1. Yakasai IA, Bappa LA. Diagnosis and management of adnexal masses in pregnancy. J Surg Tech Case Rep. 2012; 4:79–85.

2. Hakoun AM, AbouAl-Shaar I, Zaza KJ, Abou-Al-Shaar H, A Salloum MN. Adnexal masses in pregnancy: an updated review. Avicenna J Med. 2017; 7:153–7.

3. Kumari I, Kaur S, Mohan H, Huria A. Adnexal masses in pregnancy: a 5-year review. Aust N Z J Obstet Gynaecol. 2006; 46:52–4.

4. Bernhard LM, Klebba PK, Gray DL, Mutch DG. Predictors of persistence of adnexal masses in pregnancy. Obstet Gynecol. 1999; 93:585–9.

5. Nelson MJ, Cavalieri R, Graham D, Sanders RC. Cysts in pregnancy discovered by sonography. J Clin Ultrasound. 1986; 14:509–12.

6. Lee SJ, Oh HR, Na S, Hwang HS, Lee SM. Ultrasonographic ovarian mass scoring system for predicting malignancy in pregnant women with ovarian mass. Obstet Gynecol Sci. 2022; 65:1–13.

7. Oehler MK, Wain GV, Brand A. Gynaecological malignancies in pregnancy: a review. Aust N Z J Obstet Gynaecol. 2003; 43:414–20.
8. Horowitz NS. Management of adnexal masses in pregnancy. Clin Obstet Gynecol. 2011; 54:519–27.

9. Gholson R, Pullen J, Miles A, Gardner MO, Doyle NM. Adnexal masses in pregnancy: does magnitude matter? Obstetrics & Gynecology. 2014; 123:192S–3S.
10. Hoover K, Jenkins TR. Evaluation and management of adnexal mass in pregnancy. Am J Obstet Gynecol. 2011; 205:97–102.

11. Condous G, Khalid A, Okaro E, Bourne T. Should we be examining the ovaries in pregnancy? Prevalence and natural history of adnexal pathology detected at first-trimester sonography. Ultrasound Obstet Gynecol. 2004; 24:62–6.

12. Giuntoli RL 2nd, Vang RS, Bristow RE. Evaluation and management of adnexal masses during pregnancy. Clin Obstet Gynecol. 2006; 49:492–505.

13. Kwon YS, Mok JE, Lim KT, Lee IH, Kim TJ, Lee KH, et al. Ovarian cancer during pregnancy: clinical and pregnancy outcome. J Korean Med Sci. 2010; 25:230–4.

14. Mohaghegh P, Rockall AG. Imaging strategy for early ovarian cancer: characterization of adnexal masses with conventional and advanced imaging techniques. Radiographics. 2012; 32:1751–73.

15. Ha HI, Chang HK, Park SJ, Lim J, Won YJ, Lim MC. The incidence and survival of cervical, ovarian, and endometrial cancer in Korea, 1999–2017: Korea Central Cancer Registry. Obstet Gynecol Sci. 2021; 64:444–53.

16. de Haan J, Verheecke M, Van Calsteren K, Van Calster B, Shmakov RG, Mhallem Gziri M, et al. Oncological management and obstetric and neonatal outcomes for women diagnosed with cancer during pregnancy: a 20-year international cohort study of 1170 patients. Lancet Oncol. 2018; 19:337–46.

17. Kaijser J. Towards an evidence-based approach for diagnosis and management of adnexal masses: findings of the international ovarian tumour analysis (IOTA) studies. Facts Views Vis Obgyn. 2015; 7:42–59.
18. Sassone AM, Timor-Tritsch IE, Artner A, Westhoff C, Warren WB. Transvaginal sonographic characterization of ovarian disease: evaluation of a new scoring system to predict ovarian malignancy. Obstet Gynecol. 1991; 78:70–6.
19. Lerner JP, Timor-Tritsch IE, Federman A, Abramovich G. Transvaginal ultrasonographic characterization of ovarian masses with an improved, weighted scoring system. Am J Obstet Gynecol. 1994; 170:81–5.

20. DePriest PD, Shenson D, Fried A, Hunter JE, Andrews SJ, Gallion HH, et al. A morphology index based on sonographic findings in ovarian cancer. Gynecol Oncol. 1993; 51:7–11.

21. Cao L, Wei M, Liu Y, Fu J, Zhang H, Huang J, et al. Validation of American college of radiology ovarian-adnexal reporting and data system ultrasound (O-RADS US): analysis on 1054 adnexal masses. Gynecol Oncol. 2021; 162:107–12.

22. Rossi A, Braghin C, Soldano F, Isola M, Capodicasa V, Londero AP, et al. A proposal for a new scoring system to evaluate pelvic masses: pelvic masses score (PMS). Eur J Obstet Gynecol Reprod Biol. 2011; 157:84–8.

23. Wang WH, Zheng CB, Gao JN, Ren SS, Nie GY, Li ZQ. Systematic review and meta-analysis of imaging differential diagnosis of benign and malignant ovarian tumors. Gland Surg. 2022; 11:330–40.

24. Glanc P, Brofman N, Kornecki A, Abrams J, Farine D, Salem S. Visualization of the ovaries in early pregnancy by transvaginal sonography. J Obstet Gynaecol Can. 2007; 29:228–31.

25. Chiang G, Levine D. Imaging of adnexal masses in pregnancy. J Ultrasound Med. 2004; 23:805–19.

26. Glanc P, Brofman N, Salem S, Kornecki A, Abrams J, Farine D. The prevalence of incidental simple ovarian cysts >or= 3 cm detected by transvaginal sonography in early pregnancy. J Obstet Gynaecol Can. 2007; 29:502–6.
27. Frates MC, Doubilet PM, Durfee SM, Di Salvo DN, Laing FC, Brown DL, et al. Sonographic and Doppler characteristics of the corpus luteum: can they predict pregnancy outcome? J Ultrasound Med. 2001; 20:821–7.

28. Parsons AK. Imaging the human corpus luteum. J Ultrasound Med. 2001; 20:811–9.

29. Guerriero S, Ajossa S, Melis G. Imaging the human corpus luteum. J Ultrasound Med. 2001; 20:1376–7.

30. Kobayashi H, Yoshida A, Kobayashi M, Yamada T. Changes in size of the functional cyst on ultrasonography during early pregnancy. Am J Perinatol. 1997; 14:1–4.

31. Glanc P, Salem S, Farine D. Adnexal masses in the pregnant patient: a diagnostic and management challenge. Ultrasound Q. 2008; 24:225–40.
32. Foulk RA, Martin MC, Jerkins GL, Laros RK. Hyperreactio luteinalis differentiated from severe ovarian hyperstimulation syndrome in a spontaneously conceived pregnancy. Am J Obstet Gynecol. 1997; 176:1300–2. discussion 1302–4.

33. Mashiach S, Bider D, Moran O, Goldenberg M, Ben-Rafael Z. Adnexal torsion of hyperstimulated ovaries in pregnancies after gonadotropin therapy. Fertil Steril. 1990; 53:76–80.

34. Gorkemli H, Camus M, Clasen K. Adnexal torsion after gonadotrophin ovulation induction for IVF or ICSI and its conservative treatment. Arch Gynecol Obstet. 2002; 267:4–6.

35. Wiser A, Levron J, Kreizer D, Achiron R, Shrim A, Schiff E, et al. Outcome of pregnancies complicated by severe ovarian hyperstimulation syndrome (OHSS): a follow-up beyond the second trimester. Hum Reprod. 2005; 20:910–4.

36. Manganiello PD, Adams LV, Harris RD, Ornvold K. Virilization during pregnancy with spontaneous resolution postpartum: a case report and review of the English literature. Obstet Gynecol Surv. 1995; 50:404–10.
37. Shadinger LL, Andreotti RF, Kurian RL. Preoperative sonographic and clinical characteristics as predictors of ovarian torsion. J Ultrasound Med. 2008; 27:7–13.

38. Vijayaraghavan SB. Sonographic whirlpool sign in ovarian torsion. J Ultrasound Med. 2004; 23:1643–9. quiz 1650–1.

39. Lee EJ, Kwon HC, Joo HJ, Suh JH, Fleischer AC. Diagnosis of ovarian torsion with color Doppler sonography: depiction of twisted vascular pedicle. J Ultrasound Med. 1998; 17:83–9.

40. Chiou SY, Lev-Toaff AS, Masuda E, Feld RI, Bergin D. Adnexal torsion: new clinical and imaging observations by sonography, computed tomography, and magnetic resonance imaging. J Ultrasound Med. 2007; 26:1289–301.
41. Desai SK, Allahbadia GN, Dalal AK. Ovarian torsion: diagnosis by color Doppler ultrasonography. Obstet Gynecol. 1994; 84:699–701.
42. Leeners B, Damaso F, Ochsenbein-Kölble N, Farquhar C. The effect of pregnancy on endometriosis-facts or fiction? Hum Reprod Update. 2018; 24:290–9.

43. Asch E, Levine D. Variations in appearance of endometriomas. J Ultrasound Med. 2007; 26:993–1002.

44. Patel MD, Feldstein VA, Chen DC, Lipson SD, Filly RA. Endometriomas: diagnostic performance of US. Radiology. 1999; 210:739–45.

45. Jain KA. Endometrioma with calcification simulating a dermoid on sonography. J Ultrasound Med. 2006; 25:1237–41.

46. Coccia ME, Rizzello F, Palagiano A, Scarselli G. The effect of the hormonal milieu of pregnancy on deep infiltrating endometriosis: serial ultrasound assessment of changes in size and pattern of deep endometriotic lesions. Eur J Obstet Gynecol Reprod Biol. 2012; 160:35–9.

47. Ueda Y, Enomoto T, Miyatake T, Fujita M, Yamamoto R, Kanagawa T, et al. A retrospective analysis of ovarian endometriosis during pregnancy. Fertil Steril. 2010; 94:78–84.

48. Benaglia L, Somigliana E, Calzolari L, Busnelli A, Cardellicchio L, Ragni G, et al. The vanishing endometrioma: the intriguing impact of pregnancy on small endometriotic ovarian cysts. Gynecol Endocrinol. 2013; 29:863–6.

49. Adusumilli S, Hussain HK, Caoili EM, Weadock WJ, Murray JP, Johnson TD, et al. MRI of sonographically indeterminate adnexal masses. AJR Am J Roentgenol. 2006; 187:732–40.

50. Sammour RN, Leibovitz Z, Shapiro I, Degani S, Levitan Z, Aharoni A, et al. Decidualization of ovarian endometriosis during pregnancy mimicking malignancy. J Ultrasound Med. 2005; 24:1289–94.

51. Alcázar JL, Laparte C, Jurado M, López-García G. The role of transvaginal ultrasonography combined with color velocity imaging and pulsed Doppler in the diagnosis of endometrioma. Fertil Steril. 1997; 67:487–91.

52. Aslam N, Ong C, Woelfer B, Nicolaides K, Jurkovic D. Serum CA125 at 11–14 weeks of gestation in women with morphologically normal ovaries. Bjog. 2000; 107:689–90.

53. Resnik R, Creasy RK, Iams JD, Lockwood CJ, Moore T, Greene MF. Creasy and resnik’s maternal-fetal medicine: principles and practice e-book. 6th ed. Philadelphia: Elsevier Health Sciences;2008.
54. Koonings PP, Campbell K, Mishell DR Jr, Grimes DA. Relative frequency of primary ovarian neoplasms: a 10-year review. Obstet Gynecol. 1989; 74:921–6.

55. Pepe F, Lo Monaco S, Rapisarda F, Raciti G, Genovese C, Pepe P. An unusual case of multiple and bilateral ovarian dermoid cysts. Case report. G Chir. 2014; 35:75–7.
56. Ayhan A, Aksu T, Develioglu O, Tuncer ZS, Ayhan A. Complications and bilaterality of mature ovarian teratomas (clinicopathological evaluation of 286 cases). Aust N Z J Obstet Gynaecol. 1991; 31:83–5.

57. Comerci JT Jr, Licciardi F, Bergh PA, Gregori C, Breen JL. Mature cystic teratoma: a clinicopathologic evaluation of 517 cases and review of the literature. Obstet Gynecol. 1994; 84:22–8.
58. Rumack CM, Wilson SR, Charboneau JW. Diagnostic ultrasound. 1 and 2:3rd ed. New York: Mosby Year Book;1991. p. 220–6.
59. Osto M, Brooks A, Khan A. Ovarian cystic teratoma in pregnant women: conservative management or prophylactic oophorectomy? Cureus. 2021; 13:e17354.

60. Bromley B, Benacerraf B. Adnexal masses during pregnancy: accuracy of sonographic diagnosis and outcome. J Ultrasound Med. 1997; 16:447–52. quiz 453–4.

61. Patel MD, Feldstein VA, Lipson SD, Chen DC, Filly RA. Cystic teratomas of the ovary: diagnostic value of sonography. AJR Am J Roentgenol. 1998; 171:1061–5.

62. Kawamoto S, Sato K, Matsumoto H, Togo Y, Ueda Y, Tanaka J, et al. Multiple mobile spherules in mature cystic teratoma of the ovary. AJR Am J Roentgenol. 2001; 176:1455–7.

63. Hricak H, Ascher S. Pocketradiologist - gynecologic: top 100 diagnoses. 1st ed. Salt Lake City: Amirsys;2004.
64. Saha S, Robertson M. Meigs’ and pseudo-meigs’ syndrome. Australas J Ultrasound Med. 2012; 15:29–31.

65. Zhang H, Zhang H, Gu S, Zhang Y, Liu X, Zhang G. MR findings of primary ovarian granulosa cell tumor with focus on the differentiation with other ovarian sex cord-stromal tumors. J Ovarian Res. 2018; 11:46.

66. Timor-Tritsch IE, Lerner JP, Monteagudo A, Murphy KE, Heller DS. Transvaginal sonographic markers of tubal inflammatory disease. Ultrasound Obstet Gynecol. 1998; 12:56–66.

67. Pectasides D, Pectasides E, Economopoulos T. Fallopian tube carcinoma: a review. Oncologist. 2006; 11:902–12.

68. Skírnisdóttir I, Garmo H, Wilander E, Holmberg L. Borderline ovarian tumors in Sweden 1960–2005: trends in incidence and age at diagnosis compared to ovarian cancer. Int J Cancer. 2008; 123:1897–901.

69. Sherman ME, Mink PJ, Curtis R, Cote TR, Brooks S, Hartge P, et al. Survival among women with borderline ovarian tumors and ovarian carcinoma: a population-based analysis. Cancer. 2004; 100:1045–52.

70. Aggarwal P, Kehoe S. Ovarian tumours in pregnancy: a literature review. Eur J Obstet Gynecol Reprod Biol. 2011; 155:119–24.

71. Kane A, Uzan C, Rey A, Gouy S, Camatte S, Pautier P, et al. Prognostic factors in patients with ovarian serous low malignant potential (borderline) tumors with peritoneal implants. Oncologist. 2009; 14:591–600.

72. Du Bois A, Ewald-Riegler N, Du Bois O, Harter P. Borderline-tumoren des ovars-eine systematische Übersicht. Geburtshilfe Frauenheilkd. 2009; 69:807–33.
73. du Bois A, Ewald-Riegler N, de Gregorio N, Reuss A, Mahner S, Fotopoulou C, et al. Borderline tumours of the ovary: a cohort study of the Arbeitsgmeinschaft Gynäkologische Onkologie (AGO) study group. Eur J Cancer. 2013; 49:1905–14.
74. Helpman L, Beiner ME, Aviel-Ronen S, Perri T, Hogen L, Jakobson-Setton A, et al. Safety of ovarian conservation and fertility preservation in advanced borderline ovarian tumors. Fertil Steril. 2015; 104:138–44.

75. Uzan C, Kane A, Rey A, Gouy S, Duvillard P, Morice P. Outcomes after conservative treatment of advanced-stage serous borderline tumors of the ovary. Ann Oncol. 2010; 21:55–60.

76. Franchi D, Boveri S, Radice D, Portuesi R, Zanagnolo V, Colombo N, et al. Ultrasonographic diagnosis and longitudinal follow-up of recurrences after conservative surgery for borderline ovarian tumors. Am J Obstet Gynecol. 2016; 215:e1–756e9.
77. Auekitrungrueng R, Tinnangwattana D, Tantipalakorn C, Charoenratana C, Lerthiranwong T, Wanapirak C, et al. Comparison of the diagnostic accuracy of international ovarian tumor analysis simple rules and the risk of malignancy index to discriminate between benign and malignant adnexal masses. Int J Gynaecol Obstet. 2019; 146:364–9.

78. Patel-Lippmann KK, Sadowski EA, Robbins JB, Paroder V, Barroilhet L, Maddox E, et al. Comparison of international ovarian tumor analysis simple rules to society of radiologists in ultrasound guidelines for detection of malignancy in adnexal cysts. AJR Am J Roentgenol. 2020; 214:694–700.

79. Bent CL, Sahdev A, Rockall AG, Singh N, Sohaib SA, Reznek RH. MRI appearances of borderline ovarian tumours. Clin Radiol. 2009; 64:430–8.

80. Bazot M, Nassar-Slaba J, Thomassin-Naggara I, Cortez A, Uzan S, Daraï E. MR imaging compared with intraoperative frozen-section examination for the diagnosis of adnexal tumors; correlation with final histology. Eur Radiol. 2006; 16:2687–99.

81. Leiserowitz GS, Xing G, Cress R, Brahmbhatt B, Dalrymple JL, Smith LH. Adnexal masses in pregnancy: how often are they malignant? Gynecol Oncol. 2006; 101:315–21.

82. Zhao XY, Huang HF, Lian LJ, Lang JH. Ovarian cancer in pregnancy: a clinicopathologic analysis of 22 cases and review of the literature. Int J Gynecol Cancer. 2006; 16:8–15.

83. Gezginç K, Karataylı R, Yazıcı F, Acar A, Celik C, Capar M. Ovarian cancer during pregnancy. Int J Gynaecol Obstet. 2011; 115:140–3.

84. Committee opinion No. 696: nonobstetric surgery during pregnancy. Obstet Gynecol. 2017; 129:777–8.
85. Yuen PM, Ng PS, Leung PL, Rogers MS. Outcome in laparoscopic management of persistent adnexal mass during the second trimester of pregnancy. Surg Endosc. 2004; 18:1354–7.

86. Al-Fozan H, Tulandi T. Safety and risks of laparoscopy in pregnancy. Curr Opin Obstet Gynecol. 2002; 14:375–9.

87. Amant F, Halaska MJ, Fumagalli M, Dahl Steffensen K, Lok C, Van Calsteren K, et al. Gynecologic cancers in pregnancy: guidelines of a second international consensus meeting. Int J Gynecol Cancer. 2014; 24:394–403.

88. Hong JY. Adnexal mass surgery and anesthesia during pregnancy: a 10-year retrospective review. Int J Obstet Anesth. 2006; 15:212–6.

89. Visser BC, Glasgow RE, Mulvihill KK, Mulvihill SJ. Safety and timing of nonobstetric abdominal surgery in pregnancy. Dig Surg. 2001; 18:409–17.

90. Kodama M, Grubbs BH, Blake EA, Cahoon SS, Murakami R, Kimura T, et al. Feto-maternal outcomes of pregnancy complicated by ovarian malignant germ cell tumor: a systematic review of literature. Eur J Obstet Gynecol Reprod Biol. 2014; 181:145–56.

91. Elit L, Bocking A, Kenyon C, Natale R. An endodermal sinus tumor diagnosed in pregnancy: case report and review of the literature. Gynecol Oncol. 1999; 72:123–7.

92. Buller RE, Darrow V, Manetta A, Porto M, DiSaia PJ. Conservative surgical management of dysgerminoma concomitant with pregnancy. Obstet Gynecol. 1992; 79:887–90.
93. Morice P, Uzan C, Gouy S, Verschraegen C, Haie-Meder C. Gynaecological cancers in pregnancy. Lancet. 2012; 379:558–69.

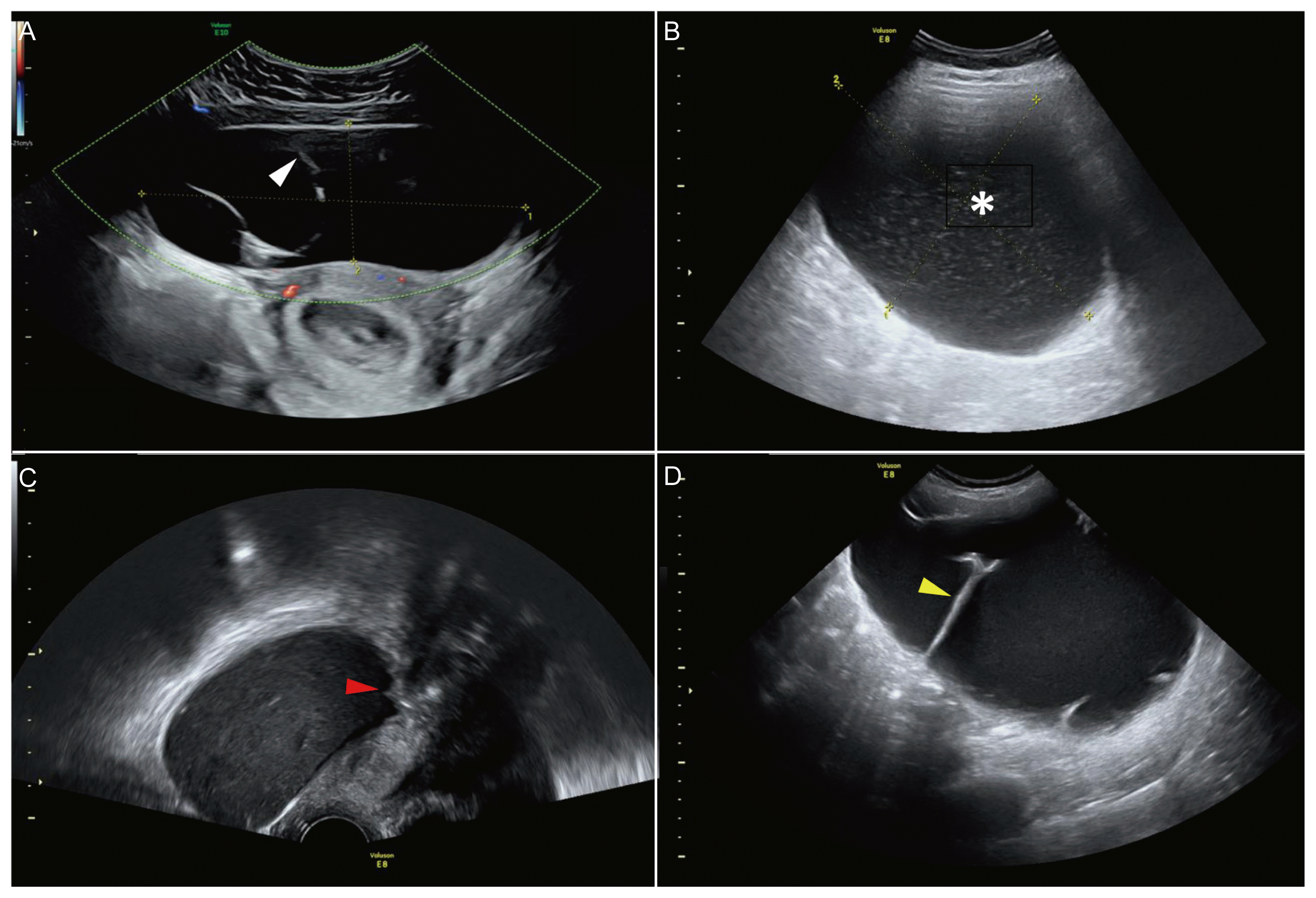
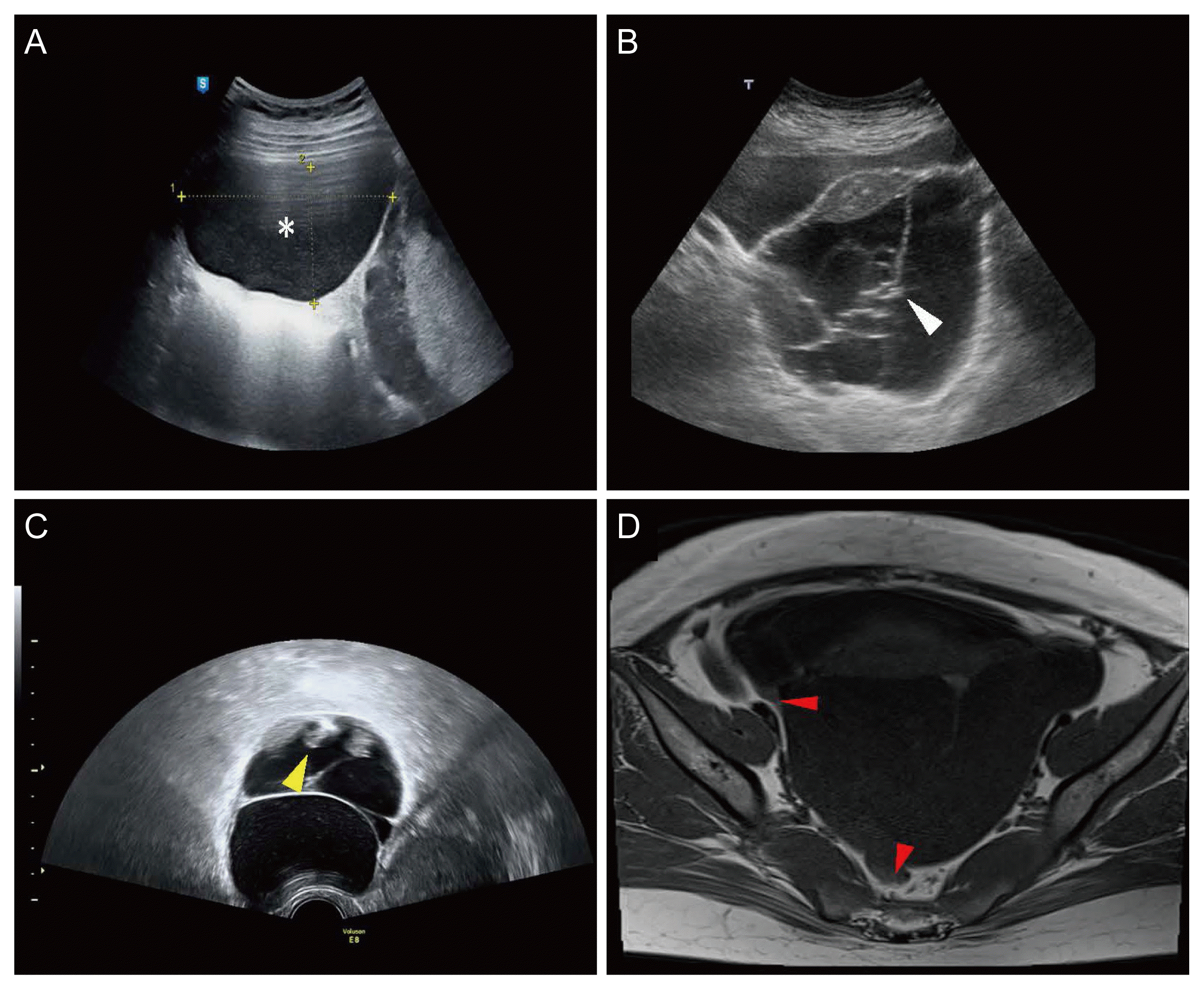




 PDF
PDF Citation
Citation Print
Print




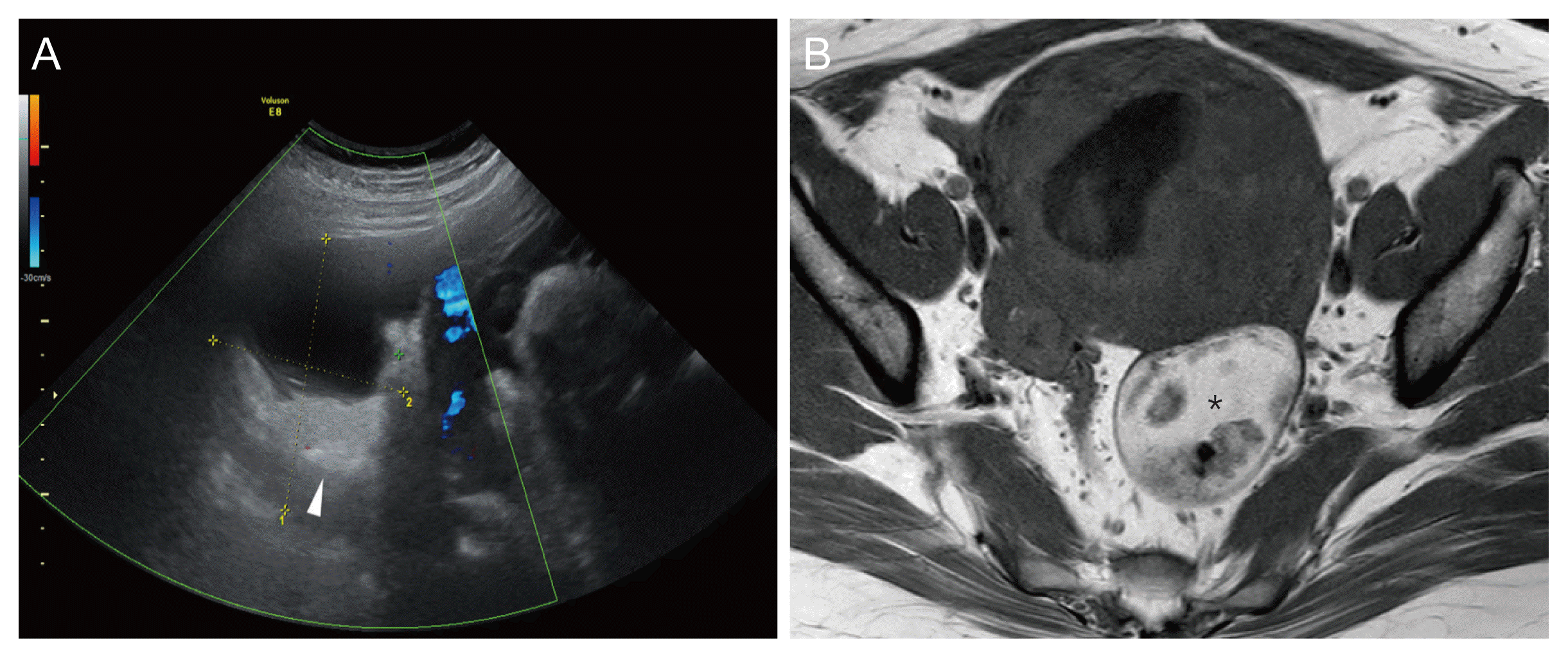
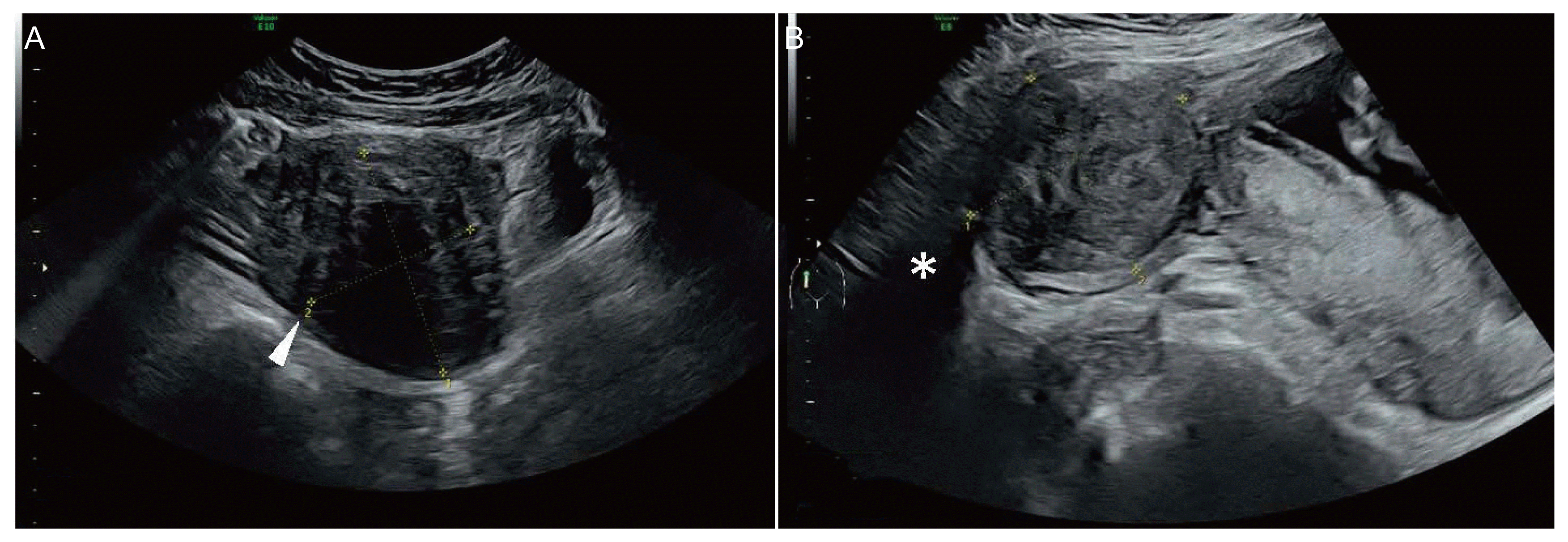
 XML Download
XML Download