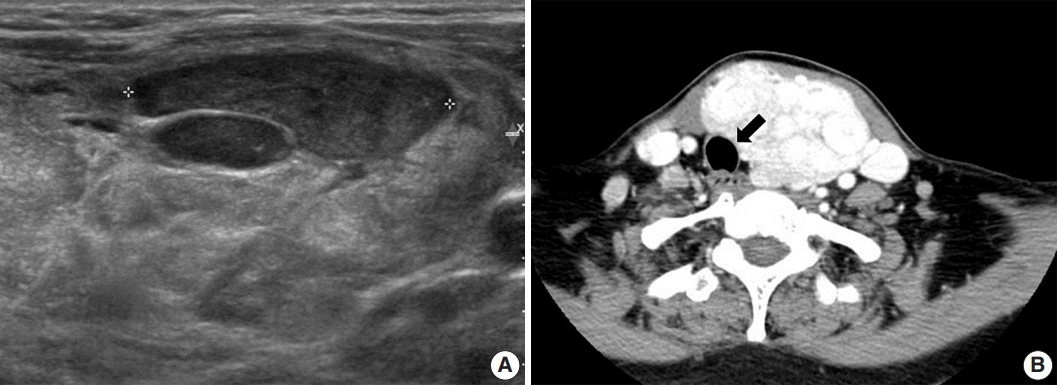
A 52-year-old-woman visited our endocrinology clinic with multiple thyroid nodules. She had undergone a right lobectomy for benign nodules 30 years prior. She had remaining left multiple thyroid nodules diagnosed as nodular hyperplasia from fine-needle aspiration cytology in 2005. She received ultrasonography (US) and computed tomography (CT) exams in 2021 to evaluate the surgical site for symptoms of neck swelling. The US revealed an enlarged left lobe with numerous iso- and hypoechoic nodules. Several nodules had cystic changes, while others were solid with well-defined margins and increased vascularity. The intermediate and low suspicion nodules measured up to 3.5 cm. In addition, the CT revealed multinodular masses in the left thyroid lobe, causing laryngotracheal airway displacement (
Fig. 1A,
B). The patient underwent a subsequent core needle biopsy on the 3.5 cm-sized, intermediate suspicious mass in the left lobe.
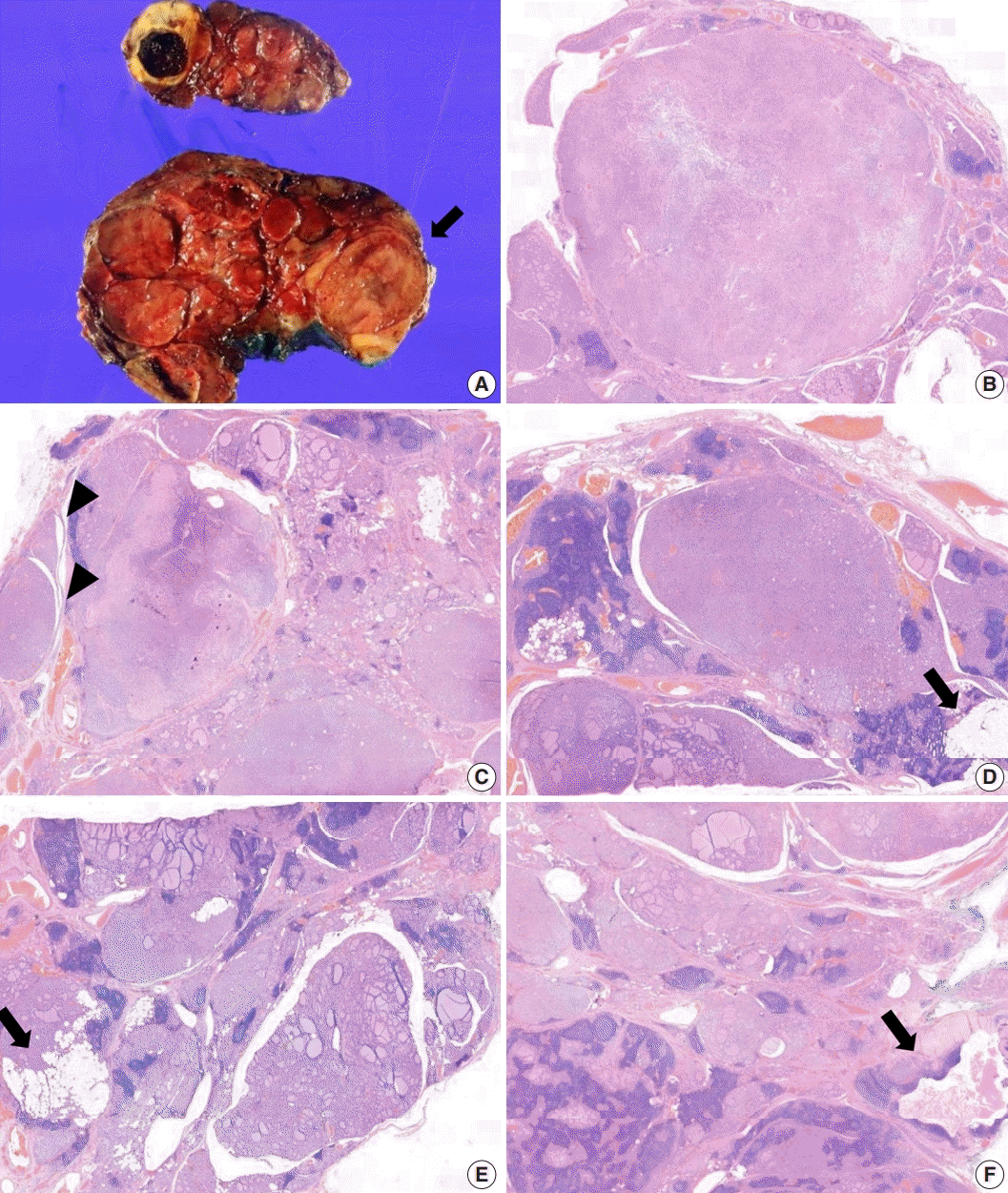
The core needle biopsy of the thyroid revealed monomorphic microfollicular cells with fibrous capsules. A follicular neoplasm was suspected, and a left thyroid gland lobectomy was performed. The resected specimen of the left lobe was enlarged, measuring 9×7 cm, and consisted of well-circumscribed nodular lesions (
Fig. 2A).
Grossly, multiple well-circumscribed tan nodular lesions with suspicious encapsulation and unencapsulated small nodules with calcification and infarction were identified. Adjacent to the nodules, yellowish fatty components were present. The nodules were too numerous with inconspicuous margins to determine the exact number.
Microscopically, follicular adenomas with monomorphic microfollicular patterns of growth (
Fig. 2B,
C), numbering more than 20 up to 3.5×2.3 cm in size, were identified. They were well-demarcated with a fibrous capsule containing medium to large vessels. Capsular or vascular invasion was not observed. In addition, numerous adenomatous hyperplasias without a thin fibrous band were noted. A mixture of lipomatous metaplasia with mixed mature fat and follicles and chronic lymphocytic thyroiditis was characteristic (
Fig. 2D,
E). A 0.5-cm-sized lymphoepithelial cyst composed of stratified squamous epithelium linings, lymphoid germinal centers, and luminal debris with cholesterol clefts was present in the background of the adjacent chronic lymphocytic thyroiditis (
Fig. 2F). Based on these aspects, it was considered to exhibit hamartomatous morphology, and PHTS was suspected. According to the literature indicating PTEN staining for sensitive and specific detection of CS, PTEN immunohistochemistry was performed. In addition, to exclude papillary thyroid carcinoma, the slides showing subtle nuclear atypia were subjected to HBME-1, CD56, cytokeratin 19, and galectin-3 immunohistochemical staining. Small adenomatous nodules with mild nuclear atypia maintained CD56 expression and were negative for HBME1. Galectin-3 and cytokeratin 19 were focally expressed in areas of thyroiditis and fibrosis with calcifications, but were considered negative.
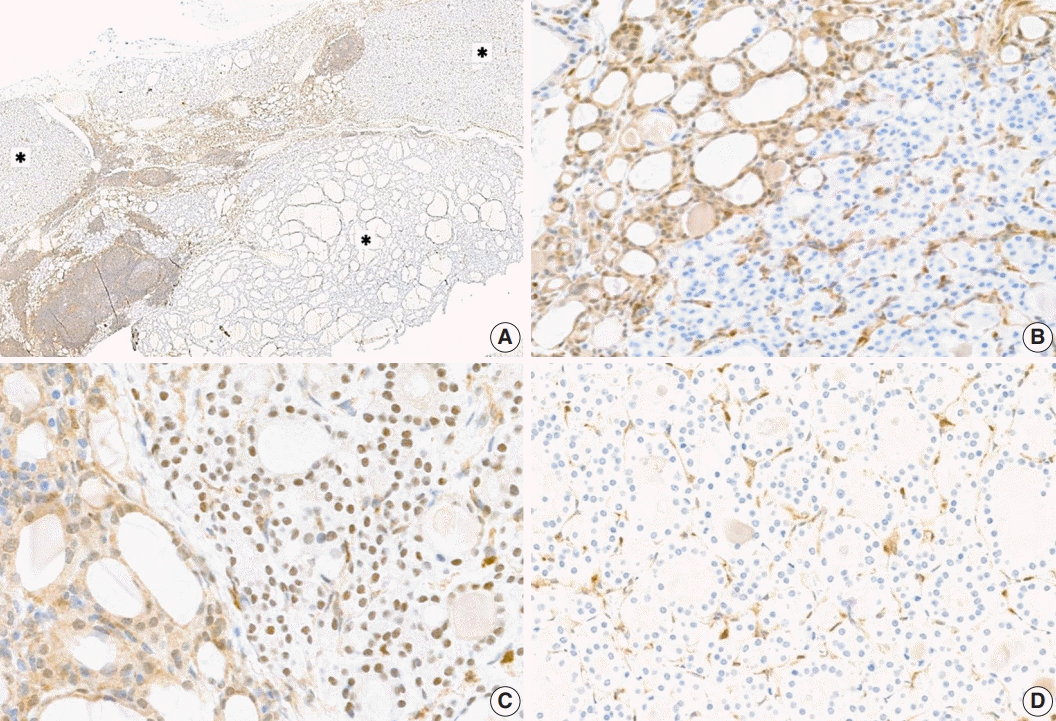
Proliferative thyroid lesions were all negative for PTEN proteins, whereas the normal background thyroid tissue was positive (
Fig. 3A,
B). PTEN proteins in normal tissue and endothelial cells within the tumor exhibited nuclear and cytoplasmic expression, but the loss of expression was observed in the follicular adenoma components (
Fig. 3C,
D).
The immunohistochemistry result of PTEN was compatible with PTHS. Further examination of the patient’s history revealed a family history of uterine disease and thyroid cancer in her mother and aunt. Moreover, the patient had received sclerotherapy for an arteriovenous malformation in 2002, a hysterectomy for leiomyomas in 2011, a polypectomy for multiple gastric polyps, and an excisional biopsy of the breasts for intraductal papillomas in 2021. Reviewing her medical history, we found that the endometrial glands of the uterus specimen showed nuclear atypia, crowding, and complex architecture with loss of PTEN expression. Her breast specimen exhibited a PTEN loss within the intraductal papilloma. Under the recommendation of clinical correlation and genetic counseling for the PTHS, the patient underwent an additional endoscopy exam and skin biopsy. The patient had a lipoma in the proximal jejunum, fundic gland polyps in the stomach, and multiple facial plaques diagnosed as syringomas in her forehead.
The next-generation sequencing analysis revealed a heterozygous germline mutation in chromosome 10q23 (c.260_ 281delinsCATAT). A subsequent genetic analysis by polymerase chain reaction and direct sequencing revealed that the mutation induces a frameshift and leads to a stop codon ending the transcription process (p.Gln87Profs*14). The mRNA reference sequence was NM.000314.4. Therefore, the patient’s diagnosis was confirmed as PHTS.
DISCUSSION
PTEN encodes a tumor suppressor, a dual-specificity phosphatase that downregulates the anti-apoptotic/pro-proliferative phosphatidylinositol 3kinase/protein kinase B signaling pathway, and the mitogen-activated kinase pathway [
1,
7]. Inactivation of PTEN occurs through frameshift mutations, inactivating missense mutations, and copy number losses, typically in exons 5, 7, and 8 [
4]. PHTS includes CS, BRRS, which is a congenital disorder characterized by macrocephaly [
8] and PS, which is a hereditary disorder displaying characteristic hyperostosis [
8]. Based on the clinical manifestations, our patient was suspected of CS among PHTS.
According to the National Comprehensive Cancer Network guidelines for CS/PTHS, genetic testing can be recommended when a patient satisfies one major and more than three or four minor criteria (
Table 1) [
9]. Genetic testing was relevant for our patient as she had multiple gastrointestinal hamartomas and cutaneous facial papules among the major criteria, and a lipoma, thyroid structural lesions, and vascular anomaly meeting the minor criteria. Mucocutaneous lesions are present in 90% to 100% of CS cases [
1,
4,
6], and cutaneous lesions are usually the first signs of the disease [
4,
10], with facial papules being the most frequent lesions [
4].
Thyroid nodules, adenomas, and goiters have been reported in 30% to 68% of adults and 2% to 14% of children with
PTEN mutations [
2,
11,
12]. Several studies have provided detailed descriptions of the clinical features and pathology of thyroid nodules in
PTEN mutation carriers [
2]. Harach et al. [
5] were the first to note the histologic findings of multiple adenomatous goiters and multiple follicular adenomas, including adenolipomas, particularly in children and young adults, should alert clinicians to the possibility of CS [
1]. In a pathologic study evaluating thyroidectomy specimens from patients with CS and BRRS, Laury et al. [
6] observed that multiple adenomatous nodules were the most common finding (75%), followed by papillary thyroid carcinomas (60%), lymphocytic thyroiditis (55%), C-cell hyperplasia (55%), and follicular carcinoma (25%) [
13]. For the diagnostic criteria of PHTS, only follicular thyroid cancer is specified as a major criterion because it appears to be over-represented in mutation carriers (25%) compared with the general population (15%) [
2].
Thyroid lesions in patients with CS mainly manifest in females and present at a younger age (mean diagnosis age, 33.7 years) compared to sporadic thyroid nodules [
14]. Grossly, solid yellow-tan thyroid nodules are identified with variations in size and number. Specifically, the thyroid nodules in CS do not present with abundant colloids as seen in sporadic goiters. Microscopically, the nodules are well-delineated, with no, partial, or complete encapsulation and variable growth patterns. The nodules present with microscopic features of follicular adenomas, cellular hyperplastic nodules, adenolipomas or lipomatous metaplasias, Hurthle cell or clear cell nodules, C-cell hyperplasia, papillary microcarcinomas, or follicular carcinoma in the background of chronic lymphocytic thyroiditis. The adenomatous nodules have a microfollicular architecture composed of small cellular follicles without a capsule. Meanwhile, follicular adenomas present with a welldefined fibrous capsule and possess an architectural pattern that differs from the surrounding thyroid tissue without capsular or vascular invasion. While these thyroid pathologic findings are not specific to CS patients [
13], PTEN immunohistochemistry can be helpful for the identification of CS. PTEN immunohistochemical staining shows a complete or heterogeneous loss in adenomatous nodules, specifically in CS patients.
Although CS has a reported incidence of 1 in 200,000 [
15], it seems that the prevalence has been underestimated because of the complex clinical criteria and non-specific manifestations that are similar to those found in the general population. However, the diagnosis of CS is essential because these patients have an increased cancer risk [
13]. The projected estimated lifetime risks of cancer in individuals with PTHS range from 85%–89% for any cancer, 67%–85% for female breast cancer, 6%–38% for thyroid cancer, 2%–28% for endometrial cancer, 2%–34% for renal cancer, and 9%–20% for colorectal cancer [
16-
19]. The characteristic thyroid pathology and PTEN immunohistochemistry are indicators of PHTS, diagnosis of which is crucial for initiation of cancer screenings and genetic counseling.
To summarize, we report a case of a patient who presented with unusual thyroid pathology, exhibiting numerous follicular adenomas, adenomatous nodules with cellular and microfollicular proliferations intermixed with lipomatous metaplasia, and lymphocytic thyroiditis. The patient was diagnosed with PTHS based on the loss of PTEN immunohistochemical staining not only in the thyroid specimen but also in the breast and uterus specimens, and the diagnosis was confirmed by the molecular study. This case indicates the importance of recognizing the thyroid histologic findings of PTHS to suspect CS.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download