1. Kim JW, Kwon GY, Roh JL, et al. Carcinoma ex pleomorphic adenoma of the salivary glands: distinct clinicopathologic features and immunoprofiles between subgroups according to cellular differentiation. J Korean Med Sci. 2011; 26:1277–85.

2. Bahrami A, Perez-Ordonez B, Dalton JD, Weinreb I. An analysis of
PLAG1 and
HMGA2 rearrangements in salivary duct carcinoma and examination of the role of precursor lesions. Histopathology. 2013; 63:250–62.

3. Ohtake S, Cheng J, Ida H, et al. Precancerous foci in pleomorphic adenoma of the salivary gland: recognition of focal carcinoma and atypical tumor cells by p53 immunohistochemistry. J Oral Pathol Med. 2002; 31:590–7.

4. Di Palma S, Skalova A, Vanieek T, Simpson RH, Starek I, Leivo I. Non-invasive (intracapsular) carcinoma ex pleomorphic adenoma: recognition of focal carcinoma by HER-2/neu and MIB1 immunohistochemistry. Histopathology. 2005; 46:144–52.

5. Ihrler S, Weiler C, Hirschmann A, et al. Intraductal carcinoma is the precursor of carcinoma ex pleomorphic adenoma and is often associated with dysfunctional p53. Histopathology. 2007; 51:362–71.

6. Ihrler S, Guntinas-Lichius O, Agaimy A, Wolf A, Mollenhauer M. Histological, immunohistological and molecular characteristics of intraductal precursor of carcinoma ex pleomorphic adenoma support a multistep carcinogenic process. Virchows Arch. 2017; 470:601–9.

7. Di Palma S. Carcinoma ex pleomorphic adenoma, with particular emphasis on early lesions. Head Neck Pathol. 2013; 7 Suppl 1:S68–76.

8. Logasundaram R, Amarawickrama H, Premachandra D, Hellquist H. Intracapsular (
in situ) carcinoma ex pleomorphic adenoma with unusual clinical and histological features. Eur Arch Otorhinolaryngol. 2008; 265:1563–6.

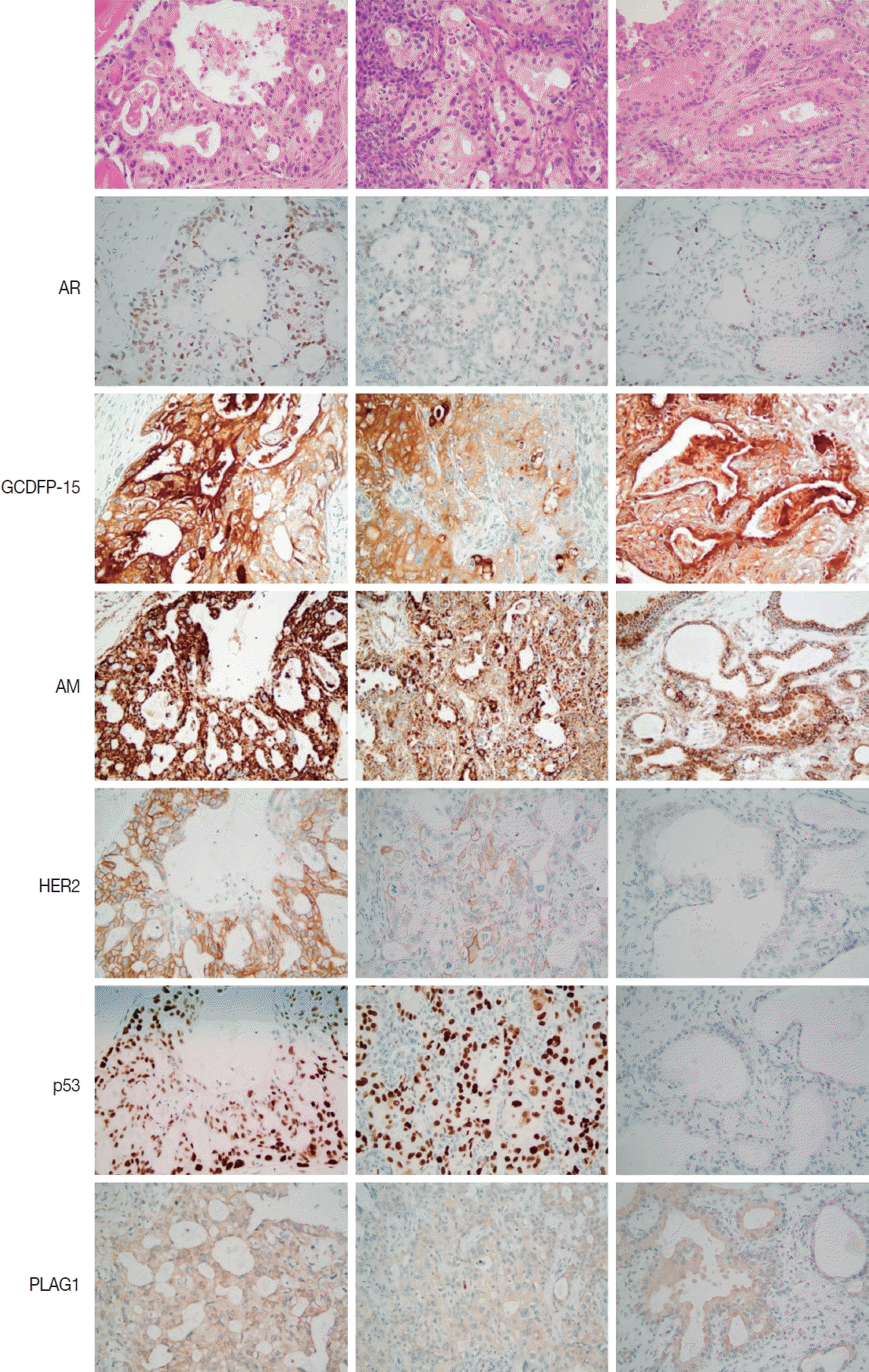
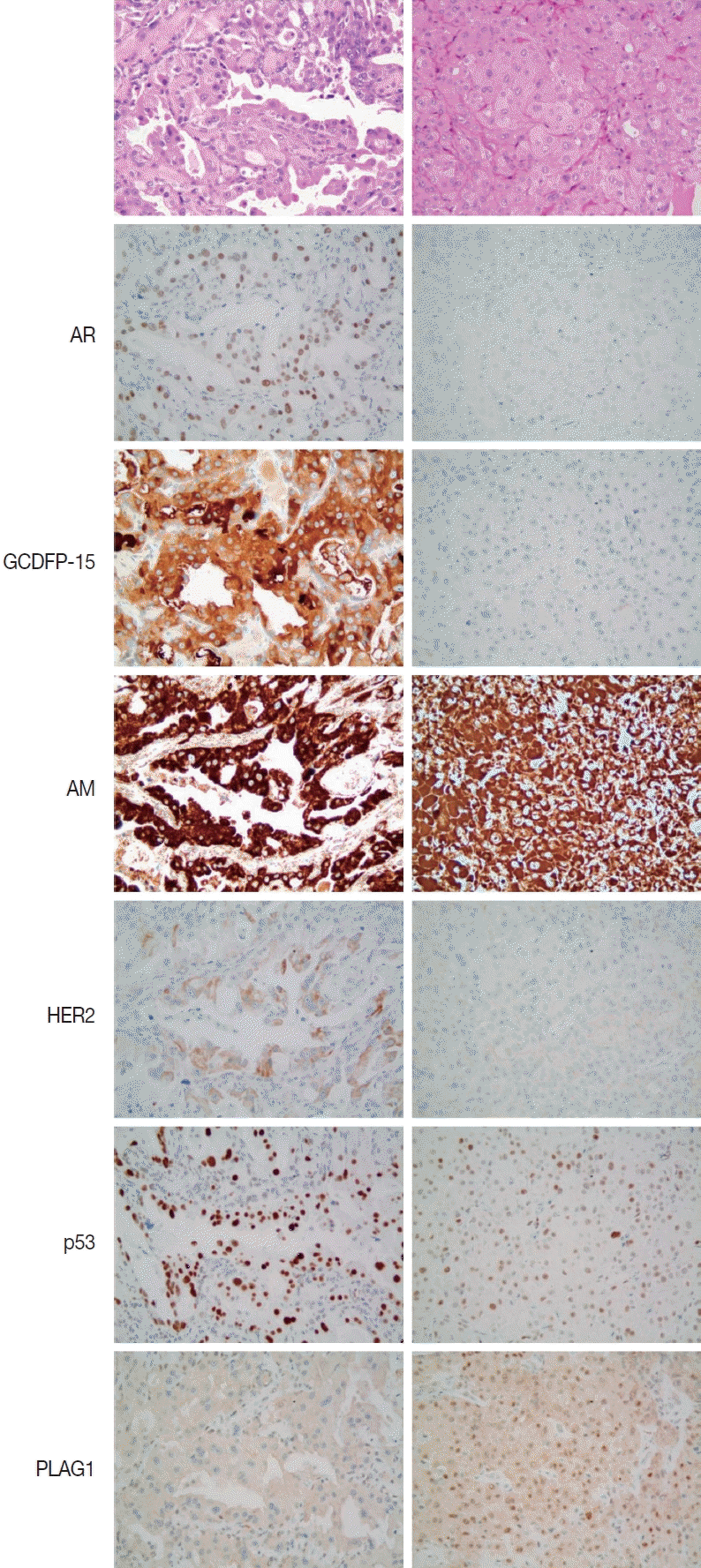
9. Kapadia SB, Barnes L. Expression of androgen receptor, gross cystic disease fluid protein, and CD44 in salivary duct carcinoma. Mod Pathol. 1998; 11:1033–8.
10. Williams L, Thompson LD, Seethala RR, et al. Salivary duct carcinoma: the predominance of apocrine morphology, prevalence of histologic variants, and androgen receptor expression. Am J Surg Pathol. 2015; 39:705–13.
11. Nasser SM, Faquin WC, Dayal Y. Expression of androgen, estrogen, and progesterone receptors in salivary gland tumors: frequent expression of androgen receptor in a subset of malignant salivary gland tumors. Am J Clin Pathol. 2003; 119:801–6.

12. Nakajima Y, Kishimoto T, Nagai Y, et al. Expressions of androgen receptor and its co-regulators in carcinoma ex pleomorphic adenoma of salivary gland. Pathology. 2009; 41:634–9.

13. DeRoche TC, Hoschar AP, Hunt JL. Immunohistochemical evaluation of androgen receptor, HER-2/neu, and p53 in benign pleomorphic adenomas. Arch Pathol Lab Med. 2008; 132:1907–11.
14. Schmitt FC, Soares R, Cirnes L, Seruca R. P53 in breast carcinomas: association between presence of mutation and immunohistochemical expression using a semiquantitative approach. Pathol Res Pract. 1998; 194:815–9.

15. Wolff AC, Hammond ME, Allison KH, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. Arch Pathol Lab Med. 2018; 142:1364–82.
16. Chiosea SI, Thompson LD, Weinreb I, et al. Subsets of salivary duct carcinoma defined by morphologic evidence of pleomorphic adenoma,
PLAG1 or
HMGA2 rearrangements, and common genetic alterations. Cancer. 2016; 122:3136–44.

17. Katabi N, Ghossein R, Ho A, et al. Consistent
PLAG1 and
HMGA2 abnormalities distinguish carcinoma ex-pleomorphic adenoma from its de novo counterparts. Hum Pathol. 2015; 46:26–33.

18. D’Arcy C, Quinn CM. Apocrine lesions of the breast: part 2 of a two-part review. Invasive apocrine carcinoma, the molecular apocrine signature and utility of immunohistochemistry in the diagnosis of apocrine lesions of the breast. J Clin Pathol. 2019; 72:7–11.

19. Jones C, Damiani S, Wells D, Chaggar R, Lakhani SR, Eusebi V. Molecular cytogenetic comparison of apocrine hyperplasia and apocrine carcinoma of the breast. Am J Pathol. 2001; 158:207–14.

20. D’Arcy C, Quinn C. Apocrine lesions of the breast: part 1 of a twopart review: benign, atypical and
in situ apocrine proliferations of the breast. J Clin Pathol. 2019; 72:1–6.

21. Mazoujian G, Pinkus GS, Davis S, Haagensen DE Jr. Immunohistochemistry of a gross cystic disease fluid protein (GCDFP-15) of the breast: a marker of apocrine epithelium and breast carcinomas with apocrine features. Am J Pathol. 1983; 110:105–12.
22. Espinosa CA, Rua L, Torres HE, Fernandez Del Valle A, Fernandes RP, Devicente JC. Sclerosing polycystic adenosis of the parotid gland: a systematic review and report of 2 new cases. J Oral Maxillofac Surg. 2017; 75:984–93.

23. Gnepp DR. Salivary gland tumor “wishes” to add to the next WHO Tumor Classification: sclerosing polycystic adenosis, mammary analogue secretory carcinoma, cribriform adenocarcinoma of the tongue and other sites, and mucinous variant of myoepithelioma. Head Neck Pathol. 2014; 8:42–9.

24. Skalova A, Hyrcza MD, Leivo I. Update from the 5th edition of the World Health Organization classification of head and neck tumors: salivary glands. Head Neck Pathol. 2022; 16:40–53.

25. Petersson F, Tan PH, Hwang JS. Sclerosing polycystic adenosis of the parotid gland: report of a bifocal, paucicystic variant with ductal carcinoma in situ and pronounced stromal distortion mimicking invasive carcinoma. Head Neck Pathol. 2011; 5:188–92.
26. Di Palma S, Lambros MB, Savage K, et al. Oncocytic change in pleomorphic adenoma: molecular evidence in support of an origin in neoplastic cells. J Clin Pathol. 2007; 60:492–9.

27. Triantafyllou A, Thompson LD, Devaney KO, et al. Functional histology of salivary gland pleomorphic adenoma: an appraisal. Head Neck Pathol. 2015; 9:387–404.

28. Han MW, Roh JL, Choi SH, et al. Prognostic factors and outcome analysis of salivary duct carcinoma. Auris Nasus Larynx. 2015; 42:472–7.

29. Luk PP, Weston JD, Yu B, et al. Salivary duct carcinoma: clinicopathologic features, morphologic spectrum, and somatic mutations. Head Neck. 2016; 38 Suppl 1:E1838–47.

30. Valstar MH, Mast H, Ten Hove I, et al. Malignant transformation of salivary gland pleomorphic adenoma: proof of principle. J Pathol Clin Res. 2021; 7:432–7.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download