Abstract
Objective
Hypertensive disease during pregnancy increases the risk of maternal morbidity and mortality and leads to the development of multi-organ dysfunction, including kidney dysfunction. Complicated pregnancies require careful postpartum management to prevent sequelae. It is believed that kidney injury can consistently occur even after delivery; therefore, defining the chronicity and endpoint is essential for establishing diagnostic criteria. However, data on the prevalence of persistent renal complications following hypertensive disease during pregnancy are limited. In this study, we evaluated the risk of developing renal disorders in patients with a history of hypertensive disease during pregnancy.
Methods
Participants who gave birth between 2009 and 2010 were followed up for 8 years after delivery. The risk of renal disorder development after delivery was determined according to a history of hypertensive disease during pregnancy. Different factors that could affect the course of pregnancy, including age, primiparity, multiple pregnancy, preexisting hypertension, pregestational diabetes, hypertensive disease during pregnancy, gestational diabetes, postpartum hemorrhage, and cesarean section, were adjusted for using the Cox hazard model.
Results
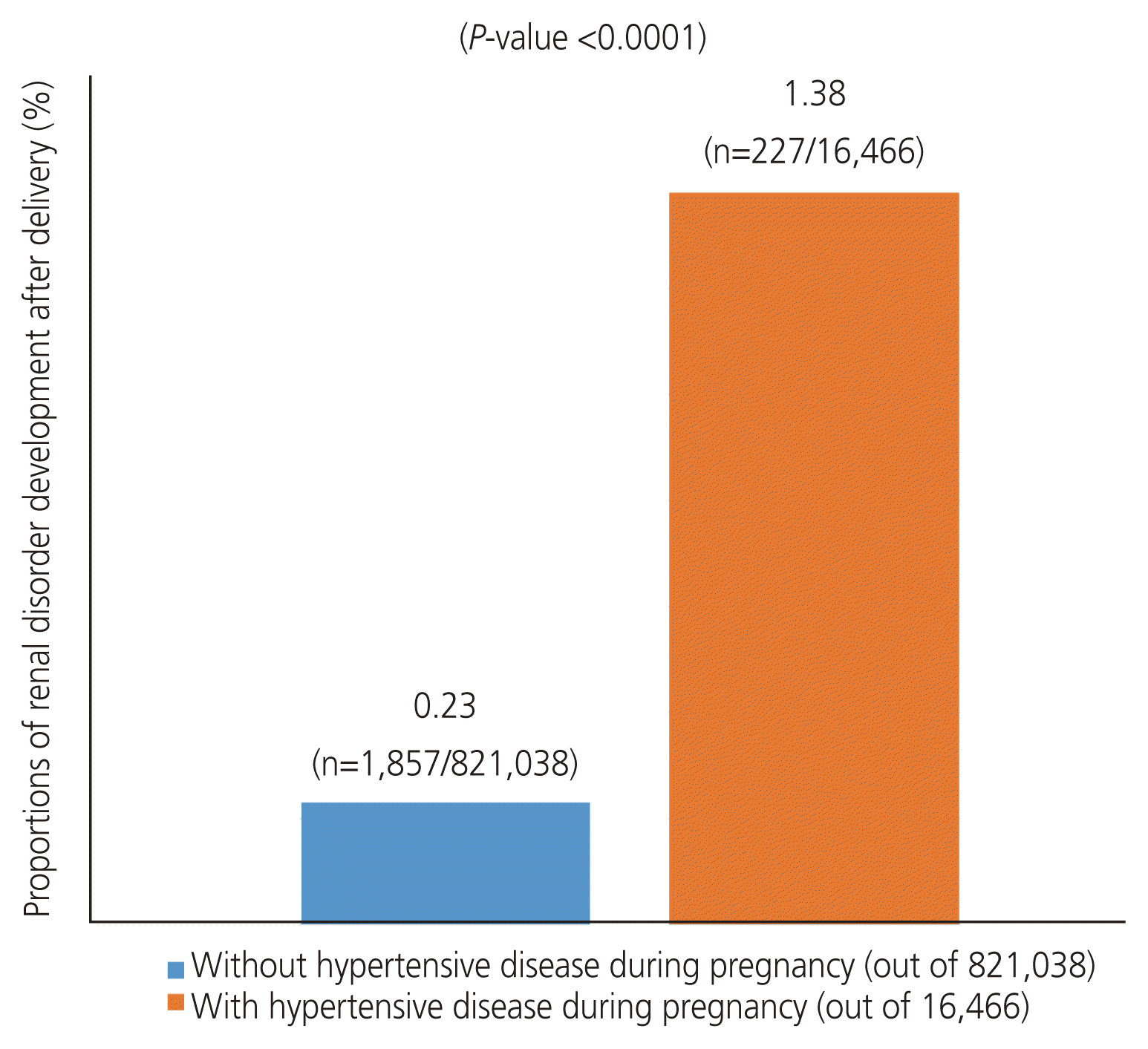
Women with hypertension during pregnancy had a higher risk of developing renal disorders after delivery (0.23% vs. 1.38%; P<0.0001). This increased risk remained significant even after adjusting for covariates (adjusted hazard ratio, 3.861; 95% confidence interval [CI], 3.400–4.385] and 4.209 [95% CI, 3.643–4.864]; respectively).
Preeclampsia is defined as newly developed hypertension accompanied by one of the end-organ damage signs, such as proteinuria in the last half of pregnancy. The American College of Obstetricians and Gynecologists defines renal insufficiency caused by hypertensive disorders during pregnancy as a serum creatinine level greater than 1.1 mg/dL or a doubled serum creatinine concentration in cases without renal disease [1]. Hypertensive diseases during pregnancy are one of the leading causes of maternal morbidity and mortality and occur as a complication of pregnancy in 2–8% of cases worldwide [2]. Therefore, the importance of investigating preeclampsia is consistently being highlighted, and several investigations concluded that preeclampsia may be associated with renal disease development even several years after pregnancy [3].
Several studies have been conducted to define the relationship between high blood pressure during pregnancy and postpartum renal disorders in Europe and America, but data regarding Asian populations are lacking [4–7]. Considering that the prevalence or pathophysiology of renal diseases varies among different races or ethnicities, it is important to evaluate whether the causality is different within Asian populations [8,9]. We conducted a population-based cohort study using data from the Korea National Registers, including the Korean National Health Insurance (KNHI). We aimed to evaluate whether high blood pressure is a common predisposing factor for renal disorders, after delivery, in Korea.
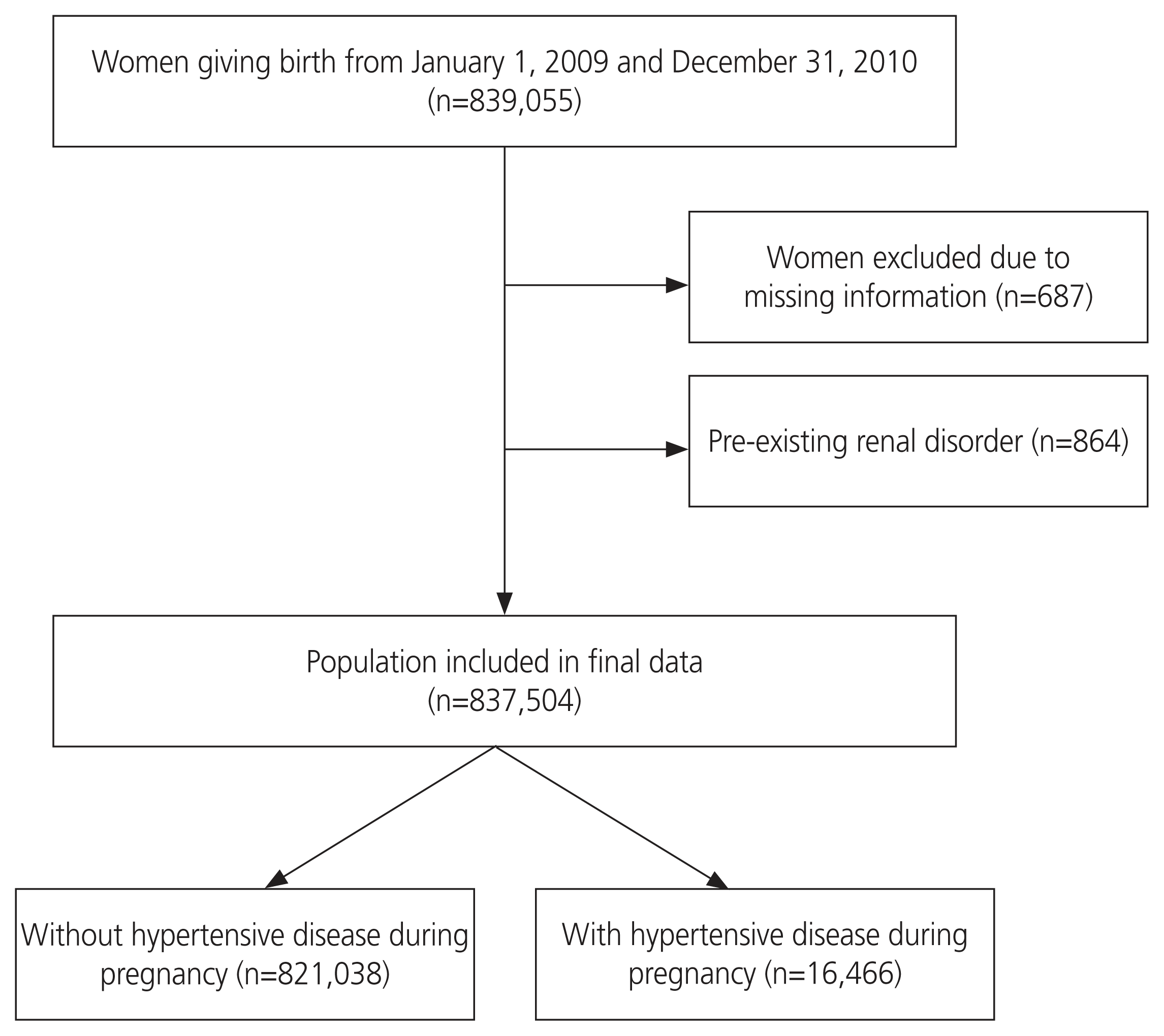
Women who gave birth between January 1, 2009 and December 31, 2010 were selected and followed up for a postpartum period of 8 years. Births did not include abortions but only live births. The participants were divided into two subgroups: those with or without hypertensive disease during pregnancy. Hypertensive disease during pregnancy was confirmed using the International Classification of Disease, 10th revision codes (ICD-10 codes) and included gestational hypertension, preeclampsia, eclampsia, and superimposed preeclampsia, but not chronic hypertension, as shown in Table 1. Personal characteristics and other obstetric characteristics and complications, such as age, primiparity, multiple pregnancy, preexisting hypertension (HTN), gestational diabetes, postpartum hemorrhage, and delivery by cesarean section were also identified. The causality between the development of renal disorders after delivery and hypertensive disease during pregnancy, as well as other obstetric factors, was analyzed. Maternal baseline characteristics were derived from the KNHI database, and pregnancy complications, including hypertensive disease, were extracted using ICD-10 codes. Renal disease was defined according to ICD-10 codes, including different stages of chronic kidney disease and unspecified kidney failure, as shown in Table 2.
The current study is a population-based study and the data were collected from the KNHI. The KNHI is known as the sole public health insurer, which covers the whole population of South Korea and, is, therefore, suitable for nationwide studies [10]. Furthermore, the KNHI provides broad medical records such as disease codes and patient hospitalization and medical histories.
Continuous variables and frequencies are presented as means and standard deviations. Categorical variables are presented as percentages. Chi-square and t-tests were used to compare categorical and continuous variables, respectively. To compare the risk of developing renal disease after delivery, covariates such as age, primiparity, multiple pregnancy, preexisting HTN, pregestational diabetes, hypertensive disease during pregnancy, gestational diabetes, postpartum hemorrhage, and delivery by cesarean section were adjusted for, and hazard ratios were calculated using Cox regression analysis. A P-value of <0.0001 was considered statistically significant. SAS for Windows (version 9.4; SAS Inc., Cary, NC, USA) was used for statistical analyses.
As demonstrated in Fig. 1, among the 839,055 women who gave birth between January 1, 2009 and December 31, 2010, 687 were excluded because of missing information and 864 because of preexisting renal disorders. Thus, 837,504 women were included in the final analysis. Participants were divided into two groups: those with hypertensive disease during pregnancy (n=16,466) and those without it (n=821,038).
Table 3 shows the obstetric characteristics of the two groups. The group with hypertensive disease during pregnancy had a higher mean age, a larger number of women over 35 years of age, and a higher rate of primiparity and multiple pregnancy. In addition, preexisting hypertension, pregestational diabetes, and gestational diabetes were more common in women with hypertensive disease during pregnancy while delivery by cesarean section was high and postpartum hemorrhage occurred more frequently in this group (P<0.0001).
Women with hypertensive disease during pregnancy had a higher incidence of renal disorder development 8 years after delivery than those without. Among the 821,038 women who did not develop hypertensive disease during pregnancy, 1,857 developed renal disorders. Furthermore, among the 16,466 women with hypertensive disease during pregnancy, 227 developed renal disorder (0.23% vs. 1.38%; P<0.0001), as shown in Fig. 2.
The correlation between each covariate and occurrence of renal disorders was also analyzed (Table 4). Even after adjusting for covariates, hypertensive disease during pregnancy was a significant risk factor for future renal disease (adjusted hazard ratio [aHR] 4.209, 95% confidence interval [CI] 3.643–4.864).
Adults aged 35 years or older had a higher risk of developing renal disorders (aHR 1.123, 95% CI 1.005–1.254) than those under 35 years. There was no correlation between primiparity and multiple pregnancies and the risk of developing renal disorders. In addition, the risk of developing renal disorders was greater if hypertension and diabetes were present before or during pregnancy (aHR 3.861, 95% CI 3.400–4.385; aHR 2.302, 95% CI 2.014–2.631; aHR 4.209, 95% CI 3.643–4.864; aHR 1.389, 95% CI 1.101–1.752). Renal disorders occurred more frequently in cases of delivery by cesarean section (aHR 1.311, 95% CI 1.199–1.433).
The incidence of kidney disease 8 years after delivery was higher in women with hypertension during pregnancy than in those without hypertension. This increased risk remained significant even after adjusting for covariates.
These results indicate that women with hypertensive disease during pregnancy are more susceptible to developing renal disorders 8 years after delivery than those without hypertensive disease during pregnancy. Therefore, women with hypertensive disease during pregnancy should be educated about the possibility of developing renal disorders, and the need for early screening and active treatment should be explained. It is also necessary to emphasize that the risk of postpartum renal disease increases in the presence of both hypertension and diabetes before or during pregnancy. In addition, delivery by cesarean section and an age of 35 years or older can be considered predisposing factors for an increased risk of renal complications after delivery. Widespread awareness among clinicians regarding the association between hypertension during pregnancy and renal disorders could improve long-term renal outcomes.
Increased renal function is crucial for maintaining physiological adaptations during pregnancy. During pregnancy, effective renal plasma flow is increased, and renal hyperfiltration causes a 40–60% increase in glomerular filtration rate (GFR) after 20 weeks of pregnancy. In women with hypertension during pregnancy, renal function is significantly decreased due to systemic endothelial dysfunction, which results in proteinuria and lower GFR [11]. This is due to glomerular endotheliosis, which describes the loss of endothelial fenestrae of the renal capillary lumens caused by glomerular endothelial swelling [12].
To fully understand the pathophysiology of preeclampsia, researchers have studied genetically modified rats that produce a preeclamptic phenotype. In these rats, renal histologic properties were changed as described above, which, in turn, increased proteinuria due to increased glomerular permeability [13]. Assuming that permanent glomerular scarring did not occur during preeclamptic pregnancy, glomerular endotheliosis can be reversed by eliminating the cause, such as the birth of the fetus and placental delivery [11,14].
The current study suggests that renal function recovery is hindered after delivery. In addition, underrecovery after delivery may be potentiated by age, mode of delivery, and the presence of other pregnancy complications such as gestational diabetes. Moreover, there is a population-based cohort study suggesting that women with gestational diabetes possess greater risk of developing chronic kidney disease [15].
A meta-analysis, which included seven cohort studies in European and Middle Eastern regions, was undertaken to accumulate and provide an understanding of whether women with preeclampsia express a greater risk of subsequent renal disorder [16]. The participants were followed for an average of 7.1 years after delivery [16]. Women with preeclampsia and severe preeclampsia showed a four-fold and eight-fold increased risk of microalbuminuria, respectively [16]. The current study followed the participants for a similar period after delivery (8 years) and showed that hypertensive disease during pregnancy can contribute to the development of renal disorder after delivery. However, most of the study population in the meta-analysis was from European and Middle Eastern regions, whereas the current study only included Koreans [16].
Although studies on the prevalence of persistent renal complications after preeclampsia are continuously accumulating, the findings are conflicting. These findings are often dependent on the sample size, follow-up duration, endpoint definition, and other variables [5]. Additionally, it is worth noting that the meta-analysis did not include retrospective studies, which tend to show lower occurrence of proteinuria after delivery. In a study conducted among a Norwegian population, only 1.1% of preeclamptic women reported micro-albuminuria 10 years after delivery [6]. Another study followed patients for 4–5 years after delivery and reported no change in the albumin-to-creatinine ratio [7]. Furthermore, it has also been suggested that a higher incidence of persistent proteinuria can be observed if evaluated shortly after delivery (21%), followed by a decline to 14% at 3 months after delivery, whereas the incidence is only 2% at 2 years postpartum [8]. This suggests that the glomerular endothelium requires a reasonable amount of time to recover from the preeclamptic change [12]. Overall, the research on renal outcome after preeclampsia is ongoing, and further study is required to fully understand the causality under different circumstances.
The current study is the first to demonstrate that the risk of developing renal disorders after delivery is higher in pregnancies complicated by hypertension in an Asian population. This nationwide, population-based study included a large number of patients. This demonstrates that the results obtained in this study are representative of the South Korean population with a minimum risk of selection bias.
However, the hypothesis proven in this study is limited to the Korean population and cannot be applied to other Asian populations. Furthermore, the outcomes were obtained using ICD-10 codes, which may be less accurate than extracting the outcomes from medical records. In addition, obesity is also known as a potent risk factor for developing renal disorders [17]. Therefore, the inclusion of pre-pregnancy body mass index could further clarify the causality between hypertensive disease during pregnancy and the development of renal disorders.
In future, it will be necessary to evaluate whether the same hypothesis can be applied to other Asian populations. Furthermore, studies should be conducted to determine whether hypertensive diseases during pregnancy can contribute to the development of other vasculitis after delivery after delivery. Additional studies could also concentrate on whether the severity of hypertensive disease during pregnancy can increase the risk of postpartum renal complications and various diseases related to vasculitis differently. In addition, a prospective study could be useful for identifying risk factors and identifying at-risk individuals with hypertensive disease during pregnancy who might develop renal disorders after delivery.
In summary, hypertensive disease during pregnancy can be considered a contributing factor to the development of renal disorders, even after delivery, in Korea. A similar relationship in other Asian populations is possible. Therefore, it is important to actively monitor and treat patients complicated by high blood pressure during pregnancy to prevent further decrease in renal function.
Notes
References
1. Szczepanski J, Griffin A, Novotny S, Wallace K. Acute kidney injury in pregnancies complicated with preeclampsia or HELLP syndrome. Front Med (Lausanne). 2020; 7:22.

2. Abalos E, Cuesta C, Grosso AL, Chou D, Say L. Global and regional estimates of preeclampsia and eclampsia: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2013; 170:1–7.

3. Vikse BE, Irgens LM, Leivestad T, Skjaerven R, Iversen BM. Preeclampsia and the risk of end-stage renal disease. N Engl J Med. 2008; 359:800–9.

4. Kristensen JH, Basit S, Wohlfahrt J, Damholt MB, Boyd HA. Pre-eclampsia and risk of later kidney disease: nationwide cohort study. BMJ. 2019; 365:l1516.

5. Sandvik MK, Hallan S, Svarstad E, Vikse BE. Preeclampsia and prevalence of microalbuminuria 10 years later. Clin J Am Soc Nephrol. 2013; 8:1126–34.

6. Mangos GJ, Spaan JJ, Pirabhahar S, Brown MA. Markers of cardiovascular disease risk after hypertension in pregnancy. J Hypertens. 2012; 30:351–8.

7. Berks D, Steegers EAP, Molas M, Visser W. Resolution of hypertension and proteinuria after preeclampsia. Obstet Gynecol. 2009; 114:1307–14.

8. Dreyer G, Hull S, Aitken Z, Chesser A, Yaqoob MM. The effect of ethnicity on the prevalence of diabetes and associated chronic kidney disease. QJM. 2009; 102:261–9.

9. Peralta CA, Katz R, DeBoer I, Ix J, Sarnak M, Kramer H, et al. Racial and ethnic differences in kidney function decline among persons without chronic kidney disease. J Am Soc Nephrol. 2011; 22:1327–34.

10. Song SO, Jung CH, Song YD, Park CY, Kwon HS, Cha BS, et al. Background and data configuration process of a nationwide population-based study using the korean national health insurance system. Diabetes Metab J. 2014; 38:395–403.

11. Paauw ND, Luijken K, Franx A, Verhaar MC, Lely AT. Long-term renal and cardiovascular risk after preeclampsia: towards screening and prevention. Clin Sci (Lond). 2016; 130:239–46.

12. Hladunewich M, Karumanchi SA, Lafayette R. Pathophysiology of the clinical manifestations of preeclampsia. Clin J Am Soc Nephrol. 2007; 2:543–9.

13. Gillis EE, Williams JM, Garrett MR, Mooney JN, Sasser JM. The dahl salt-sensitive rat is a spontaneous model of superimposed preeclampsia. Am J Physiol Regul Integr Comp Physiol. 2015; 309:R62–70.

14. Lopes van Balen VA, Spaan JJ, Cornelis T, Spaanderman MEA. Prevalence of chronic kidney disease after preeclampsia. J Nephrol. 2017; 30:403–9.

15. Barrett PM, McCarthy FP, Evans M, Kublickas M, Perry IJ, Stenvinkel P, et al. Does gestational diabetes increase the risk of maternal kidney disease? A Swedish national cohort study. PLoS One. 2022; 17:e0264992.

Table 1
ICD-10 codes for hypertensive disease during pregnancy
Table 2
ICD-10 codes for renal disease
Table 3
Obstetrical characteristics of the participants
Table 4
Risk factors with adjusted hazard ratio for developing renal disorder 8-year after delivery




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download